Abstract
The high frequency of memory T cells present in primates is thought to represent a major barrier to tolerance induction in transplantation. Therefore, it is crucial to characterize these memory T cells and determine their functional properties. High numbers of memory T cells were detected in peripheral blood and all lymphoid tissues except lymph nodes, which were essentially the site of naïve T cells. The majority of CD8+ memory T cells were effector memory cells located in the blood and bone marrow while most CD4+ memory T cells were central memory cells present in the spleen. Next, memory T cells from over 100 monkeys were tested for their response to alloantigens by ELISPOT. Memory alloreactivity mediated via direct but not indirect allorecognition was detected in all animals. The frequency of allospecific memory T cells varied dramatically depending upon the nature of the responder/stimulator monkey combination tested. MHC gene matching was generally associated with a low-memory alloreactivity. Nevertheless, low anamnestic alloresponses were also found in a significant number of fully MHC-mismatched monkey combinations. These results show that selected donor/recipient combinations displaying a low memory alloresponsiveness can be found. These combinations may be more favorable for transplant tolerance induction.
Keywords: Alloreactivity, memory T cells, MHC genes, nonhuman primates
Introduction
Memory represents the hallmark of adaptive immunity (1). Following antigen encounter, some T-cell clones expand and differentiate into memory T cells that can persist for a lifetime (2–4). Upon reexposure to the same antigen, these cells mount a faster and stronger immune response as compared to naïve T cells that had never seen this antigen (5–7). This anamnestic response is driven by the high frequency of memory T cells, their elevated affinity for the antigen and their low requirement for costimulation (8). Memory T cells, like ‘stem cells’, can divide in the absence of antigen. Their number in vivo is fairly stable with homeostasis being maintained by IL-7 and IL-15 cytokines. T-cell memory is mediated via two processes: (1) Protective memory mediated by effector memory T cells (TEMs) and (2) Reactive memory ensured by central memory T cells (TCMs) (1,5).
The presence of allospecific memory T cells in individuals results from previous exposure to alloantigens via transplantation, blood transfusion or pregnancy. In addition, microbial infections presumably induce the differentiation/expansion of memory T cells to antigens that can cross-react with allo-MHC antigens. This has been observed in mice after exposure to lymphocytic choriomeningitis virus (LCMV) and Leishmania parasites (9–12). It is believed that antigen mimicry between self-MHC/microbial peptide X and allo-MHC/peptide Y complexes accounts for the high frequency of T cells recognizing allo-MHC molecules (13–17). In contrast to laboratory mice, significant numbers of alloreactive memory T cells are present in monkeys (wild caught) and humans prior to transplantation (10, 18, 19). The high frequency of alloreactive memory T cells found in primates is thought to contribute to their resistance to tolerization protocols. This is supported by the demonstration in mice that alloreactive memory T cells, generated after microbial infection, skin allografting or acquired through adoptive transfer, invariably prevent transplant tolerance induction via mixed chimerism or costimulation blockade approaches (11,20–22). This implies that deletion or inactivation of the host's donor-reactive memory T cells could enhance our ability to induce drug-free transplant survival in primates. Alternatively, selection of donors eliciting a low anamnestic response in the host might also lower the threshold necessary to accomplish transplant tolerance in primates.
In the present study, we investigated the frequencies, phenotypes, alloantigen recognition pathways and lymphokine secretion patterns of memory T cells isolated from the peripheral blood and lymphoid tissues of cynomolgus monkeys. We show that the level of memory alloreactivity varies greatly depending upon the responder/stimulator monkey pair tested, a phenomenon influenced in part by the degree of MHC gene disparity among these monkeys. The implications of this finding for the design of tolerance protocols in clinical transplantation are discussed.
Materials and Methods
Animals
Male cynomolgus monkeys caught in the wild (Mauritius Island) and weighing 3–5 kg were used (Charles River Primates, Wilmington, MA).
Cynomolgus MHC genotyping
First, genomic DNA was prepared from PBMC and splenocytes. A panel of 17 microsatellite loci spanning approximately 5 Mb of the MHC region were amplified from the genomic DNA with fluorescent-labeled PCR primers and fragment size analysis was determined. The microsatellite haplotypes for each animal were converted to predicted MHC genotypes based on our previous cloning and sequencing work with cynomolgus monkeys (23,24).
Flow cytometric analyses and cell sorting
PBMC, peripheral lymph nodes (PLN), spleen and bone marrow cells were labeled with a combination of the following mAbs: CD3 PerCP (SP 34– 2), CD4 PerCP (L-200), CD8 PerCP (RPA-T8), CD8 APC (RPA-T8), CD95 FITC (DX2), CD95 APC (DX2), and CD28 PE (CD28.2) (BD Pharmingen, San Jose, CA). The fluorescence of the stained samples was analyzed using FACS Calibur and FACS Scan flow cytometers and Cell Quest Software (BD). Cells were gated on lymphocytes and sorted into CD95−CD28+ naïve and CD95+CD28low/high memory populations using a FACS Vantage cell sorter (BD Immunocytometry System). The purity of sorted cells was consistently > 95%.
Measurement of direct and indirect alloresponses by ELISPOT
ELISPOT plates (Millipore, Bedford, MA) were precoated with 5 μg/mL of capture antibodies against type 1 (γIFN, IL-2) and type 2 (IL-4 or IL-10) cytokines (Mabtech, Sweden) in PBS and stored overnight at 4°C. The plates were blocked for 1 h with PBS BSA 1% followed by three washes in PBS. A total of 1.5 × 105 responding cells were added to each well in 100 μL complete RPMI 1640 (Mediatech, Cellgro) supplemented with 10% normal monkey serum and L-glutamine, penicillin/streptomycin and Hepes buffer (Invitrogen, NY). The responding cells were cocultured with an equal number of irradiated donor PBMCs as stimulating cells (1.5 × 105 cells/well) (for direct allostimulation), or donor sonicates (for indirect allostimulation) (6–12 million PBMCs in 50 mL culture tubes were sonicated over ice for 2–3 min (Microson Cell Disruptor, Misonix Inc.) (25), or unstimulated in medium alone or with PHA at 1 μg/mL (Sigma). After 12–48 h incubation at 37°C, the plates were washed and biotinylated detection antibodies (Mabtech) were added (4°C OVN). After four washes with PBS/Tween, streptavidin-horseradish-peroxidase conjugate in PBS BSA 0.5% (Dako, Glostrup, Denmark) was added for 2 h at room temperature, followed by six washes. The development was performed with aminoethylcarbazole (10 mg/mL in N,N-dimethylformamide; D4254, Sigma) freshly prepared in 0.1 M sodium acetate buffer (pH 5) mixed with 30% H2O2. The resulting spots were counted with an ELISPOT image analyzer (CTL Inc., Cleveland, OH).
Statistical methods
The statistical analyses were performed using STATView software (Abacus Concepts Inc., Berkeley, CA). p-Values were calculated using paired t-test. A p-value <0.05 was considered statistically significant. The statistical analyses of the relationships between MHC matching and memory alloreactivity were performed with a rank ANOVA using generalized estimating equation to control for the fact that certain responders had multiple representations.
Results
Phenotype, localization and frequency of memory T cells in cynomolgus monkeys
The monoclonal antibodies anti-CD95 and anti-CD28 were used to detect naïve T cells (CD95−CD28+) as well as central (TCMs, CD95+CD28+) and effector (TEMs, CD95+CD28−) memory T cells as previously described by Pircher et al. (26). Figure 1A shows the frequencies of naïve and TCMs and TEMs among peripheral blood T cells of a representative cynomolgus monkey. The memory T cells (CD95+) represented approximately 48% of all T cells and were equally divided between central (CD28+, 23.5%) and effector (CD28−, 24.5%) memory T cells. The remaining T cells (CD95−, 52%) corresponded to naïve T cells. It is noteworthy that these relative proportions were extremely stable with less that 5% variation among 48 monkeys tested. In another set of experiments, CD95+CD28− and CD95+CD28+ T cells were backgated and analyzed for their expression of CD44 and CD62L markers (traditionally used for phenotyping mouse memory T cells [TMEMs]). CD95+CD28+ T cells were consistently CD44highCD62L+ (TCMs) while all CD95+CD28− T cells were CD44highCD62L− (TEMs) (data not shown). On the other hand, all naïve T cells were CD95−CD44low (data not shown). This confirms the validity of CD95 and CD28 as markers for central and TEMs. This also shows that CD44 and CD62L markers, traditionally used in mouse models, can be utilized to detect memory T cell subsets in cynomolgus monkeys.
Figure 1. Phenotype and distribution of naïve and memory T cells.
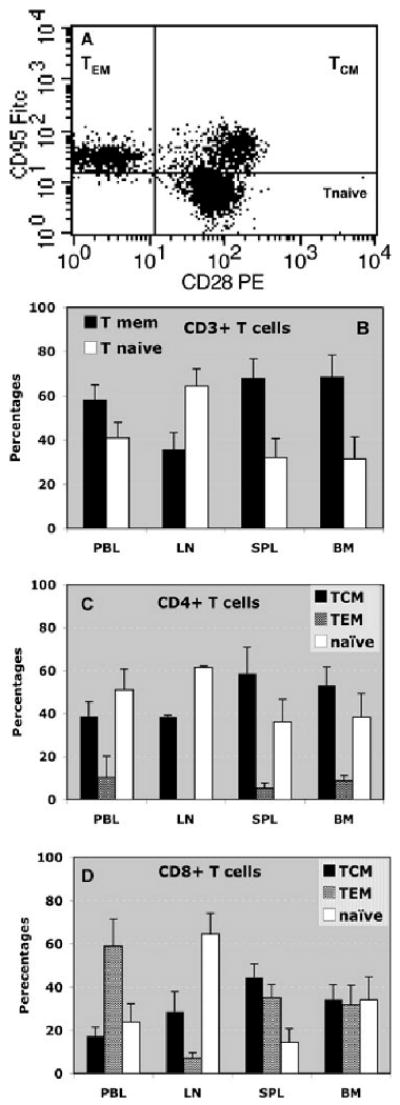
(A) T cells from the peripheral blood were isolated and stained with anti-CD95 FITC (DX2) and CD28 PE (CD28.2) monoclonal antibodies and analyzed via cytofluorometry. This plot shows the distribution of CD95−CD28+ (naïve T cells, 52% of total T cells), CD95+CD28+ (central memory T cells, 23.5% of total T cells) and CD95+CD28− (effector memory T cells, 24.5% of total T cells). This plot is representative of 32 monkeys tested individually. The variation of the percentages of T cell subsets between monkeys was less than 5%. (B)–(D) shows the distribution of naïve T cells, memory T cells (TMEMs) and memory T cell subsets (TCM, central memory T cells; TEM, Effector memory T cells) in different lymphoid organs and tissues (PBL, peripheral blood; LN, lymph nodes; SPL, spleen; BM, bone marrow). The average percentages ± SD of different subsets among CD3+ T cells (B), CD4+ T cells (C) or CD8+ T cells (D) were obtained with 5–12 monkeys tested individually.
Next, we investigated the tissue distribution and frequencies of naïve and memory T-cell subsets in monkeys. As shown in Figure 1B, the majority of the CD3+ T cells collected from the lymph nodes expressed a naïve phenotype while the peripheral blood, spleen and bone marrow were primarily populated with memory T cells. In the lymph nodes, most CD4+ T cells were naïve T cells while in the spleen and bone marrow most CD4+ T cells were memory T cells (Figure 1C). In all lymphoid tissues, the vast majority of CD4+ T cells displaying memory markers were TCMs (Figure 1C). Most of the CD8+ T cells (60–80%) collected from the peripheral blood, bone marrow and spleen of the monkeys expressed a memory phenotype (Figure 1D). In contrast, a large proportion of the CD8+ T cells isolated from the lymph nodes were naïve T cells (Figure 1D). Finally, equal frequencies of central and effector memory were found among CD8+ T cells from the spleen and bone marrow while in the peripheral blood, CD8+ memory T cells were principally TEMs (Figure 1D). In summary, large proportions of memory T cells are present in peripheral blood and all lymphoid tissues with the exception of the lymph nodes, which is essentially the site of naïve T cells. Many CD8+ memory T cells are TEMs located in the blood while most CD4+ memory T cells are TCMs found primarily in the spleen and bone marrow.
Variability of alloreactive memory T cells in different monkey combinations
We compared the frequencies of memory T cells secreting IL-2 and γIFN via direct allorecognition in a series of responder/stimulator monkey combinations. Peripheral blood memory T cells from a panel of individual monkeys were isolated by FACS sorting and stimulated in vitro with irradiated allogeneic stimulator cells (mixed lymphocyte reaction). The number of cytokine-producing TMEMs was determined by ELISPOT in each single responder/stimulator combination. Figure 2 shows the frequencies of donor-reactive memory T cells secreting IL-2 (Figure 2A) or γIFN (Figure 2B) in each of 164 and 250 monkey combinations, respectively. The histograms represent the distributions of the frequencies of donor-reactive TMEMs among all the donor–recipient pairs tested that is the percentages of monkey combinations (Y-axis) producing a memory response comprised within a certain range of memory alloreactivity (X-axis). For instance, 36.8% of the monkey pairs displayed a frequency of donor-reactive memory T cells secreting IL-2 comprised between 1 and 50 cells per million TMEMs (Figure 2A). The magnitude of the memory T-cell alloresponse varied dramatically among all the responder/stimulators combinations tested (3–432 spots for IL-2 and 10–3624 spots for γIFN). This heterogeneity was not due to experimental variability as most combinations were tested at least twice in separate experiments (each including triplicate wells) and displayed similar spot numbers (variability <10%). The frequencies of alloreactive memory T cells secreting IL-2 were consistently lower than those of T cells producing γIFN. This presumably reflects the predominance of alloreactive effector TMEMs producing γIFN in the peripheral blood. Interestingly, there was a strong correlation between the frequencies of IL-2- and γIFN-producing TMEMs (r2 = 0.2158, p = 0.00002) (Figure 2C). We surmise that the secretion of IL-2 cytokine influences the production of γIFN, a phenomenon we previously reported in mouse transplant models (27). It is noteworthy that the frequencies of alloreactive naïve T cells did not vary significantly among various donor–recipient pairs. This suggests that the observed differences in anamestic alloreactivity reflect expansions of memory T cells (immunologic history) presumably due to infections rather than intrinsic differences in the naïve T-cell repertoire.
Figure 2. Distribution of the frequencies of donor-reactive TMEMs among all the stimulator-responder pairs.
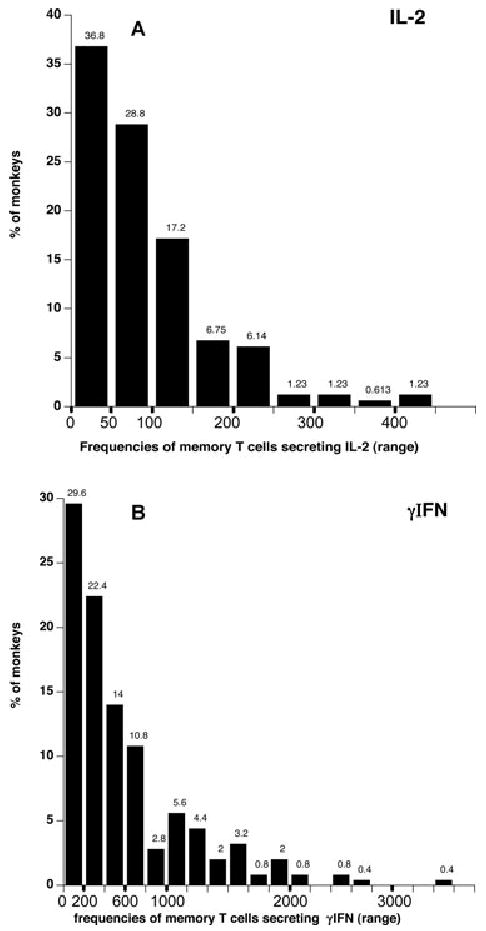
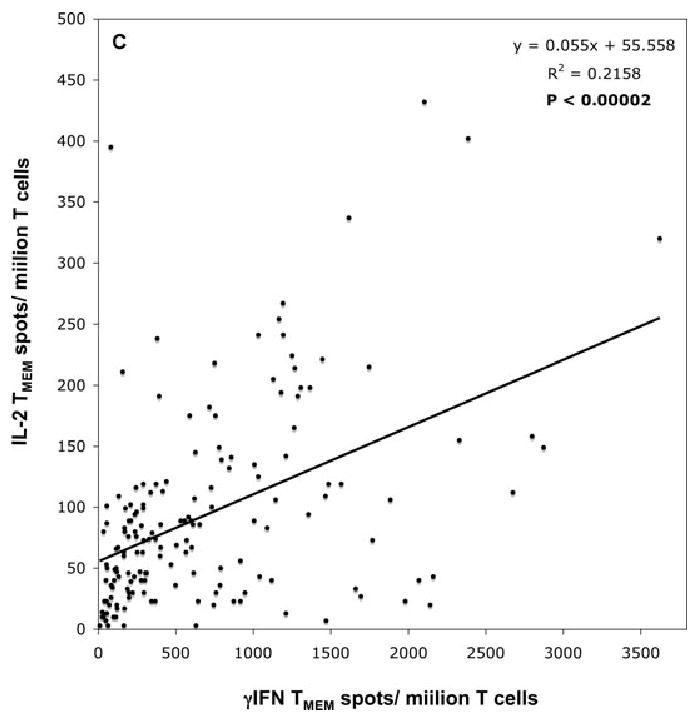
(A) and (B) shows the frequencies of donor-reactive memory T cells secreting IL-2 (panel A) or γIFN (panel B) for each of 164 and 250 monkey combinations, respectively. The histograms represent the distributions of the frequencies of donor-reactive TMEMs among all the stimulator/responder pairs tested that is the percentages of monkey combinations (Y-axis) exhibiting a memory response whose level is comprised within a certain range of T-cell frequency (X-axis). The actual percentages are indicated above each bar. (C) shows the relationship between the frequencies of IL-2- and γIFN-producing memory T cells. R corresponds to the coefficient of correlation. The line shows the linear regression curve. The p-value was calculated using a paired t-test.
Next, memory T cells from each of 11 monkeys were tested individually against a panel of stimulator cells. Alternatively, stimulator cells from each of 14 monkeys were tested individually for their ability to elicit a memory T-cell response by a series of responder cells. As shown in Table 1, individual monkeys displayed a high-memory alloreactivity against certain stimulators but a low reactivity toward others. Alternatively, the same stimulator cells could elicit high-memory T-cell responses in some but not other allogeneic responders. These results emphasize that the magnitude of the memory T-cell alloresponse depends upon the combination tested.
Table 1.
Frequencies of alloreactive memory T cells in different responder/stimulator combinations
| Responders | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3605 | 3805 | 4205 | 4305 | 3005 | 4005 | 3405 | 3905 | 4105 | 405 | 2504 | |
| Stimulators | |||||||||||
| 105 | 281 | 2101 | 47 | 1279 | 593 | ||||||
| 405 | 305 | 2174 | 627 | 325 | |||||||
| 805 | 201 | 1494 | 165 | 439 | |||||||
| 3705 | 124 | 1719 | 402 | 177 | |||||||
| 5400 | 442 | 1333 | 707 | 144 | 395 | 117 | 144 | ||||
| 6400 | 111 | 747 | 757 | 516 | 402 | 137 | 171 | ||||
| 301 | 34 | 194 | 422 | 194 | 472 | 101 | 141 | ||||
| 3501 | 372 | 456 | 968 | 462 | 720 | 238 | 305 | ||||
| 5601 | 67 | 111 | 1139 | 124 | 546 | 221 | 174 | ||||
| 5801 | 338 | 546 | 1300 | 573 | 392 | 278 | 302 | ||||
| 7001 | 298 | 285 | 690 | 268 | 408 | 154 | 134 | ||||
| 1502 | 64 | 439 | 573 | 456 | 436 | 469 | 281 | ||||
| 2102 | 40 | 288 | 660 | 714 | 402 | 372 | 121 | ||||
| 5602 | 67 | 171 | 235 | 248 | 221 | 121 | 291 | ||||
The response of individual responders to a series of stimulators and conversely the response evoked in various responders by the same stimulator were measured. The numbers correspond to the frequencies of γIFN-producing cells per million of purified memory T cells.
Functional properties of alloreactive naïve and memory T cells
We investigated the cytokine secretion patterns of naïve and memory T cells recognizing alloantigens in monkeys displaying high frequencies of alloreactive memory T cells. To test this, naïve and memory T cells were isolated from the peripheral blood of eight monkeys by FACS sorting and tested for their ability to mount an alloresponse via the direct (intact alloMHC displayed on donor APCs) or indirect (donor peptides presented by self-MHC on recipient APCs) allorecognition pathway. The frequencies of naïve and memory T cells secreting IL-2 and γIFN (type 1 cytokines) and IL-4 and IL-10 (type 2 cytokines) were determined using an ELISPOT assay as previously described (25). As shown in Figure 3A only a few naïve T cells (50 cells/million naïve T cells) produced IL-2 cytokine. In contrast, memory T cells mounted a potent direct alloresponse characterized by high frequencies of T cells secreting type 1 cytokines, IL-2 and γIFN. A few but significant number of memory T cells producing IL-4 type 2 cytokines (>50 cells/million TMEMs) were observed (Figure 3B). No cytokine secretion was observed following stimulation of T cell cultures with no APCs (medium) or control syngeneic APCs (Figure 3A and B). No indirect alloreactivity was detected with either naïve or memory T cells upon exposure to syngeneic APCs + allogeneic sonicates (data not shown). The majority of allospecific memory T cells secreting IL-2 were CD4+ memory T cells while most of the T cells producing γIFN corresponded to CD8+ memory T cells (Figure 3C).
Figure 3. Functional properties of alloreactive memory T cells.
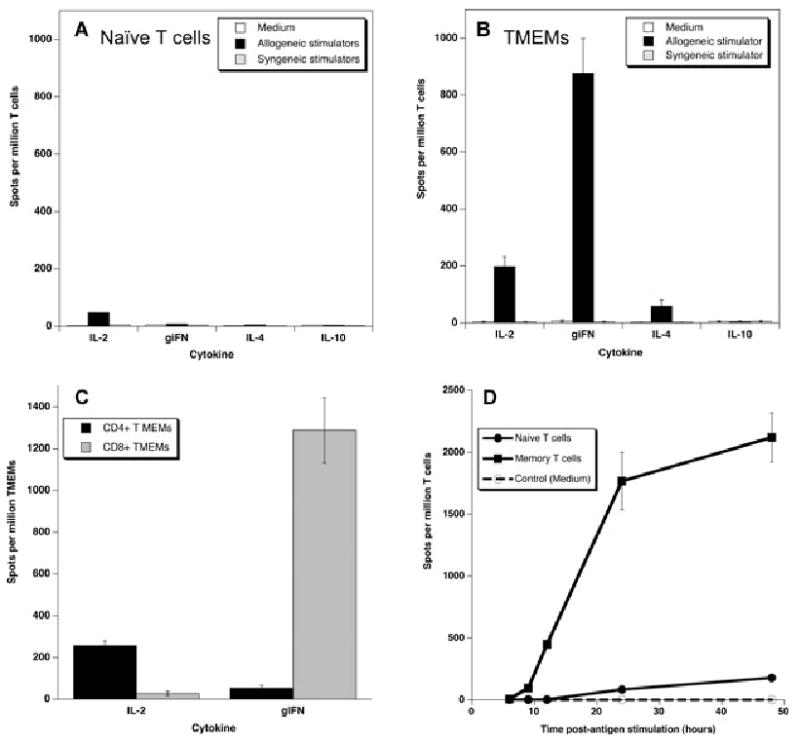
(A) and (B): The frequencies of naïve (panel A) and memory (panel B) alloreactive T cells secreting type 1 cytokines (IL-2 and γIFN) and type 2 cytokines (IL-4 and IL-10) via direct alloreognition were measured using an ELISPOT assay. Negative controls included T cells stimulated with medium and T cells stimulated with syngeneic stimulators (direct pathway) or sonicates (indirect pathway). (C) CD4+ and CD8+ memory T cells were isolated by FACS sorting and tested for their ability to secrete IL-2 or γIFN upon stimulation with allogeneic stimulators (direct pathway). (D) Naïve and memory T cells were isolated and cultured for different periods of time (4–48 h) with allogeneic stimulators. The frequencies of activated T cells producing either IL-2 or γIFN were measured by ELISPOT. The data represent the average numbers of cytokine-producing spot per million T cells ± SD (based on triplicate wells). The results are representative of 3–5 monkeys tested individually in 2–3 separate experiments.
Next, we compared the kinetics of direct alloresponses mediated by naïve and memory T cells. Naïve and memory T cells were isolated from the peripheral blood and cultured for various periods of time with irradiated allogeneic stimulator cells and the frequencies of activated T cells secreting γIFN were measured by ELISPOT. As shown in Figure 3D, a significant number of memory T cells producing γIFN could be detected as early as 8 h after allogeneic stimulation. The number of γIFN spots reached a peak at 24 h and remained stable at 48 h postantigen stimulation. The kinetics were quite different with naïve T cells for which the direct alloresponse was detectable only 24 h after alloantigen stimulation and reached a maximum level at 48 h (Figure 3D). At all time points, the magnitude of the naïve T-cell response was significantly lower than that observed with memory T cells (Figure 3D). Therefore, memory T cells respond in an accelerated and elevated fashion to alloantigens as compared to their naïve counterparts. This observation supports the concept that the vigorous in vitro alloresponse recorded in primary MLR (in nontransplanted animals) is chiefly mediated by allospecific memory T cells.
Relationships between MHC gene expression and the level of memory alloreactivity in cynomolgus monkeys
We investigated whether the degree of MHC gene disparity between monkeys used as responders or stimulators impacts memory alloreactivity. The MHC class I (A and B genes) and II (DP, DQ and DR) genes expressed by our cynomolgus monkeys were characterized using genetic techniques (23,24). The frequencies of alloreactive peripheral blood memory T cells producing IL-2 or γIFN were measured by ELISPOT in several responder/stimulator combinations using purified peripheral blood memory T cells. Figure 4A shows the frequencies of γIFN-producing memory T cells measured in a series of responder/stimulator pairs (n = 111) sharing from 0 (fully disparate) to 4 (no disparity) MHC class I alleles. Each dot represents the memory response measured in an individual responder/stimulator monkey pair. The line represents the linear regression curve corresponding to the relationship between the levels of MHC class I gene sharing and the magnitude of γIFN T cell memory alloreactivity. Figure 4B shows the frequencies of IL-2 producing memory T cells measured in a series of responder/stimulator pairs (n = 98) displaying various levels of MHC class II gene (DP and DQ/DR) disparity. MHC class II DR and DQ genes were considered as a whole given that none of the monkeys displayed any recombination between these two genes. The regression curves shown in Figure 4A and B show that there was an inverse correlation between the level of IL-2 and γIFN memory alloreactivity and the degree of MHC class II and I gene sharing between responders and stimulators, respectively. However, it is important to note that this was not an absolute rule given that a substantial number of fully MHC class I- and II-disparate pairs displayed low γIFN and IL-2 memory responses, respectively.
Figure 4. Influence of MHC disparity between responders and stimulators on the levels of memory alloreactivity.
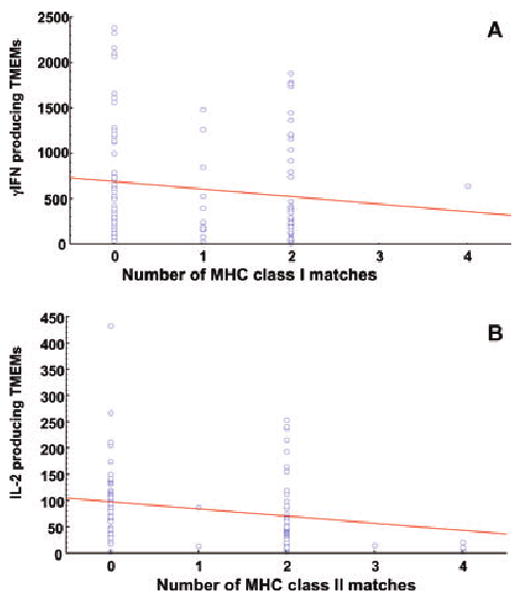
The frequencies of memory T cells producing γIFN (4A) or IL-2 (4B) as measured by ELISPOT in a series of responder/stimulator pairs (n = 98 for IL-2 and n = 111 for γIFN) sharing from 0 (fully disparate) to 4 (no disparity) alleles at the MHC class I (A and B alleles, panel A) or II (DP and DQ/DR alleles, panel B) loci. MHC class II DR and DQ genes were considered as a whole as none of the monkeys displayed a recombination between these two genes. Each dot represents the memory response measured for an individual responder/stimulator monkey pair. The lines represents the linear regression curves corresponding to the relationships between the levels of MHC class gene sharing and the magnitude of T cell memory alloreactivity.
Next, we investigated whether the correlation between the level of MHC gene sharing and the memory alloreactivity was statistically significant. The statistical analyses were performed with a rank ANOVA using generalized estimating equation to control for the fact that certain responders had multiple representations. As shown in Table 2, we observed a statistically significant correlation between the degree of MHC class I disparity (all class I genes considered) and the frequency of γIFN-secreting memory T cells (p = 0.0199). This result presumably reflects the association of γIFN production and the activation of peripheral blood CD8+ TEMs recognizing MHC class I alloantigens (Figure 3C). Apparently, sharing of MHC class IA but not B genes between responders and stimulators was correlated with a low γIFN memory alloreactivity (Table 2). It is noteworthy that a low but significant correlation (p = 0.0402) was also observed between the γIFN memory response and MHC class II DQ/DR matching. This may be due to the activation of some CD4+ MHC class II-restricted TEMs. Alternatively, this may reflect the influence of CD4+ T cell help (via IL-2 secretion) on CD8+ T cells producing γIFN.
Table 2.
Correlations between the levels of memory T cell alloreactivity and the degree of MHC gene sharing between allogeneic monkey responder/stimulator pairs
| TMEM | Matching at MHC locus | Number of combinations tested | p-Value |
|---|---|---|---|
| γIFN | All class I genes | 111 | 0.0199 |
| Class IA | 144 | 0.0014 | |
| Class IB | 167 | 0.0701 | |
| All class II genes | 154 | 0.0657 | |
| Class II DR/DQ | 161 | 0.0402 | |
| Class II DP | 186 | 0.1908 | |
| IL-2 | All class I genes | 83 | 0.3895 |
| Class IA | 108 | 0.1729 | |
| Class IB | 103 | 0.8050 | |
| All class II genes | 98 | 0.0023 | |
| Class II DR/DQ | 105 | 0.0016 | |
| Class II DP | 103 | 0.0018 |
We compared the memory T-cell responses (γIFN or IL-2) in a series of responders differing from their stimulators by different MHC class I or II genes. The statistical relevance (p-values) of the correlations between the degree of gene sharing at a given locus and the level of the memory response was analyzed with a rank ANOVA using generalized estimating equation to control for the fact that certain responders had multiple representations. The p-values <0.05 (shown in bold) were considered statistically significant.
There was a highly significant correlation between the degree of MHC class II gene disparity and the magnitude of the IL-2 memory T-cell alloreactivity (p = 0.0023). This phenomenon was observed with both DR/DQ and DP genes (p = 0.016 and 0.018, respectively). This result is consistent with the fact that IL-2 is primarily produced by CD4+ memory T cells recognizing donor MHC class II molecules in a direct fashion (Figure 3C). In contrast, sharing of MHC class I genes had no effect on the frequency of memory T cells producing IL-2. Therefore, MHC gene matching between allogeneic responders and stimulators was usually associated with a lower memory T cell alloresponsiveness. However, it is important to note that a significant number of monkeys displayed low memory responsiveness to fully MHC class I- or II-disparate allogeneic stimulators (Figure 4A and B).
Discussion
Selective suppression of donor-specific T cells responsible for transplant rejection has been achieved in many experimental mouse models. However, these protocols have seldom been successfully extended to transplant tolerance in primates. Wild-caught monkeys and humans display higher frequencies of potentially alloreactive memory T cells than laboratory mice, a feature that may render them resistant to tolerance induction (28–30). Memory T cells capable of recognizing allogeneic MHC molecules in a direct fashion were detected in all the monkeys prior to any treatment. It is likely that memory T cells recognizing alloantigens directly correspond to cross-reactive T cells previously exposed to microbial peptides presented in a self-MHC context that is Self-MHC + X (microbial peptide) = AlloMHC + Y (donor peptide) (31). This type of mimicry is thought to account in part for the large fraction of the T-cell repertoire directed to alloantigens in individuals that have never been exposed to alloantigens (32,33). Alternatively, we found no TMEMs with indirect alloreactivity in any of the monkeys tested, an observation consistent with previous findings in rodents and swine (25,34,35). This observation suggests that these monkeys have never been exposed to allogeneic MHC molecules, a phenomenon probably accounting for the absence of alloantibodies in these animals. It is important to emphasize that humans who have been exposed to allogeneic MHC molecules through blood transfusion, a prior transplant or pregnancy may possess indirectly activated memory T cells. Indeed, some patients, even without a history of pregnancy or blood transfusions, display significant levels of alloantibodies pretransplantation (36,37), a feature that may reflect the presence of memory T cells initially activated through the indirect pathway. It is noteworthy that the monkeys tested in our study were males, a feature excluding any alloantigen exposure via pregnancy in this group. Therefore, unlike rodents and the monkeys used in this study, some patients may display memory T cells sensitized through indirect allorecognition pretransplantation.
We observed a possibly clinically important variability in the level of memory alloreactivity pretransplantation depending upon the monkey combination examined. This feature appeared to reflect in part disparities at the MHC genetic locus since a majority of the monkey pairs sharing MHC class I and II genes displayed low frequencies of donor-reactive memory T cells. A higher degree of MHC class II sharing between responders and stimulators was associated with lower IL-2 and γIFN memory responses by TMEMs. Indeed, the absence of MHC class II mismatches is traditionally associated with a lack of direct alloresponsiveness by CD4+ T cells as evidenced by weakly reactive MLR (38). The subsequent lack of CD4+ T cell help might result in a poor effector response by γIFN-producing T cells (Figure 3C). Also, the presence of shared MHC class II molecules on allogeneic stimulator cells could promote the suppression of memory T cells by regulatory CD4+FoxP3+ T cells (Tregs) whose activation is governed by the recognition of self-MHC class II molecules on target cells (39). Matching of MHC class I genes was usually associated with a low γIFN memory T-cell response by TMEMs. Consistent with these observations, haplo-matched combinations also exhibited a low anamnestic alloreactivity (data not shown). We hypothesize that (1) recipients transplanted with MHC-matched allografts may be particularly susceptible to donor-specific tolerance due to their low antidonor memory T cell responsiveness and, (2) MHC class II matching may be sufficient to enhance transplant tolerance induction in primates by averting the CD4+ T-cell memory alloresponse and the IL-2 secretion thereby limiting effector T-cell functions by γIFN-producing CD8+ T cells while promoting donor-specific regulatory T-cell immunity.
It is important to note that while MHC matching was usually associated with a low memory T-cell response, a significant number of monkeys displayed a low anamnestic response to some fully MHC gene-disparate animals. This suggests that certain MHC gene disparities are poorly immunogenic in selected responder/stimulator combinations. This is supported by the observation that certain monkeys displayed a high memory response to some but not other fully mismatched allogeneic monkeys. This may also reflect the influence of non-MHC genes or antigens in the development of the alloreactive anamnestic response. Indeed, differences in the memory T-cell repertoire due to microbial infections may account for the sensitization of some monkeys against particular allo-MHC molecules. For instance T cells from humans primed to EBV peptides presented by HLA-B8 also react to the allo-MHC molecule HLA-B4402. Therefore, HLA-B8 individuals may possess TMEMs directed to HLA-B4402 allogeneic subjects as a result of an EBV infection. Altogether, our observations show that MHC gene matching may be helpful but is not essential for selecting donor/recipient combinations in whom tolerance induction is possible.
In summary, our results show that nonhuman primates display significant numbers of alloreactive memory T cells prior to transplantation. However, the level of memory alloreactivity is extremely variable depending upon the nature of the responder/stimulator pair considered. MHC matching between potential transplant recipients and donors is generally associated with a low anamnestic alloresponse. However, the contrary is not true since a significant number of individuals display a low memory reactivity to fully MHC gene disparate allogeneic animals. Several lines of evidence suggest that the presence of donor-reactive memory T cells in recipients represents a major barrier to transplant tolerance induction. Our study suggests that selection of donor–recipient pairs with a low-memory alloreactivity rather than MHC gene matching may be helpful to the success of tolerance induction in clinical transplantation.
Acknowledgments
This work was supported by grants from the MGH ECOR and NIAID U19 AI066705 grants to GB NIH 19 DK080652 and HL018446 To ABC and NIH U191066705 and PO1HL18646 to JCM.
References
- 1.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 2.Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. doi: 10.1016/j.coi.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 4.Swain SL, Agrewala JN, Brown DM, Roman E. Regulation of memory CD4 T cells: Generation, localization and persistence. Adv Exp Med Biol. 2002;512:113–120. [PubMed] [Google Scholar]
- 5.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 6.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 7.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 8.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: Intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 9.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170:4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 10.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: An overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 11.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 13.Benichou G. Direct and indirect antigen recognition: The pathways to allograft immune rejection. Front Biosci. 1999;4:D476–D480. doi: 10.2741/benichou. [DOI] [PubMed] [Google Scholar]
- 14.Benichou G, Fedoseyeva EV. The contribution of peptides to T cell allorecognition and allograft rejection. Int Rev Immunol. 1996;13:231–243. doi: 10.3109/08830189609061750. [DOI] [PubMed] [Google Scholar]
- 15.Lechler R, Lombardi G. The structural basis of alloreactivity. Immunol Res. 1990;9:135–146. doi: 10.1007/BF02918204. [DOI] [PubMed] [Google Scholar]
- 16.Lechler R, Lombardi G. Structural aspects of allorecognition. Curr Opin Immunol. 1991;3:715–721. doi: 10.1016/0952-7915(91)90102-7. [DOI] [PubMed] [Google Scholar]
- 17.Sayegh MH, Watschinger B, Carpenter CB. Mechanisms of T cell recognition of alloantigen. The role of peptides. Transplantation. 1994;57:1295–1302. doi: 10.1097/00007890-199405150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lakkis FG, Sayegh MH. Memory T cells: A hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402–2410. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 19.Valujskikh A, Lakkis FG. In remembrance of things past: Memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 20.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Tan JT, Adams AB, et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J Immunol. 2001;167:4987–4995. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 22.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6:647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 23.Pendley CJ, Becker EA, Karl JA, et al. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics. 2008;60:339–351. doi: 10.1007/s00251-008-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor SL, Blasky AJ, Pendley CJ, et al. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59:449–462. doi: 10.1007/s00251-007-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 26.Pitcher CJ, Hagen SI, Walker JM, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 27.Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4 +and CD8+ T cells in allorecognition: Lessons from corneal transplantation. J Immunol. 2001;167:1891–1899. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 29.Illigens BM, Yamada A, Fedoseyeva EV, et al. The relative contribution of direct and indirect antigen recognition pathways to the alloresponse and graft rejection depends upon the nature of the transplant. Human Immunology. 2002;63:912–925. doi: 10.1016/s0198-8859(02)00449-4. [DOI] [PubMed] [Google Scholar]
- 30.Obhrai JS, Oberbarnscheidt MH, Hand TW, Diggs L, Chalasani G, Lakkis FG. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051–4058. doi: 10.4049/jimmunol.176.7.4051. [DOI] [PubMed] [Google Scholar]
- 31.Rogers NJ, Lechler RI. Allorecognition. Am J Transplant. 2001;1:97–102. [PubMed] [Google Scholar]
- 32.Matzinger P, Bevan MJ. Hypothesis: Why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977;29:1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- 33.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 34.Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992;175:305–308. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haller GW, Lima B, Kunisaki SM, et al. MHC alloantigens elicit secondary, but not primary, indirect in vitro proliferative responses. J Immunol. 2002;169:3613–3621. doi: 10.4049/jimmunol.169.7.3613. [DOI] [PubMed] [Google Scholar]
- 36.Jordan SC, Pescovitz MD. Presensitization: The problem and its management. Clin J Am Soc Nephrol. 2006;1:421–432. doi: 10.2215/CJN.01651105. [DOI] [PubMed] [Google Scholar]
- 37.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 38.McDevitt HO. Discovering the role of the major histocompatibility complex in the immune response. Annu Rev Immunol. 2000;18:1–17. doi: 10.1146/annurev.immunol.18.1.1. [DOI] [PubMed] [Google Scholar]
- 39.LeGuern C. Regulation of T-cell functions by MHC class II self-presentation. Trends Immunol. 2003;24:633–638. doi: 10.1016/j.it.2003.10.010. [DOI] [PubMed] [Google Scholar]


