Abstract
Aim
We tested the hypothesis that early recovery of cortical SEP would be associated with milder hypoxic-ischemic injury and better outcome after resuscitation from CA.
Methods
Sixteen adult male Wistar rats were subjected to asphyxial cardiac arrest. Half underwent 7 min of asphyxia (Group CA7) and half underwent 9 min (Group CA9). Continuous SEPs from median nerve stimulation were recorded from these rats for 4hr immediately following CA, and at 24, 48, and 72hr. Clinical recovery was evaluated using the Neurologic Deficit Scale.
Results
All rats in group CA7 survived to 72hr, while only 50% of rats in group CA9 survived to that time. Mean NDS values in the CA7 group at 24, 48, and 72hr after CA were significantly higher than those of CA9. The N10 (first negative potential at 10 ms) amplitude was significantly lower within 1 hr after CA in rats that suffered longer CA durations. SEPs were also analyzed by separating the rats into good (NDS ≥ 50) vs. bad (NDS < 50) outcomes at 72hr, again showing significant difference in N10 and peak-to-peak amplitudes between the two groups. In addition, a smaller N7 potential was consistently observed to recover earlier in all rats.
Conclusions
The diminished recovery of N10 is associated with longer CA times in rats. Higher N10 and peak-to-peak amplitudes during early recovery are associated with better neurologic outcomes. N7, which may represent thalamic activity, recovers much earlier than cortical responses (N10), suggesting failure of thalamocortical conduction during early recovery.
Introduction
Approximately 450,000 Americans suffer cardiac arrests (CA) annually, with 80% of them occurring out-of-hospital.1 The survival rate of out-of-hospital CA is less than 10%.2 Brain injury remains a leading cause of morbidity, with only 3–7% of survivors returning to their previous levels of function.2
Currently, neuroprotective therapies such as hypothermia are applied without objective measures of neuronal function or therapeutic response. Though electroencephalography (EEG) remains a mainstay of neurologic monitoring, it is susceptible to external factors such as anesthesia, medications, or temperature. There exists a strong need to develop robust, noninvasive neurophysiologic markers that can be used to optimize neuroprotective treatments in real-time. To achieve this goal, the evolution of neurophysiologic signals must first be defined.
Previous experiments indicate that synchronous activity between the thalamus and cortex is necessary to maintain consciousness.3 Neuronal injury from global hypoxia may contribute to coma by disrupting these thalamocortical circuits.4 Prolonged thalamocortical dissociation (for up to 90 min) has been reported in rats that experienced severe asphyxial CA of 7 min duration. 5 We believe that faster resolution of this thalamocortical dissociation is pivotal to neurological recovery. Somatosensory evoked potentials (SEP) analyze integrity and function of thalamocortical circuits because these signals are conducted through the thalamic ventral posterolateral (VPL) nucleus to the primary sensory cortex. Previous work has demonstrated the existence of a matrix of specialized neurons in the thalamus which are implicated in thalamo-cortico-thalamic interactions necessary for consciousness.6 These neurons are abundant in the VPL nucleus, making it a good target for functional studies.6
SEPs have been extensively studied for evaluation of neurologic function after resuscitation and are the most accurate neurophysiologic predictor of poor outcome after CA. The bilateral absence of the N20 response (the primary somatosensory cortical response to median nerve electrical stimulation with a latency of 20 ms) reliably predicts poor neurologic outcome.7–9 While the current application of SEP is as a static measure of prognosis (i.e. presence or absence of N20 at 24–72 hr), the evolution of the SEP signal during the early stages of recovery from CA has not been characterized nor studied in relation to outcomes.10 Previous studies have indicated that early measurement of SEP (less than 3 hr after CA) may be predictive of neurologic outcome in humans.11
Using a well-studied and validated model of graded asphyxial CA in rats, we tested the hypothesis that SEP signal would recover at measurably different rates depending on the extent of hypoxic-ischemic injury.12, 13 Furthermore, we tested the hypothesis that neurologic outcomes could be predicted by examining the extent of SEP recovery during the early stages of post-CA recovery.
Materials and Methods
A. Asphyxia Rat Model of CA and Resuscitation Protocol
All use of rodents was approved by the Johns Hopkins School of Medicine Animal Care and Use Committee. Sixteen male Wistar rats (350±25g) underwent experimentation using an asphyxia model of CA, as previously described. Eight rats underwent 7 min of asphyxia, and 8 rats underwent 9 min of asphyxia. All rats were allowed free access to food and water, and were housed in a temperature controlled environment with regular day-night cycles.
Rats were intubated and mechanically ventilated using a pressure controlled ventilator (Kent Scientific). Anesthesia was achieved using 1.5% isoflurane in a 50% N2/50% O2 carrier gas. Throughout the entire CA protocol, rats were kept normothermic (37±1°C) using a thermo-regulated heating pad (Physitemp Instruments) based on rectal probe temperatures. The femoral artery and vein just distal to the inguinal ligament were canulated in order to obtain arterial blood gases (ABG), monitor blood pressure, and provide drugs. An initial ABG was checked to ensure appropriate ventilation and oxygenation. Ventilator adjustments were made to normalize the ABG before proceeding with the protocol. Following 15 min of baseline SEP recording, isoflurane was turned off for 5 min prior to cessation of mechanical ventilation and clamping of the breathing circuit. During the last 2 min of isoflurane washout, 2 mg/kg of vecuronium was administered and the gas mixture was switched to room air. Time to CA (from start of asphyxia to mean arterial pressure < 10 mmHg) was recorded. Total asphyxia time for each rat was either 7 or 9 min. Cardiopulmonary resuscitation (CPR) was performed with external chest compressions, mechanical ventilation, and intravenous epinephrine and sodium bicarbonate boluses. Isoflurane was restarted at 0.5% after 45 min to maintain animal comfort during ongoing SEP measurements, and was titrated up to 1.5% as needed.
B. Data Recording and SEP Stimulation
One week prior to CA, each rat was implanted with 5 epidural screw electrodes placed over the primary somatosensory cortices of the fore and hind limbs, and a ground electrode over the right frontal lobe. Electrodes were implanted to a depth such that light contact with the dura matter was made without penetration into the brain. Electrodes, wires, and the exposed skull were covered by dental cement. SEP signals were recorded using Tucker Davis Technologies (TDT) data acquisition systems and software. Stimulation of the median nerves was achieved though insertion of two pairs of 1 cm stainless steel needle electrodes in the distal forelimbs. Direct current stimulation was applied with pulse duration of 200 μs, 0.6 mA, and frequency of 0.5 Hz. Signals were sampled at a frequency of 6.1 kHz. Along with SEP data, ECG and continuous arterial blood pressure waveforms were also recorded. Baseline SEP signals were recorded for 15 min prior to CA. Continuous SEP signals were then recorded from 5 min prior to asphyxia to 1 hr after initiating CPR. Subsequently, SEP was recorded in alternating 15 min blocks for the remainder of the first 4 hr after CPR. After the initial 4 hr period, the rats were returned to their cages and were allowed free access to food and water. Subsequent recordings of 15 min duration were made at 24, 48, and 72 hr after CPR.
C. Clinical Evaluation
Rats were clinically evaluated by serial Neurologic Deficit Scale (NDS) assessments at 24, 48, and 72 hr after CPR. The NDS has been previously validated as an outcome measure in rats after global hypoxic-ischemic injury. It is fashioned after a standard human neurologic exam and incorporates elements of outcome scales for rats, dogs, and piglets.12 The NDS score ranges from 0 to 80, where 80 represents a neurologically normal rat (Table 1). As with previous studies, the primary outcome measure was defined as NDS score at 72 hr, and rats that died prior to the evaluation were assigned a score of 0.
Table 1.
Animal characteristics by experimental group (Mean±SD). Time to CA defined from start of asphyxia to mean arterial pressure < 10 mmHg. Time to return of spontaneous circulation (ROSC) measured from start of CPR.
| 7 min (CA7) | 9 min (CA9) | p-value | |
|---|---|---|---|
| Weight (g) | 363±12 | 358±12 | 0.23 |
| Mean time to CA (s) | 182±33 | 188±35 | 0.37 |
| Mean time to ROSC (s) | 27.9±2.7 | 30.7±1.9 | 0.02 |
| Baseline ABG | |||
| pH | 7.44±0.02 | 7.46±0.04 | 0.18 |
| pCO2 | 41.1±5.4 | 37.7±8.7 | 0.40 |
| pO2 | 204.8±36.4 | 178.6±49.9 | 0.30 |
| HCO3 | 27.6±3.4 | 26.4±4.5 | 0.60 |
D. Signal Analysis Methods
Traditional signal analysis methods were used to analyze the acquired SEPs. Recorded SEPs were averages of 20 single sweeps. During final analysis, SEPs were averaged over a 6 min period (180 sweeps) at regular time intervals for peak detection. Signals were analyzed using MATLAB to determine the amplitudes and latencies of the initial significant negative (upward deflection) potential. Automatic peak-detection algorithms were used to aid in this process. This potential is referred to as the N10, given its approximate latency of 10 ms. This N10 potential likely represents signal from the primary somatosensory cortex triggered by afferent stimulation relayed through the thalamic VPL nucleus, equivalent to the human N20. Previous studies have demonstrated an initial cortical response between 7 and 13 ms latency in rats.5, 14–16 The amplitude of N10 is normalized to the baseline signal recorded just prior to the initiation of the CA protocol. The latencies were measured from the time of the stimulus artifact seen on the recorded signals, and were normalized before analysis. When a N10 peak was not detected, the latency was excluded from statistical analysis. Other important peaks, the N7 (negative potential at 7 ms latency) and P15 (positive potential at 15 ms latency), were detected and analyzed using similar methods as the N10. Peak-to-peak amplitudes were calculated as the difference between N10 and P15.
E. Statistical Analysis
Based on preliminary experiments, we estimated that a total sample size of 16 rats would be needed to generate significant results. Comparison of NDS scores between the two experimental groups was performed using the Mann-Whitney test. This test is equivalent to the Student’s t-test for parametric distributions but does not assume normality of population samples. Comparisons of SEP amplitudes and latencies were performed using Student’s t-test, one-tailed, assuming unequal variances. Repeated measures analysis of variance (ANOVA) was also performed on the SEP amplitudes. A p-value of less than 0.05 was considered statistically significant for the above tests.
SEP amplitudes and latencies of rats that underwent 7 min vs. 9 min of asphyxia were compared. In order to correlate NDS and SEPs, we also compared rats dichotomized to good (NDS ≥ 50) vs. bad (NDS < 50) outcomes at 72 hr. Since this cohort of rats all experienced severe asphyxial CA without induced hypothermia, outcomes were skewed toward lower NDS scores than prior cohorts. We, therefore, chose a NDS cutoff of 50 in order to produce balanced sample sizes in the 2 groups. The NDS cutoff of 50 also dichotomizes rats into 2 functionally distinct groups. Rats with NDS <50 were immobile and reacted minimally to environmental stimuli. Rats with NDS >50 generally retained some mobility and reacted briskly to stimuli.
Results
Sixteen rats were included in the experiment; 8 rats were randomly selected for 7 min asphyxia duration (Group CA7) and the other 8 rats were selected for 9 min asphyxia (Group CA9). Characteristics of the rats in each group are summarized in Table 1.
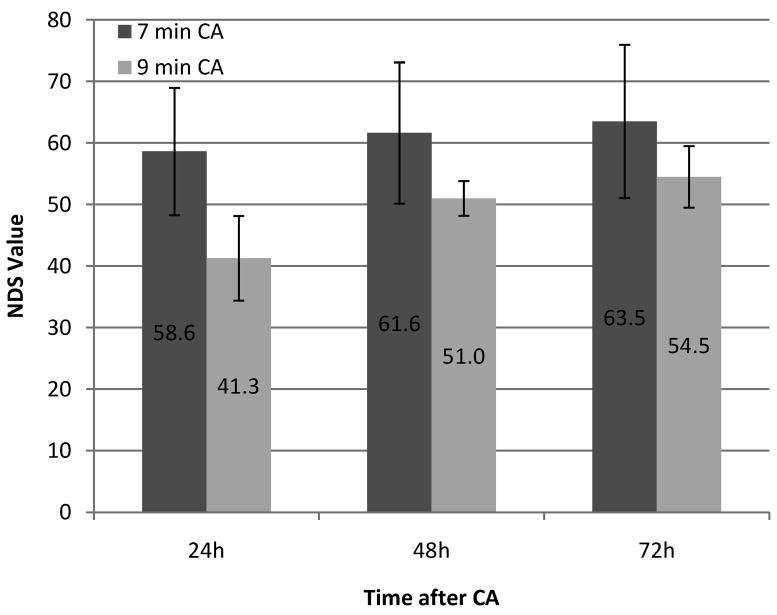
All rats in group CA7 survived to 72 hr, while only 4 rats in group CA9 survived to that time. NDS scores were assessed at 24, 48, and 72 hr after CA, which are shown by group and time in Figure 1. As a group, CA7 neurologic outcomes were better than CA9 on all days. Mean±SD NDS values in the CA7 group at 24, 48, and 72 hr after CA were 58.6±10.4, 61.6±11.5, and 63.5±12.4; while in group CA9, they were 41.3±6.9 (p-value 0.002), 51.0±2.8 (p-value 0.040), and 54.5±5.0 (p-value 0.019), respectively.
Figure 1.
Serial individual and mean (line) NDS scores at 24h, 48h, and 72h after cardiac arrest of 7 and 9 min CA rats.
The primary measures of SEP signal recovery were N10 amplitude and latency. Peak-to-peak amplitudes were also analyzed for correlation with neurologic outcomes as measured by the NDS at 72 hr. One rat’s SEP data from the CA9 group was excluded from statistical analysis because it did not exhibit normal baseline SEP signals (i.e. distorted N10 and P15 bilaterally).
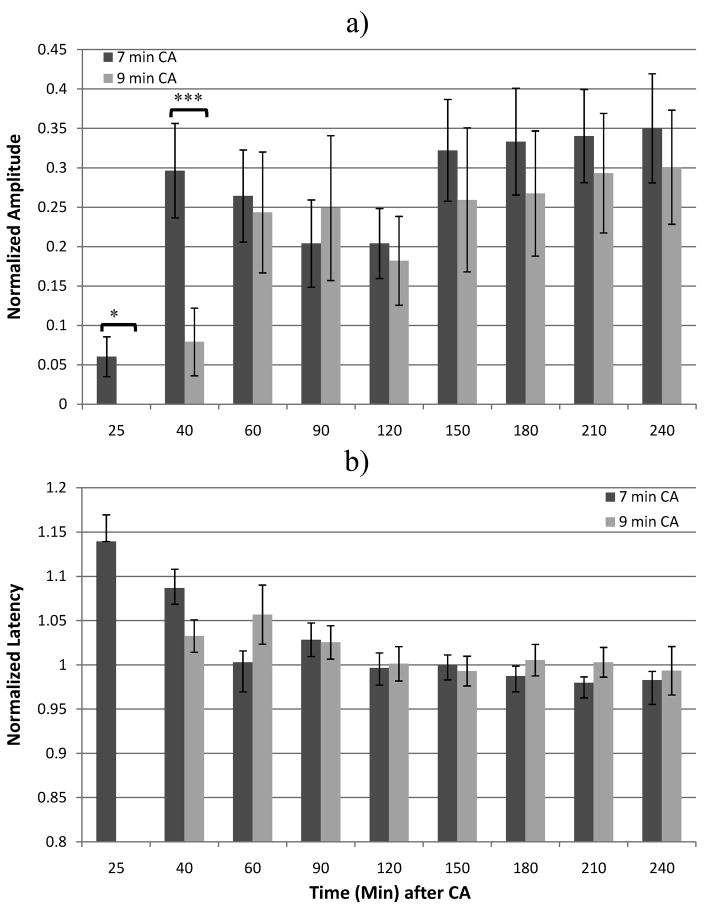
When comparing groups CA7 and CA9, the N10 amplitudes were similar, with the CA7 group showing slightly higher amplitudes during the early period after ROSC. The differences in means achieved significance at 25 and 40 min after onset of asphyxia, with Student’s t-Test p-values of 0.016 and 0.023, respectively (Figure 2a). The normalized N10 latencies, as measured from the onset of the median nerve stimulus, were not significantly different between groups CA7 and CA9 (Figure 2b).
Figure 2.
Means of N10 (a) amplitudes and (b) latencies between rats that underwent 7 vs. 9 min of asphyxial CA. *p=0.02, ** p=.01, *** p=.005.
Next, we tested for correlations between SEP and neurologic outcomes using the NDS score. Rats were divided into 2 groups based on NDS scores at 72 hr. Group 1 (G1) contained all rats with NDS of 50 or greater at 72 hr and Group 2 (G2) contained the remaining rats, including the ones that died prior to the final NDS assessment at 72 hr. This division produced a G1 size of 9 rats, and a G2 size of 7 rats. As described above, 1 G2 rat’s SEP was excluded from statistical analysis due to aberrant baseline signals. N10 amplitudes of the G1 rats were significantly higher at most time points during the initial 4 h period after CA (Figure 3a). Peak-to-peak amplitude measurements were also analyzed, which showed similarly significant differences between groups G1 and G2 (data not shown). The N10 latencies did not show any significant difference between G1 and G2 (Figure 3b). Using repeated measures ANOVA, there was a significant difference between N10 amplitudes in G1 and G2 during the initial 4 hr period (p=0.05). Repeated measures ANOVA also detected a significant difference in peak-to-peak amplitudes between the two groups between 2–4 hr (p=0.02).
Figure 3.
Post CA N10 (a) amplitudes and (b) latencies between G1 and G2. Statistically significant differences denoted by asterisks. *p=0.02, ** p=.01, *** p=.005.
Finally, an additional negative potential at approximately 7 ms latency (N7) was consistently detected. During baseline recordings, this signal was often obscured by the rising slope of the much larger N10 potential. However, after the N10 was suppressed by hypoxic-ischemic brain injury, the N7 became more apparent during the early stages of recovery (0–60 min after CA). It is notable that, though the N10 recovered after a mean interval of 67 min post-CA, the N7 recovered much earlier. The mean interval prior to return of any measurable N7 signal was 15.1±2.7 min for group CA7, and 19.6±6.1 min for group CA9 (p-value = 0.007). Comparing the return of N7 based on outcomes (G1 vs. G2), the mean interval prior to N7 recovery was 16.3±3.4 min for G1 and 18.7±6.8 min for G2 (p-value = 0.12). The evolution of the two potentials is demonstrated in Figure 4. N7 amplitude could not be reliably measured because the potential becomes obscured by recovery of N10.
Figure 4.
SEP recordings from single rat showing (a) baseline signal with N7 obscured by N10, (b) early post-CA signal with only N7 visible, (c) beginning recovery of N10, and (d) eventual recovery of both potentials.
Based on continuous measurement of SEPs after CA, the recovery of potentials can be summarized in the following way: 1) Electrical silence during and immediately after CA; 2) Reappearance of the N7 signal without other distinguishable short-latency potentials at 10 to 25 min after start of CA; 3) Slow recovery of major short-latency potentials (N10, P15) at 25 min to 4 h; 4) Gradual recovery of potentials approaching baseline morphology and amplitude by 24 to 72 h. This pattern of SEP evolution was seen in virtually all rats (Figure 5).
Figure 5.
Evolution pattern of SEPs during and after cardiac arrest with example potentials.
Discussion
The present study supports the hypothesis that SEP signals evolve at different rates depending on the extent of hypoxic-ischemic injury after CA. The experiment demonstrated earlier and more robust recovery of cortical N10 amplitudes after resuscitation from moderate (7 min) compared to severe (9 min) hypoxic-ischemic injury. This finding suggests that N10 amplitude may be an early indicator of the extent of primary hypoxic-ischemic injury.
The results also support the hypothesis that SEPs early after CA may be used as an indicator of future neurologic outcome. There was a significant difference between rats with good outcomes (NDS >50) and bad outcomes (NDS <50) in terms of mean N10 and peak-to-peak amplitudes during the initial recovery phase (0 to 4 hr). Previous studies have tested for an association between early SEP and neurologic outcomes, but have only analyzed presence or absence of cortical potentials.11 The present study is unique in using quantitative methods to distinguish between good vs. bad outcomes even when cortical potentials were consistently present. However, significant overlap exists in the distribution of individual amplitude values, which could reduce sensitivity and specificity as a predictor of neurologic outcome.
An important observation was the earlier reappearance of the N7 potential compared to N10. We believe this potential is analogous to the N18 potential in humans, which may be generated from the thalamus or thalamocortical projections. 17, 18 In the Macaque monkey, a similar potential at 7 ms latency is also recorded from a widespread cortical distribution after median nerve stimulation. The source of this potential is theorized to be from a thalamic generator.19 We believe that the reappearance of the N7 signal well before recovery of the N10 signal is an important observation. It provides insights into the period of disrupted thalamo-cortical conduction that precedes emergence from coma.5 In this experiment, the N7 potential uniformly recovered before the N10 potential, by as much as several hours. This finding suggests much earlier recovery of thalamic function compared to cortical activity. There exists a significant difference in the time to recovery of N7 between groups CA7 and CA9, with the former group recovering an average of 4.5 min earlier. However, there was only a trend for earlier N7 recovery in group G1 compared to G2 (P-value = 0.12). Thus, the time to recovery of N7 is indicative of extent of initial injury, but is not strongly predictive of neurologic outcome.
Even though CA9 rats had significantly worse outcomes, the early N10 amplitudes and peak-to-peak amplitudes had only a modest correlation with 72-hr NDS scores. This finding implies that recovery was heterogeneous despite similar initial graded injuries. This heterogeneity is likely multi-factorial and may be accounted for by ongoing neuronal damage occurring during recovery. This phenomenon of delayed cell death after hypoxic-ischemic brain injury is seen in many animals, including humans.20
The anesthesia used, isoflurane, may have introduced some suppression effects on the SEP signal.21 However, our baseline recordings demonstrated significant suppression only at isoflurane concentrations of greater than 1%. There was minimal inter-animal variability in the amount of anesthesia needed to prevent awakening during electrical stimulation; most rats required a constant low concentration of 0.5% isoflurane post CA, minimizing its effects as a confounder. Also, though each animal’s body temperature was closely regulated during the initial 4 hr after CA, it was not logistically possible to do so for the subsequent 72 h. Animals were allowed to auto-regulate their body temperatures, which might have affected the extent of injury.22, 23
SEP signals provide additional information beyond amplitudes and latencies. Advanced measures such as signal shape, slope, and frequency content may also relate to neurological outcome. These measures will be tested in future experiments. Though previous observations support the theory that the N7 potential is generated from the thalamus, we cannot confirm this hypothesis based on the current results alone. Further experiments using depth microelectrodes and cortical multiunit recordings to measure local field potentials after CA are required. Finally, our data will allow for the better design of experiments examining SEP in relation to therapeutic hypothermia.
Conclusion
The present study has demonstrated a significant and measurable difference in SEP signals based on neurologic injury in rats after CA. Following hypoxic-ischemic brain injury, SEP evolves in a predictable manner and is associated with outcome. These findings suggest the disruption of thalamocortical conduction early after CA. We are hopeful that this work will lay the foundation for using SEP as a neurophysiologic monitor during the process of coma recovery after CA.
Acknowledgments
Role of the Funding Source
This work was supported by grants RO1 HL071568 from the National Institute of Health and 09SDG2110140 from the American Heart Association. The study sponsors participated in the design of this experiment as well as in the preparation of this manuscript.
Footnotes
Conflict of Interest Statement
No authors have any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young GB. Clinical practice. neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 2.Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. Management of brain injury after resuscitation from cardiac arrest. Neurol Clin. 2008;26:487–506. ix. doi: 10.1016/j.ncl.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiff ND, Plum F. The role of arousal and “gating” systems in the neurology of impaired consciousness. J Clin Neurophysiol. 2000;17:438–452. doi: 10.1097/00004691-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Muthuswamy J, Kimura T, Ding MC, Geocadin R, Hanley DF, Thakor NV. Vulnerability of the thalamic somatosensory pathway after prolonged global hypoxic-ischemic injury. Neuroscience. 2002;115:917–929. doi: 10.1016/s0306-4522(02)00369-x. [DOI] [PubMed] [Google Scholar]
- 6.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Bolton CF, Young B. Prediction of outcome in patients with anoxic coma: A clinical and electrophysiologic study. Crit Care Med. 1996;24:672–678. doi: 10.1097/00003246-199604000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Young GB, Doig G, Ragazzoni A. Anoxic-ischemic encephalopathy: Clinical and electrophsiological associations with outcome. Neurocrit Care. 2005;2:159–164. doi: 10.1385/NCC:2:2:159. [DOI] [PubMed] [Google Scholar]
- 9.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 10.Gendo A, Kramer L, Hafner M, et al. Time-dependency of sensory evoked potentials in comatose cardiac arrest survivors. Intensive Care Med. 2001;27:1305–1311. doi: 10.1007/s001340101008. [DOI] [PubMed] [Google Scholar]
- 11.Nakabayashi M, Kurokawa A, Yamamoto Y. Immediate prediction of recovery of consciousness after cardiac arrest. Intensive Care Med. 2001;27:1210–1214. doi: 10.1007/s001340100984. [DOI] [PubMed] [Google Scholar]
- 12.Geocadin R. A novel quantitative EEG injury measure of global cerebral ischemia. Clinical Neurophysiology. 2000;111:1779. doi: 10.1016/S1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- 13.Jia X, Koenig MA, Shin HC, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111:166–175. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison T, Hume AL. A comparative analysis of short-latency somatosensory evoked potentials in man, monkey, cat, and rat. Exp Neurol. 1981;72:592–611. doi: 10.1016/0014-4886(81)90008-x. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro SM. Somatosensory and brainstem auditory evoked potentials in the gunn rat model of acute bilirubin neurotoxicity. Pediatr Res. 2002;52:844–849. doi: 10.1203/00006450-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Freeman S, Sohmer H. A comparison of forepaw and vibrissae somatosensory cortical evoked potentials in the rat. Electroencephalogr Clin Neurophysiol. 1996;100:362–369. doi: 10.1016/0168-5597(96)95690-7. [DOI] [PubMed] [Google Scholar]
- 17.Ulas UH, Ozdag F, Eroglu E, et al. Median nerve somatosensory evoked potentials recorded with cephalic and noncephalic references in central and peripheral nervous system lesions. Clin Electroencephalogr. 2001;32:191–196. doi: 10.1177/155005940103200406. [DOI] [PubMed] [Google Scholar]
- 18.Nagahiro S, Matsukado Y, Ushio Y, Fukumura A. Diagnostic value of short latency somatosensory evoked potentials for various intracranial lesions. No Shinkei Geka. 1990;18:813–819. [PubMed] [Google Scholar]
- 19.Hayashi N, Nishijo H, Ono T, Endo S, Tabuchi E. Generators of somatosensory evoked potentials investigated by dipole tracing in the monkey. Neuroscience. 1995;68:323–338. doi: 10.1016/0306-4522(95)00126-4. [DOI] [PubMed] [Google Scholar]
- 20.Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx. 2006;3:154–169. doi: 10.1016/j.nurx.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson DO, Drummond JC, Todd MM. Effects of halothane, enflurane, isoflurane, and nitrous oxide on somatosensory evoked potentials in humans. Anesthesiology. 1986;65:35–40. doi: 10.1097/00000542-198607000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Markand ON, Warren C, Mallik GS, King RD, Brown JW, Mahomed Y. Effects of hypothermia on short latency somatosensory evoked potentials in humans. Electroencephalogr Clin Neurophysiol. 1990;77:416–424. doi: 10.1016/0168-5597(90)90002-u. [DOI] [PubMed] [Google Scholar]
- 23.Seyal M, Mull B. Mechanisms of signal change during intraoperative somatosensory evoked potential monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:409–415. doi: 10.1097/00004691-200210000-00004. [DOI] [PubMed] [Google Scholar]