Abstract
Objective
To visualize tumor angiogenesis using the MRI contrast agent, Gd-DTPA-anti-VEGF receptor 2 antibody conjugate, with a 4.7-Tesla MRI instrument in a mouse model.
Materials and Methods
We designed a tumor angiogenesis-targeting T1 contrast agent that was prepared by the bioconjugation of gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA) and an anti-vascular endothelial growth factor receptor-2 (VEGFR2) antibody. The specific binding of the agent complex to cells that express VEGFR2 was examined in cultured murine endothelial cells (MS-1 cells) with a 4.7-Tesla magnetic resonance imaging scanner. Angiogenesis-specific T1 enhancement was imaged with the Gd-DTPA-anti-VEGFR2 antibody conjugate using a CT-26 adenocarcinoma tumor model in eight mice. As a control, the use of the Gd-DTPA-anti-rat immunoglobulin G (Gd-DTPA-anti-rat IgG) was imaged with a tumor model in eight mice. Statistical significance was assessed using the Mann-Whitney test. Tumor tissue was examined by immunohistochemical analysis.
Results
The Gd-DTPA-anti-VEGFR2 antibody conjugate showed predominant binding to cultured endothelial cells that expressed a high level of VEGFR2. Signal enhancement was approximately three-fold for in vivo T1-weighted MR imaging with the use of the Gd-DTPA-anti-VEGFR2 antibody conjugate as compared with the Gd-DTPA-rat IgG in the mouse tumor model (p < 0.05). VEGFR2 expression in CT-26 tumor vessels was demonstrated using immunohistochemical staining.
Conclusion
MR imaging using the Gd-DTPA-anti-VEGFR2 antibody conjugate as a contrast agent is useful in visualizing noninvasively tumor angiogenesis in a murine tumor model.
Keywords: Magnetic resonance (MR) contrast agent, Molecular Imaging, Angiogenesis, Bioconjugation
Monitoring in vivo angiogenesis offers a potentially valuable surrogate marker for the detection of tumors and the evaluation of chemotherapy and drug efficacy. Generally, tumors cannot grow beyond 1-2 mm3 in diameter without the development of a vascular supply (1). Angiogenesis, the formation of new blood vessels, is required for malignant tumor growth and metastasis. Recently, several studies have shown that angiogenesis is a dynamic process by which the blood supply of a tumor is provided by preexisting blood vessels and endothelial precursor cells (2). Vascular endothelial growth factor (VEGF) is a prototypical proangiogenic molecule, and VEGF has been implicated in several steps throughout the angiogenesis process (3). Findings in other studies have shown that VEGF is expressed at high levels for a broad spectrum of malignancies including carcinoma of the breast (4), colon (5), ovary (6), and brain (7).
MRI is a highly useful noninvasive imaging method with sub-millimeter resolution and high tissue contrast. Furthermore, MRI enhanced with contrast agent can be used to characterize microvessels of tumors quantitatively and can thereby be used to assess angiogenesis (8). For instance, Gd-based contrast agent can be used to detect early tumor with the use of MRI instrument (9). The use of Gd-based contrast agents provides strong positive T1 relaxation contrast. In addition, Gd-based contrast agents have traditionally been used for non-specific contrast-enhanced clinical MRI. Recently, this approach has been successfully used to image the neovasculature in angiogenic tumors with MRI (10-12).
The use of Gd-based contrast agents; however, cannot provide molecular-specific information. For visualization of molecular information for cell surface antigens and/or receptors in vivo, the use of molecular targeted agents can be used. This method relies on the specific labeling of extracellular cell surface receptors or antigens with an MRI targeted contrast agent. MRI contrast agent can be specifically targeted with a monoclonal antibody (mAb) that binds with high affinity to a specific receptor or antigen. Imaging of tumors that express VEGFR2 has been attempted using a compound synthesized by the conjugation of avidin-Gd and biotinylated anti-VEGFR2 antibody for the visualization of tumor angiogenesis (13). However, avidin and biotin are expected to cause potentially unfavorable effects such as difficulties in humanization and tissue clearance of the avidin from the patient's body. As a result, we used a direct conjugation method for Gd-DTPA and anti-VEGFR2 antibody, as previously described by our laboratory (14).
Based on the above considerations, we hypothesized that the Gd-DTPA targeted for tumor vasculature via the conjugation with an anti-VEGF R2 antibody that binds to tumor cells would result in increased contrast enhancement during the MR imaging of solid tumors using a murine model. The purpose of this study was to evaluate the use of a novel contrast agent, the Gd-DTPA-anti-VEGFR2 antibody conjugate, to visualize tumor angiogenesis with 4.7-Tesla (T) animal MR imaging using a mouse model.
MATERIALS AND METHODS
Preparation of the Gd-DTPA-anti VEGFR2 Antibody Conjugate
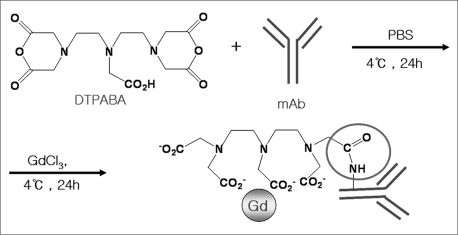
The preparation of the contrast agent, Gd-DTPA-anti-VEGFR2 antibody conjugate (Fig. 1), was based on a similar method previously described by our laboratory (14) for the preparation of the contrast agent Gd-DTPA-anti-intercellular adhesion molecule 1 (ICAM-1). The VEGFR2 antibody was purified from the culture supernatant of rat hybridoma using protein A/G-coupled affinity chromatography. Purified antibodies were identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), Western blot analysis, and bicinchoninic acid (BCA) assay. Diethylenetriaminepentaacetic acid bisanhydride (DTPABA) was dissolved in dimethylformamide and then added to the antibody solution. The anti-VEGFR2 antibody was conjugated to DTPABA in phosphate-buffered saline (PBS) (pH 8.5) for one hour at room temperature. The reaction solution was purified using a PD-10 column (Sephadex G-25M, Amersham Biosciences, Amersham, UK). The concentration of the antibody was measured using the BCA assay. Gd chloride was dissolved in deionized water and then added to the DTPA-antibody conjugate solution. One part of the DTPA-antibody complex was then reacted with 40 parts of Gd chloride in 0.5 M sodium acetate (pH 5.5). The solution was loaded onto a PD-10 column and was eluted from the column with 0.15 M NaCl (pH 5.5). Gd-DTPA antibody conjugates were analyzed using matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) (Voyager-DE STR 4349) spectrometry. The Gd content was determined with the aid of inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7500A, Agilent Technologies, Palo Alto, CA), and the final concentration of the conjugated antibody was measured using BCA analysis. The molar ratio of gadolinium and antibody was then calculated. The control antibody conjugate, Gd-DTPA-anti-rat-IgG, was prepared using a similar method.
Fig. 1.
Schematic diagram depicting preparation of Gd-DTPA-anti-VEGFR2 antibody is shown.
DTPABA = diethylenetriaminepentaacetic acid bisanhydride, mAb = monoclonal antibody (anti-VEGFR 2 antibody), PBS = phosphate-buffered saline, Gd = gadolinium
Cell Culture
A rat hybridoma used for the isolation of anti-VEGFR2 antibody was purchased from the American Type Culture Collection (ATCC, Manassas, VA) and was cultured in Dulbecco's Modified Eagle's Medium (DMEM) with 4 mM L-glutamine, which was adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% fetal bovine serum. The murine endothelial cell line (MS-1) and murine colon adenocarcinoma cell line (CT-26) were also obtained from ATCC and the cells were cultured according to the supplier's instructions. Cells were grown as monolayer cultures in DMEM with 10% fetal bovine serum (Invitrogen, Grand Island, NY) supplemented with 1% glutamine and 1% antibiotics. Cells were maintained in a 37℃ incubator in 5% CO2 humidified air.
For the preparation of the endothelial cell-specific contrast effect, MS-1 cells (5 × 106) were cultured and were incubated with the Gd-DTPA-VEGFR2 antibody complexes (300 nmol Gd) in the presence or absence of anti-VEGFR2 antibody (600 nmol) at 4℃ for four hours. The cells were then washed three times with PBS, harvested by scraping, and pelleted by centrifugation.
In Vitro MR Imaging
In vitro MRI was performed on a 4.7-T animal MRI instrument (Bruker, Ettlingen, Germany). The endothelial cell-specific contrast effect was assessed by determining MRI contrast effects with the endothelial MS-1 cells. An MR image of the cells in the tubes placed in a water-filled chamber was obtained with a spin echo sequence using the following imaging parameters: TR = 300 milliseconds, TE = 10 milliseconds, field of view (FOV) = 25.6 mm × 25.6 mm, slice thickness = 1 mm, pixel resolution = 100 × 100 µm, in the 4.7-T instrument. The signal intensity of T1-weighted imaging (WI) of the cell pellets was normalized against that of the surrounding water. Each experiment was performed in triplicate and the signal intensity was shown as the mean ± standard deviation.
A region of interest (ROI = 0.02 cm2) for cell and water was calculated. The average of the ROIs included areas of maximal and minimal enhancement in each slice. For the in vitro MRI study, we defined the relative signal intensity (SI) as: ([mean of ROI] cell)/([mean of ROI] water).
Mouse Tumor Model
Male Balb/c nude mice (n = 16, aged 6 weeks and each weighing 20-25 g) were purchased from the Central Animal Laboratory (Seoul, South Korea) and used for this study. The Balb/c nude mice were injected subcutaneously in their back with CT-26 cells (1 × 106 cells) suspended in 0.1 mL phosphate-buffered saline. The injected cells were allowed to expand for 10 days until the tumors grew to a size approximately 0.5 cm3.
In Vivo MR Imaging
In vivo MRI was performed on a 4.7-T animal MRI instrument. T1WI was obtained at 10 minutes and at 12, 24, and 48 hours after the injection of the Gd-DTPA-anti-VEGFR2 antibody conjugate (12 µmol of Gd/kg of body weight) in eight mice, followed by the injection of the Gd-DTPA-anti-rat IgG conjugate (12 µmol of Gd/kg of body weight) in another eight mice. All the animals were examined by contrast-enhanced T1-weighted MRI using the following imaging parameters: TR = 300 milliseconds, TE = 10 milliseconds, FOV = 25.6 mm × 25.6 mm, slice thickness =1 mm, pixel resolution = 100 × 100 µm, in the 4.7-T instrument. All of the animal studies were carried out in accordance with the regulations set by the Institutional Review Board of our university.
An ROI (= 0.02 cm2) for the tumor center and muscle was calculated for the mean value. The average of the ROIs included areas of maximal and minimal enhancement in each slice. The SI was calculated using the following equations: SI = ([mean of ROI] tumor)/([mean of ROI] muscle). For the in vivo MRI study, we defined the contrast enhancement ratio (CER) as ([SIpost-SIpre]/SIpre) × 100%, where SIpre = signal intensity before administration of contrast medium and SIpost = signal intensity after contrast medium administration.
Immunohistochemical Analysis
After analyzing the mice by MRI, tumor tissue was collected and the expression of VEGFR2 was then examined by way of an immunohistochemical analysis. Tissue sections were fixed with 4% paraformaldehyde and sections were reacted with rabbit anti-mouse VEGFR2 antibody for two hours at 37℃. The primary antibody that bound to VEGFR2 and expressed in cells was then visualized with a secondary peroxidase-conjugated antibody and substrate dye. To detect the injected Gd-DTPA-anti-VEGFR2 antibody, an adjacent section was incubated with only the second antibody (goat anti-rabbit IgG antibody) for the Gd-conjugated antibody (rabbit anti-mouse VEGFR2 antibody) and was detected with the substrate dye.
Statistical Analysis
The data were presented as the mean ± standard deviation, and the significance of the data was assessed using the Mann-Whitney test. The statistical analyses were performed using commercial software (SPSS, Chicago, IL).
RESULTS
Preparation of the Gd-DTPA-Anti-VEGFR2 Antibody Conjugate
The purified antibody fraction was identified by SDS-PAGE (separating the 50 kDa and 25 kDa) of the IgG. These antibody fragments were also identified using Western blot analysis, which also detected the 50 kDa and 25 kDa. The intermediate product, DTPA antibody, and the final product, Gd-DTPA antibody, were quantified by BCA assay for their antibody content and were characterized using MALDI-TOF spectrometry analysis for the conjugated product. The molecular weight shift from anti-VEGFR2 to Gd-DTPA-anti-VEGFR2 antibody in MALDI-TOF spectrometry analysis represents the conjugation of the antibody with Gd-DTPA. A quantitative analysis of the Gd content in the Gd-DTPA-antibody was performed using ICP-MS. The molar ratio of Gd to antibody was about 1:20.
VEGFR2-Specific MRI with the Gd-DTPA-Anti-VEGFR2 Antibody Conjugate in Cultured Endothelial Cells
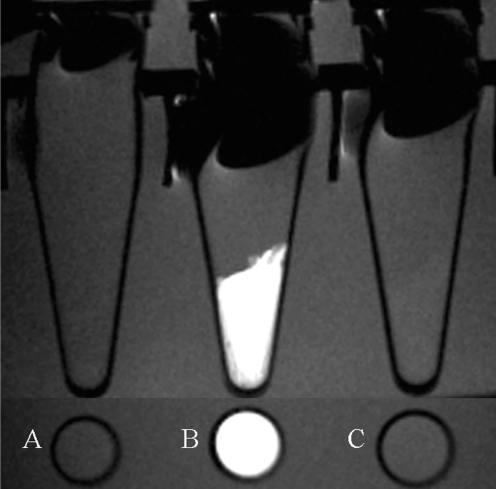
To examine whether the Gd-DTPA-anti-VEGFR2 antibody conjugate can bind to cells in a VEGFR2-specific manner, the contrast agent was incubated with MS-1 cells in the presence or absence of a competitive binding inhibitor, the anti-VEGFR2 antibody (Fig. 2). The competitive antibody was added at a three-fold higher concentration than the anti-VEGFR2 antibody content of the Gd-DTPA-anti-VEGFR2 antibody complex. The value of signal intensity showed that binding of the Gd-DTPA-anti-VEGFR2 antibody complex to the VEGFR2-expressing cells was significantly inhibited by competition with the anti-VEGFR2 antibody (Table 1).
Fig. 2.
T1-weighted MR images using Gd-DTPA-anti-VEGFR2 antibody in cultured endothelial cells are shown.
A. MS-1 cells were not treated with either contrast or control agent.
B. VEGFR2 expressing MS-1 cells treated with Gd-DTPA-anti-VEGFR2 antibody shows significant increase in high signal intensity.
C. MS-1 cells show no high signal on T1-weighted imaging with Gd-DTPA-anti-VEGFR2 antibody in presence of competitive binding inhibitor, anti-VEGFR2 antibody.
Table 1.
Signal Intensity for Murine Endothelial Cells as Determined by in Vitro MRI Analysis
Note.-*SI = relative signal intensity
Angiogenesis-Specific MRI with the Gd-DTPA-Anti-VEGFR2 Antibody Conjugate in a Mouse Tumor Model
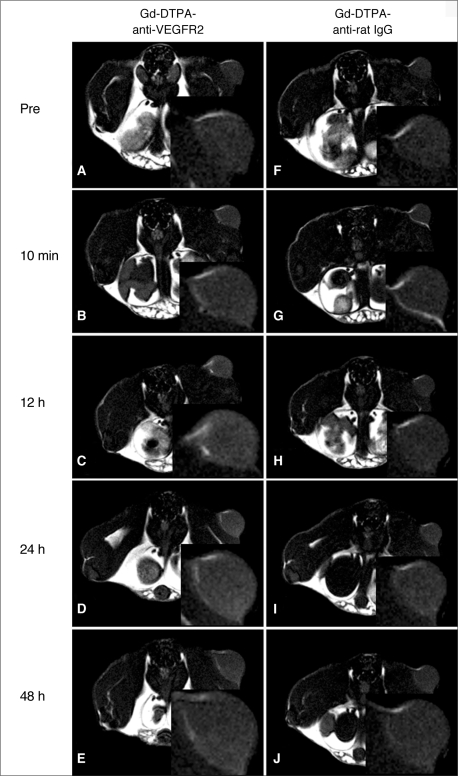
Angiogenesis-targeted MRI using the Gd-DTPA-anti-VEGFR2 antibody conjugate was examined in a mouse tumor model. The Gd-DTPA-anti-VEGFR2 antibody conjugate displayed significant signal enhancement and accumulation of tumor tissues until 48 hours after contrast agent injection (Fig. 3A-E). The Gd-DTPA-anti-rat IgG and the Gd-conjugated control antibody containing the same amount of Gd as the Gd-DTPA-anti-VEGFR2 antibody conjugate, showed no significant enhancement in T1 until 48 hours after injection (Fig. 3F-J). The CER in the mouse tumors that received Gd-DTPA-anti-VEGFR2 antibody conjugate increased at 24 hours (29%) and at 48 hours (9%) after injection. Conversely, the CER in the mouse tumors that received Gd-DTPA-anti-rat IgG was increased at 24 hours (11%) and at 48 hours (11%) after injection. The CER value was significantly different for mouse tumors that were administered Gd-DTPA-anti-VEGFR2 antibody conjugate and Gd-DTPA-anti-rat IgG (p < 0.05). The Gd-DTPA-anti-VEGFR2 antibody conjugate injected into tumors showed a peak time at 24 hours. Conversely, Gd-DTPA-anti-rat IgG injected into tumors showed a peak time at 24 hours with a low level of enhancement (Fig. 3).
Fig. 3.
In vivo dynamic MR imaging of tumors is shown at 10 minutes and at 12, 24 and 48 hours following injection of Gd-DTPA-anti-VEGFR2 antibody conjugate (A-E) as well as injection of Gd-DTPA-anti-rat IgG (F-J). Both contrast agents were injected at same dose. Gd-DTPA-anti-VEGFR2 antibody conjugate shows significant signal enhancement and accumulation in tumor tissues until 48 hours after contrast agent injection. Comparatively, Gd-DTPA-anti-rat IgG shows no significant enhancement in T1 until 48 hours after injection.
Immunohistochemical Analysis
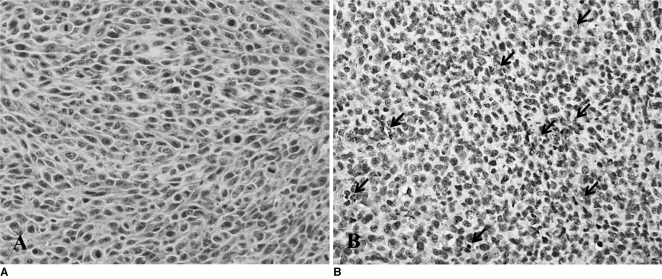
CT-26 tumor tissue was examined using immunohistochemical staining for VEGFR2 expression to assess whether the angiogenesis-specific imaging, using the Gd-DTPA-anti-VEGFR2 antibody, corresponded to VEGFR2 expression in tumors. The expression of VEGFR2 showed an extremely high level in vessels of CT-26 tumor tissue (Fig. 4).
Fig. 4.
Histopathological examination results.
A. Hematoxylin and eosin staining shows tumor tissue.
B. Tissue was stained with rabbit anti-mouse VEGFR2 (Flk-1) antibody for immunohistochemical analysis, indicating expression of VEGFR2 in vessels (arrows) of CT-26 tumor tissue (×400 magnification).
DISCUSSION
The ability of tumors to induce new blood-vessel formation has been a major focus of cancer research over the past few decades, and VEGF is now known to have a central role in this process (15). Vascular endothelial growth factor-A (VEGF-A) is one of the factors responsible for new blood-vessel formation in the VEGF family (16-20). VEGF-A, a major regulator of angiogenesis, binds and activates two tyrosine kinase receptors, VEGFR1 (Flt-1) and VEGFR2 (KDR/Flk-1). These receptors regulate physiological as well as pathological angiogenesis. VEGFR2 has a strong tyrosine kinase activity, and the protein transduces the major signals for angiogenesis. VEGFR2 expression is 3-5-fold higher in tumor vasculature as compared to normal vasculature (21). The specificity of VEGFR2 expression, the location of VEGFR2 on the surface of the tumor vessels, and the predominant role of the receptor in tumor angiogenesis, makes the receptor a highly desirable target for the development of both anti-angiogenic and vascular-targeting agents.
The targeting of contrast agents for specific tissue markers has been suggested to be useful in selecting imaging agents for the detection and evaluation of vascular pathology, including thrombosis, inflammation, tumor angiogenesis, or therapeutic angiogenesis (22, 23). For instance, P-selectin (24) and ICAM-1 (25) are known to be up-regulated on the surface of activated endothelium in tissue involved in inflammatory processes. A number of endothelial markers such as αvβ3 (26) and the VEGF receptors (15-20, 27) are up-regulated on the endothelium surface during angiogenesis.
The monitoring of tumor angiogenesis can involve several types of imaging equipment. Studies have been conducted with MRI, positron emission tomography (PET), bioluminescence imaging, and fluorescence optical imaging. Of these modalities, MRI is capable of providing both high-resolution anatomical information and functional measurements of tumor physiology (28-30).
In this study, we anticipated that we would be able to visualize tumor angiogenesis with the use of a novel contrast agent, Gd-DTPA anti-VEGF receptor 2 antibody conjugate, in a mouse model. For the in vitro study, we first showed that binding of the contrast agent, Gd-DTPA anti-VEGFR2 antibody conjugate, to MS-1 cells resulted in a higher signal intensity compared to non-treated MS-1 cells. Moreover, pre-incubation with anti-VEGFR2 antibody inhibited the signal intensity seen for the MS-1 cells. These findings indicate that Gd-DTPA anti-VEGFR2 antibody conjugate binds to the VEGFR2-expressing MS-1 cells, thereby suggesting that the Gd-DTPA-anti-VEGFR2 antibody conjugate is a useful MRI contrast agent for the visualization of tumor angiogenesis. Subsequently, we assessed tumor angiogenesis in vivo. We used two agents for contrast signal enhancement of the Gd-DTPA-anti-VEGFR2 antibody conjugate and, as a control, the Gd-DTPA-anti-rat IgG. A long period of enhancement was observed when using the Gd-DTPA-anti-VEGFR2 antibody conjugate, with an approximately three-fold enhancement ratio compared to when the Gd-DTPA-anti-rat IgG was used. The peak enhancement time when using Gd-DTPA-anti-VEGFR2 antibody conjugate was delayed compared to the peak enhancement time when using the Gd-DTPA-anti-rat IgG. These findings indicate that the Gd-DTPA-anti-VEGFR2 antibody conjugate binds to the tumor vessel endothelial cell. The elevated expression of VEGFR2 in tumor vessels was confirmed with immunohistochemical staining.
In conclusion, the results of this study suggest that the use of the MRI contrast agent, Gd-DTPA-anti-VEGFR2 antibody conjugate allows for the noninvasive visualization of tumor angiogenesis in a mouse model. These findings should have direct implications for future monitoring of anti-tumor and anti-angiogenic therapies.
Acknowledgement
This paper was supported by the Wonkwang University in 2009.
Abbreviations
- BCA
bicinchoninic acid
- CER
contrast enhancement ratio
- DTPABA
diethylenetriaminepentaacetic acid bisanhydride
- Gd-DTPA
gadolinium diethylenetriaminepentaacetic acid
- Gd-DTPA-anti-rat IgG
Gd-DTPA-anti-rat immunoglobulin G
- ICAM-1
intercellular adhesion molecule 1
- ICP-MS
inductively coupled plasma mass spectrometry
- MALDI-TOF
matrix-assisted laser desorption ionization-time of flight
- MS-1 cells
murine endothelial cells
- PBS
phosphate buffered saline
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor-2
References
- 1.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488–499. [PubMed] [Google Scholar]
- 3.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 4.Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 5.Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995;87:506–516. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- 7.Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993;91:153–159. doi: 10.1172/JCI116165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasch R, Turetschek K. MRI characterization of tumors and grading angiogenesis using macromolecular contrast media: status report. Eur J Radiol. 2000;34:148–155. doi: 10.1016/s0720-048x(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Feng XY, Henning TD, Wen L, Lu WY, Pan H, et al. MR imaging of tumor angiogenesis using sterically stabilized Gd-DTPA liposomes targeted to CD105. Eur J Radiol. 2009;70:180–189. doi: 10.1016/j.ejrad.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Li KC, Bednarski MD. Vascular-targeted molecular imaging using functionalized polymerized vesicles. J Magn Reson Imaging. 2002;16:388–393. doi: 10.1002/jmri.10174. [DOI] [PubMed] [Google Scholar]
- 11.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 12.Sipkins DA, Gijbels K, Tropper FD, Bednarski M, Li KC, Steinman L. ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J Neuroimmunol. 2000;104:1–9. doi: 10.1016/s0165-5728(99)00248-9. [DOI] [PubMed] [Google Scholar]
- 13.Poduslo JF, Curran GL, Peterson JA, McCormick DJ, Fauq AH, Khan MA, et al. Design and chemical synthesis of a magnetic resonance contrast agent with enhanced in vitro binding, high blood-brain barrier permeability, and in vivo targeting to Alzheimer's disease amyloid plaques. Biochemistry. 2004;43:6064–6075. doi: 10.1021/bi0359574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KS, Kim SH, Cai QY, Kim SY, Kim HO, Lee HJ, et al. Inflammation-specific T1 imaging using anti-intercellular adhesion molecule 1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid. Mol Imaging. 2007;6:75–84. [PubMed] [Google Scholar]
- 15.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007;38:258–268. doi: 10.1016/j.bcmd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Luo JC, Yamaguchi S, Shinkai A, Shitara K, Shibuya M. Significant expression of vascular endothelial growth factor/vascular permeability factor in mouse ascites tumors. Cancer Res. 1998;58:2652–2660. [PubMed] [Google Scholar]
- 17.Nagy JA, Masse EM, Herzberg KT, Meyers MS, Yeo KT, Yeo TK, et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995;55:360–368. [PubMed] [Google Scholar]
- 18.Rosen LS. VEGF-targeted therapy: therapeutic potential and recent advances. Oncologist. 2005;10:382–391. doi: 10.1634/theoncologist.10-6-382. [DOI] [PubMed] [Google Scholar]
- 19.Luo JC, Toyoda M, Shibuya M. Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res. 1998;58:2594–2600. [PubMed] [Google Scholar]
- 20.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 21.Ran S, Huang X, Downes A, Thorpe PE. Evaluation of novel antimouse VEGFR2 antibodies as potential antiangiogenic or vascular targeting agents for tumor therapy. Neoplasia. 2003;5:297–307. doi: 10.1016/S1476-5586(03)80023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 23.Bekeredjian R, Katus HA, Kuecherer HF. Therapeutic use of ultrasound targeted microbubble destruction: a review of non-cardiac applications. Ultraschall Med. 2006;27:134–140. doi: 10.1055/s-2005-858993. [DOI] [PubMed] [Google Scholar]
- 24.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104:2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 26.Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, et al. Tumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 27.Lu E, Wagner WR, Schellenberger U, Abraham JA, Klibanov AL, Woulfe SR, et al. Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003;108:97–103. doi: 10.1161/01.CIR.0000079100.38176.83. [DOI] [PubMed] [Google Scholar]
- 28.Provenzale JM. Imaging of angiogenesis: clinical techniques and novel imaging methods. AJR Am J Roentgenol. 2007;188:11–23. doi: 10.2214/AJR.06.0280. [DOI] [PubMed] [Google Scholar]
- 29.Lyons SK. Advances in imaging mouse tumour models in vivo. J Pathol. 2005;205:194–205. doi: 10.1002/path.1697. [DOI] [PubMed] [Google Scholar]
- 30.Lee SI, Lee SY, Yoon KH, Choi KS, Jang KY, Yoo WH, et al. Molecular MR imaging for visualizing ICAM-1 expression in the inflamed synovium of collagen-induced arthritic mice. Korean J Radiol. 2009;10:472–480. doi: 10.3348/kjr.2009.10.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]