Abstract
Objective
To measure self-reported physical and mental functioning and associated clinical features at study entry in 3 ethnic groups with systemic sclerosis (SSc).
Methods
Sixty Hispanic, 39 African American, and 104 Caucasian patients with recent-onset SSc (< 5 yrs) were assessed for perceived physical and mental functioning, using the Medical Outcomes Study Short Form-36 (SF-36) and Scleroderma-Health Assessment Questionnaire (Sclero-derma-HAQ). Socioeconomic, demographic, clinical, immunologic, immunogenetic, behavioral, and psychological variables (Interpersonal Support Evaluation List, ISEL; Illness Behavior Questionnaire, IBQ; and Arthritis Helplessness Index, AHI) were analyzed by linear regression models for associations with SF-36 and mHAQ scores as dependent variables.
Results
Perceived physical functioning scores had ethnic-specific associations with AHI > fatigue scores > IBQ > clinical variables (hypertension, skin score, and percentage predicted DLCO). Scleroderma-HAQ scores had ethnic-specific associations with IBQ > AHI scores > most clinical and laboratory variables. Decreased mental component summary (MCS) scores associated with AHI > ISEL. Ethnic-specific immunogenetic variables HLA-DQB1*0202 (Caucasian) and HLA-DRB1*11 (African American), and HLA-DQA1*0501 (Hispanic) also associated with MCS. Antinuclear autoantibodies, anti-topoisomerase I, and RNA polymerases I and III also demonstrated associations with functioning in African American and Hispanic groups.
Conclusion
Clinical, psychosocial, and immunogenetic variables had ethnic-specific associations with perceived physical and mental functioning. Consideration of ethnic-specific psychological and behavioral support in designing more personalized, relevant therapeutic interventions for the patient may improve therapeutic efficacy in SSc.
Key Indexing Terms: MEDICAL OUTCOMES STUDY SHORT FORM-36, ILLNESS BEHAVIOR QUESTIONNAIRE, SCLERODERMA HEALTH ASSESSMENT QUESTIONNAIRE, HISPANIC, INTERPERSONAL SUPPORT EVALUATION LIST, PERSONALIZED MEDICINE
Systemic sclerosis (scleroderma, SSc) is an autoimmune disease in which fibrosis of the skin and internal organs occurs in association with small-vessel vasculopathy and autoantibody production1,2. A wide spectrum of organ- and non-organ-specific impairments ensue that may lead to severe limitations in physical, work, and social activities.
The influence of health-related quality of life (HRQOL) has been increasingly appreciated in SSc3–6, and is reportedly an important contributor to disease outcomes7,8. Nonclinical variables such as social support, illness behaviors, (learned) helplessness, and perceptions of illness may be more sensitive indicators of QOL or perceived functioning than objective clinical measurements. We hypothesized that some associations to perceived functioning with clinical, psychological, and behavioral factors in SSc would be culturally defined, or ethnic-specific. Self-reported physical and mental functioning were measured in patients with SSc in the GENISOS (Genetics vs ENvironment in Scleroderma Outcomes Study) cohort at study entry, using the Medical Outcomes Study Short-Form 36 Health Survey (SF-369) and the Scleroderma-Health Assessment Questionnaire (Scleroderma-HAQ10). The primary objective of our study was to measure self-reported physical and mental function and associated clinical, laboratory, demographic, psychological, and behavioral features at study entry in 3 ethnic groups with SSc. As a secondary objective, we analyzed scores of perceived functioning for ethnic-specific associations to clinical, cultural, behavioral, and laboratory variables from the GENISOS database. From these studies, a hypothetical model of clinical, behavioral, and cultural influences was developed to explain their contributions to HRQOL in SSc. Identification of these ethnic-specific associations is predicted to enhance development of medical therapy that is more tailored to and effective for the patient with SSc.
MATERIALS AND METHODS
The GENISOS cohort has been part of a study of the Specialized Center of Research (SCOR) and is currently part of a Center for Research in Translation (CORT), both sponsored by the US National Institutes of Health. The study was designed to identify predictive factors of outcome in early SSc from the 3 largest ethnic groups in Texas11. This multicenter study is a collaborative effort between the University of Texas-Houston Health Science Center, the University of Texas Medical Branch at Galveston, and the University of Texas-San Antonio Health Science Center. Each study site obtained approval from their institutional review board and general clinical research centers (GCRC) before beginning enrollment, and written informed consent was obtained from all subjects. Baseline study visits took place in the GCRC of the participating institutions as scheduled outpatient visits and, occasionally, on the inpatient services of the respective hospitals staffed by the investigators. Patients were recruited from the 3 participating centers, surrounding private outpatient practices, and the outpatient clinics of county hospital rheumatology clinics. Our cohort represents SSc patients in the Southeastern Texas catchment area.
Patients
Patients were eligible for the study if they (1) met defined preliminary criteria for the classification of SSc12, with disease duration < 5 years (from date of first non-Raynaud’s phenomenon manifestation of scleroderma); (2) had defined ethnicity as explained below; and (3) received medical care in the geographic catchment area of the participating centers.
For our study, the term ethnic group is used to refer to both racial and ethnic identification of patients (total N = 203). Caucasian (Cauc, N = 104) and African American (AA, N = 39) patients were those that identified themselves as Caucasian or African American. Hispanic patients (Hisp, N = 60) were those of predominantly Mexican or Central American ancestry. Only those patients with all 4 grandparents of the same ethnic group were included to more clearly delineate how ethnicity contributes to the factors being examined. Thirteen patients in the catchment area who satisfied inclusion criteria for enrollment refused to participate, as described11.
Variables
The comprehensive GENISOS baseline database includes variables from the following data sets, as described11,13 and as detailed below.
Demographic and clinical variables
Socioeconomic and demographic data obtained for each patient included ethnicity, sex, age, level of education, income, health-related habits, and disease duration. Disease duration was defined as the time between the date of the first non-Raynaud’s phenomenon manifestation of scleroderma and the date to the study entry visit.
Clinical manifestations
Clinical manifestations in all organ systems were ascertained by history, physical examination, and review of medical records. Disease manifestations, medication history, and laboratory, clinical and radiologic variables were recorded on a comprehensive clinical manifestation form. The extent of skin involvement, the total skin score (TSS), was measured by the modified Rodnan skin score as the sum of the scores of 17 body areas ranging from 0 to 3 per area, and a total range 0–5114,15. SSc patients were evaluated for Raynaud’s phenomena, sclerodactyly, digital ulcers, digital pulp loss, digital pitting, digital gangrene, calcinosis, heart and lung examination, pleuropericarditis, upper and lower gastrointestinal symptoms and involvement, vasculitis, muscle weakness/myositis, active synovitis, depression, peripheral neuropathy, history of renal disease, and cerebrovascular accident. Also noted were comorbidities (systemic hypertension, thyroid disease, diabetes mellitus, malignancy) and additional autoimmune diagnoses. Results from chest radiographs (posteroanterior and lateral views), 12-lead electrocardiogram, and pulmonary function tests including spirometry, lung volumes and diffusing capacity in liters of carbon monoxide (DLCO) were also collected.
Laboratory tests
Laboratory tests included complete blood count, serum creatinine, creatine kinase, and urinalysis.
Immunological variables
Autoantibodies, including SSc associated autoantibodies, antinuclear antibodies (ANA), anti-Scl-70 (topoisomerase I, topo-I), anticentromere antibodies (ACA), anti-RNA polymerase I, II and III, anti-Sm (Smith), anti-U1-RNP, anti-Ro/SSA, anti-La/SSB, and anti-U3-RNP (fibrillarin) antibodies, were determined at baseline visit as described11,16–18.
Immunogenetic variables
Class II HLA genotyping on SSc patients and ethnically matched healthy controls was performed on extracted and purified genomic-amplified DNA using standard laboratory procedures as described11,16–18.
Fatigue ratings
Fatigue ratings were measured using the revised Fatigue Severity Scale (FSS), a 29-item validated instrument18–21 that includes statements concerning the respondent’s fatigue (how fatigue affects motivation, exercise, physical functioning, daily activities, and interference with work, family, or social life). The FSS scores each item on a scale from 0 to 7, measuring no fatigue to most fatigue possible, the final score being the mean of all scores and with higher scores indicating greater fatigue. The FSS was not previously validated in SSc patients.
Behavioral and psychological variables
Three constructs for social support, coping with illness, and learned helplessness were established. Social support was ascertained using the Interpersonal Support Evaluation List (ISEL), a 40-item validated instrument comprising 4 scales with a range of 0–3 (tangible, appraisal, belonging, and self-esteem) and a summary measure22,23. To obtain a score, the mean of the subscale items is multiplied by 10. The overall score is the sum of the subscales divided by 4. Higher scores indicate better social support. The ISEL has not been previously validated in SSc.
Coping with illness was determined using the Illness Behavior Questionnaire (IBQ), a 62-item validated instrument comprising 7 scales measuring generalized hypochondriasis, disease conviction, denial, affective disturbance, affective inhibition, psychological versus somatic, and irritability, plus a summary measure with a range 0–3524. The questionnaire also includes 2 second-order factors, which are composites of scores from the IBQ scales: (1) disease affirmation and affective state and (2) the Whitley Index of Hypochondriasis. Higher IBQ scores indicate more illness behaviors. The IBQ has not been previously validated in SSc.
Helplessness or learned helplessness was ascertained with the Arthritis Helplessness Index (AHI), a 15-item validated instrument in which higher scores indicate a higher degree of learned helplessness25,26. In the case of diseases other than rheumatoid arthritis, the word “condition” is substituted for “arthritis.” Scoring ranges from 1 to 4 on a Likert-type scale and total scores ranging from 15 to 60. A higher score indicates more perceived helplessness, or that patients have less belief that they can influence their disease course. The AHI has not been previously validated in SSc.
Perceived functioning
The SF-36 (with 4-week recall) Version 1.0 was used to evaluate patient HRQOL9,11,27. The SF-36 is a standard 36-item survey instrument designed to evaluate QOL issues by self-perceived psychological and physical limitations due to an underlying illness9. It yields a score for each of 8 domains, as well as summary scores for both physical component summary (PCS) and mental component summary (MCS). All scores are rated so that higher values indicate higher levels of perceived functioning (range 0–100). Raw SF-36 version 1.0 domains were normalized to the more current version (2.0) using a 2.0 conversion kit (SF Health Outcomes Scoring Software, QualityMetric Incorporated, Lincoln, RI, USA). The converted data can be compared with normative data for the US population as well as 2.0 version scores from other studies of rheumatic diseases. The SF-36 has been validated in patients with SSc4–6.
The Scleroderma-HAQ, measuring self-reported physical disability, was also administered28,29. The Scleroderma-HAQ includes the HAQ-Disability Index (DI) score and the addition of 5 visual analog scales (VAS) to evaluate SSc organ system symptoms (Raynaud’s phenomenon, gastrointestinal tract and lung involvement, pain, and overall disease severity29). The Scleroderma-HAQ was not corrected for assistive devices. The length of the VAS was from 0 (“does not interfere”) to 15 cm (“very severe limitation”). Scoring of the HAQ-DI was performed by linear regression analysis as described27. The Scleroderma-HAQ VAS was scored by addition of the scales. The Scleroderma-HAQ has been validated and is useful in assessing function and response to treatment in patients with SSc30,31.
Statistical analysis
Data are presented as mean ± standard deviation unless otherwise noted. Chi-square or Fisher’s exact tests were used for comparison among ethnic groups for categorical variables. Group comparisons were measured using the analysis of variance (ANOVA) or nonparametric Kruskal-Wallis tests for continuous variables. For the variables that are significantly different among 3 ethnic groups, pairwise comparison tests using Bonferroni corrections were conducted to determine which pairs are significantly different. Since the number of pairwise comparisons was 3 (Cauc vs AA, Cauc vs Hisp, and AA vs Hisp), a p-value < 0.017 was considered statistically significant due to multiple comparisons.
Socioeconomic, demographic, clinical, immunological, immunogenetic, and behavioral-psychological variables were entered as independent variables into stepwise linear regression models by the stepwise selection procedure. The PCS, MCS, and Scleroderma-HAQ scores served as dependent variables. Ethnicity was entered into multivariable analyses, along with variables with p < 0.1 in the univariate analyses.
The mean highest grade levels completed for all patient groups were above 10th grade (Table 1). The SF-36, ISEL, and IBQ instruments were written at the 8th grade level, and all patients were able to complete these forms32. Eleven patients reported English as their second language. All forms were available in Spanish and 8 patients requested forms written in Spanish. Out of the 203 enrolled patients, 6 patients did not complete the SF-36 instrument; therefore the SF-36 completion rate was 97%. The proportion of data missing from all data was < 8%. For outcome variables presented, missing data were not imputed. Significance of these data did not change by imputing missing values of independent variables.
Table 1.
Sociodemographic, functioning, and behavioral scores in SSc patients by ethnicity. Variables are stated as percentages or average ± SD. Comparing all 3 groups by ANOVA for means and by chi-square or Fisher’s exact test for proportions with Bonferroni corrections.
| Variables | All Patients, n = 203 | Caucasian, n = 104 | African American, n = 39 | Hispanic, n = 60 | p* |
|---|---|---|---|---|---|
| Female, % | 84 | 80 | 87 | 90 | 0.193 |
| Age, yrs | 50 ± 13 | 51 ± 12 | 49 ± 13 | 48 ± 14 | 0.172 |
| Highest grade completed | 12.6 ± 3.2 | 13.6 ± 3.0 | 12.7 ± 2.5 | 10.8 ± 3.3 | < 0.001 |
| Disease duration, mo | 34.1 ± 29.2 | 29.8 ± 20.2 | 33.6 ± 19.9 | 35.2 ± 19.1 | 0.218 |
| Diffuse cutaneous SSc, % | 58 | 55 | 62 | 62 | 0.617 |
| Employed, % | 40 | 50 | 34 | 27 | 0.010 |
| Insured, % | 75 | 82 | 64 | 69 | 0.060 |
| Own homes, % | 71 | 78 | 68 | 62 | 0.079 |
| Married, % | 56 | 61 | 47 | 55 | 0.359 |
| Income, monthly $US | 3147 ± 2972 | 4016 ± 3411 | 1869 ± 1310 | 2474 ± 2437 | 0.0001 |
| Unhealthy behaviors†, % | 82 | 84 | 85 | 78 | 0.631 |
| Smoke tobacco | 20 | 26 | 27 | 5 | 0.002 |
| Drink ethanol | 39 | 51 | 26 | 25 | 0.0007 |
| No exercise | 62 | 59 | 65 | 65 | 0.674 |
| Social support (ISEL) | 8.0 ± 11.6 | 8.5 ± 1.3 | 7.2 ± 1.8 | 7.8 + 1.6 | < 0.0001 |
| Illness behavior (IBQ) | 11.0 ± 5.8 | 9.8 ± 5.1 | 11.7 ± 6.2 | 12.7 ± 6.2 | 0.006 |
| Helplessness (AHI) | 40.0 ± 7.0 | 39.5 ± 7.3 | 40.3 ± 8.3 | 40.6 + 5.4 | 0.573 |
| PCS (SF-36) | 38.6 ± 7.0 | 40.4 ± 6.6 | 36.8 ± 7.4 | 36.8 ± 7.4 | 0.002 |
| MCS (SF-36) | 43.4 ± 7.0 | 42.4 ± 6.1 | 44.3 ± 8.7 | 44.7 ± 7.4 | 0.104 |
| SSc-HAQ | 0.87 ± 0.68 | 0.71 ± 0.66 | 1.19 ± 0.74 | 0.92 ± 0.69 | 0.001 |
Computed comparing all 3 groups by ANOVA for means, and by Fisher’s exact test for proportions. Caucasians vs African-Americans: alcohol drinking (p = 0.006); monthly income (p < 0.0001); ISEL (p = 0.0003); PCS (p = 0.006); SSc-HAQ (p = 0.0003). Caucasians vs Hispanics: percentage employed (p = 0.004), smoke tobacco (p = 0.0006), alcohol drinking (p = 0.001), highest grade completed (p < 0.0001), monthly income (p = 0.001), ISEL (p = 0.005), IBQ (p = 0.002), PCS (p = 0.002), physical functioning (p = 0.008). African Americans vs Hispanics: smoke tobacco (p = 0.003), highest grade completed (p = 0.006).
Unhealthy behavior: presence of smoking tobacco, drinking > 2 alcohol drinks/day, and/or no formal exercise, with sedentary lifestyle.
Disease duration: no. of months diagnosed with SSc at study entry; Diffuse cutaneous SSc disease: percentage diagnosed with diffuse cutaneous disease subset; Employed: percentage employed full-time; Insured: percentage with private insurance, Medicare, or Medicaid; ISEL: Interpersonal Support Evaluation List; IBQ: Illness Behavior Questionnaire; AHI: Arthritis Helplessness Index; PCS: physical component summary score; MCS: mental component summary score of the SF-36; Scleroderma-HAQ: Scleroderma Health Assessment Questionnaire, HAQ-DI, and 5 visual analog scale-type questions related to scleroderma, also referred to as SHAQ.
Instrument validation
Cronbach’s coefficient alpha values were computed to measure consistency of ISEL, IBQ, AHI, and FSS questionnaires as a test of reliability of these scales and their validation in SSc patients. Reliability coefficient scores above 0.70 were deemed acceptable for the validity and reliability of these tests as given by Nunnally and Bernstein33.
RESULTS
A comprehensive description of the GENISOS study population at baseline has been published11. Selected features of demographic, clinical, and behavioral-psychological variables are included in Table 1. As expected, the majority of the patients were women, although the Caucasian group had a larger proportion of men. The mean ages for all groups ranged from 48 ± 12 to 51 ± 13 years. There were no significant differences among the 3 ethnic groups at study entry for age, gender, disease duration, or percentage with diffuse cutaneous involvement. Caucasians had completed significantly more grades of formal education than African Americans or Hispanics (Caucasians 13.6 ± 3, compared to African Americans 12.7 ± 2.5 and Hispanics 10.8 ± 3.3; p < 0.001). There were significant differences in monthly incomes between Caucasian, African American, and Hispanic groups (p = 0.0001). There were no significant differences among ethnic groups for percentages of those who were employed, had medical insurance, owned homes, or were married. No differences in access to either SSc-related or non-SSc-related healthcare were seen among the 3 ethnic groups, as reported 11 (data not shown).
Unhealthy behaviors
The frequency of unhealthy behaviors, described as smoking tobacco, ingestion of > 2 drinks with alcohol/day, or no exercise (sedentary) lifestyle, are shown in Table 1. Hispanics self-reported less frequency of smoking tobacco or drinking > 2 alcohol drinks/day compared to Caucasians (5% vs 26%, p = 0.002, and 25% vs 51%, p = 0.0007, respectively). African Americans also reported less frequency of ingesting > 2 alcohol drinks/day compared to Caucasians (26% vs 51%, respectively; p = 0.006). There were no differences among the groups regarding reported exercise activity.
Social support scores
There were higher scores of social support measured by all 4 components of the ISEL (Tangible, Appraisal, Belonging, and Esteem) and for average total ISEL scores among Caucasians, shown in Table 1 (Caucasians 8.51 ± 1.3 vs African Americans 7.2 ± 1.8 and Hispanics 7.8 ± 1.6; p < 0.001). Cronbach’s alpha reliability coefficient for the ISEL was 0.82533.
Illness behavior scores
Higher overall IBQ scores were noted in African Americans and Hispanics compared to Caucasians (Caucasians 9.8 ± 5.1 compared to African Americans 11.7 ± 6.2 and Hispanics 12.7 ± 6.2; p = 0.007). Specifically, African Americans and Hispanics had higher subscale scores of general hypochondriasis, psychological versus somatic, denial scores, and a higher index of hypochondriasis, indicative of more illness-related behaviors. Cronsbach’s alpha reliability coefficient for the IBQ instrument was 0.778.
Helplessness scores
Helplessness, measured by the AHI, reflecting perceived (learned) helplessness was comparable in SSc patients among the 3 ethnic groups (Caucasians 39.5 ± 7.3, African Americans 40.3 ± 8.3, and Hispanics 40.6 ± 5.4; p = 0.240). Cronbach’s alpha reliability coefficient for the AHI was 0.979.
Perceived functioning
Perceived functioning, using the PCS and MCS of the SF-36 and the Scleroderma-HAQ, are also shown in Table 1. Both instruments showed lower reported physical functioning in all 3 ethnic groups, compared to the normative population. The PCS scores demonstrated significant differences between Caucasian (40.4 ± 6.6) and African American and Hispanic groups (36.8 ± 7.4; p = 0.002). SSc patients did not show any significant differences in the SF-36 MCS among the 3 ethnic groups (Caucasians 42.6 ± 6.1, African Americans 44.3 ± 8.7, and Hispanics 44.7 ± 7.4; p = 0.104) and were within 1 standard deviation for control populations. Lower SF-36 PCS scores in SSc patients have been reported4–6,11.
Scleroderma-HAQ scores
Scleroderma-HAQ scores were significantly lower in the Caucasian group, reflecting significantly higher levels of physical abilities compared to the African American and Hispanic groups (Caucasians 0.71 ± 0.66, African Americans 1.2 ± 0.74, and Hispanics 0.92 ± 0.69; p = 0.001).
Ethnic-specific associations with SF-36 and sclero-derma-HAQ scores (Tables 2–4)
Table 2.
Ethnic-specific associations with SF-36 and Scleroderma-HAQ scores in Caucasian subjects.
| Variable | Direction of Association | p | |
|---|---|---|---|
| (1) PCS | HLA-DRB1*0301 | 2.76 | 0.022 |
| HLA-DQB1*0604 | −4.48 | 0.032 | |
| Systemic hypertension | −2.76 | 0.031 | |
| % Predicted DLCO | −2.21 | 0.044 | |
| Fatigue severity | −3.22 | 0.0002 | |
| Helplessness | −0.37 | < 0.0001 | |
| (2) MCS | HLA-DQB1*0202 | −2.84 | 0.016 |
| Social support | 1.27 | 0.004 | |
| (3) SHAQ | HLA-DQA1*0201 | 0.30 | 0.031 |
| Systemic hypertension | 0.27 | 0.042 | |
| Total skin score | 0.01 | 0.006 | |
| Muscle weakness | 0.38 | 0.004 | |
| Fatigue severity | 0.30 | < 0.001 | |
| Helplessness | 0.02 | 0.012 |
MCS: mental component summary score; PCS: physical component summary score of the SF-36; SHAQ:SSc-HAQ, HAQ-DI with 5 questions focused on scleroderma patients; social support: scores from the ISEL; fatigue severity: scores from the Fatigue Severity Scale (FSS); helplessness: scores from the Arthritis Helplessness Index (AHI).
Table 4.
Ethnic-specific associations with SF-36 and Scleroderma-HAQ scores in Hispanic subjects.
| Variable | Direction of Association | p | |
|---|---|---|---|
| (1) PCS | ANA pattern-speckled | 4.43 | 0.003 |
| Systemic hypertension | −4.24 | 0.011 | |
| Fatigue severity | −3.26 | 0.009 | |
| Illness behavior | −0.36 | < 0.001 | |
| (2) MCS | Age | 0.17 | 0.007 |
| HLA-DQA1*0501 | −3.72 | 0.031 | |
| ANA pattern-speckled | −4.80 | 0.007 | |
| Helplessness | −0.51 | 0.001 | |
| (3) SHAQ | HLA-DQB1*03 | 0.41 | 0.005 |
| RNA polymerase I | 0.37 | 0.022 | |
| Muscle weakness | 0.69 | < 0.001 | |
| Tendon friction rub | 0.49 | < 0.001 |
ANA pattern-speckled: seropositive for ANA in speckled pattern; Muscle weakness: presence of proximal muscle weakness on physical examination, presence of myositis on muscle biopsy, or elevated serum creatine kinase on laboratory testing.
Regression analyses were performed to determine associations between laboratory values, clinically measurable variables, and scores from psychological and behavioral instruments to perceived functioning by PCS, MCS, and Scleroderma-HAQ scores in Caucasian, African American, and Hispanic groups. In Caucasians (Table 2), the presence of HLA-DRB1*0301 genotype was associated with higher PCS scores (p = 0.022). Lower PCS scores were associated with the presence of HLA-DQB1*0604 genotype, systemic hypertension, and percentage of predicted DLCO (p = 0.032, 0.031, and 0.044, respectively). Higher fatigue severity and helplessness scores were associated with lower PCS scores (p = 0.0002 and < 0.0001, respectively). The presence of HLA-DQB1*0202 genotype was associated with lower MCS scores (p = 0.016). The presence of HLA-DQA1*0201 genotype, systemic hypertension, total skin score, and muscle weakness was associated with higher Scleroderma-HAQ scores (p = 0.031, 0.042, 0.006, and 0.004, respectively). Higher fatigue severity and helplessness scores were also associated with higher Scleroderma-HAQ scores (p < 0.001 and 0.012, respectively). The associations between clinical, psychosocial, and fatigue variables with PCS and Scleroderma-HAQ scores were robust in Caucasians.
Table 3 demonstrates significant clinical and psychosocial associations with perceived functioning in the African American patients with SSc. In African Americans, lower PCS scores were significantly associated with higher illness behavior scores (p = 0.001). Lower MCS scores were associated with the presence of HLA-DQB1*11 genotype (p = 0.030) and higher helplessness scores (p < 0.001). Higher Scleroderma-HAQ scores were associated with the presence of anti-RNA polymerase III and anti-topoisomerase I autoantibodies (p = 0.003 and 0.020, respectively). Higher Scleroderma-HAQ scores were also associated with higher illness behavior scores (p < 0.001). The associations of laboratory, clinical, and behavioral variables with Sclero-derma-HAQ scores were robust in African Americans.
Table 3.
Ethnic-specific associations with SF-36 and Scleroderma-HAQ scores in African American subjects.
| Variable | Direction of Association | p | |
|---|---|---|---|
| (1) PCS | Illness behavior | −0.71 | 0.001 |
| (2) MCS | HLA-DRB1*11 | 6.75 | 0.030 |
| Helplessness | −0.66 | < 0.001 | |
| (3) SHAQ | RNA Polymerase III | 0.97 | 0.003 |
| Topo-I | 0.57 | 0.020 | |
| Illness behavior | 0.08 | < 0.001 |
Illness behavior: scores from the illness behavior questionnaire (IBQ); RNA polymerase III: seropositive for anti-RNA polymerase III; Topo-I: seropositive for anti-topoisomerase antibody.
Table 4 demonstrates significant laboratory, clinical, psychological, and behavioral associations with perceived functioning in Hispanic patients with SSc. Higher PCS scores were associated with the presence of ANA, speckled pattern (p = 0.003). Lower PCS scores were associated with the presence of systemic hypertension (p = 0.011). Lower PCS scores were also associated with higher fatigue severity and illness behavior scores (p = 0.009 and < 0.001, respectively). The associations of laboratory, clinical, and behavioral variables with PCS scores were robust in Hispanics.
Higher MCS scores were associated with increasing age and the presence of HLA-DQA1*0501 (p = 0.007 and 0.031, respectively). Lower MCS scores were associated with the presence of ANA speckled pattern and higher helplessness scores (p = 0.007 and 0.001, respectively).
Higher Scleroderma-HAQ scores were robustly associated with the presence of HLA-DQB1*03, RNA polymerase I antibody, muscle weakness, and tendon friction rub (p = 0.005, 0.02, < 0.001, and < 0.001, respectively).
Across all ethnic groups, psychological or behavioral factors were often more significantly associated with PCS, MCS, and Scleroderma-HAQ scores compared to clinical, immunogenetic, or serologic variables.
Figure 1 shows a hypothetical model to explain how upstream contributors to quality of life in patients with SSc are influenced by race/ethnicity. This template was modified from a model of previous studies using populations infected with human immunodeficiency virus7,34. Centrally, disease expression influences symptom manifestations, which in turn influence perceived functioning, all of which contribute to overall patient quality of life. Peripherally, our expanded model incorporates the relative influences of social support and behaviors and socioeconomic status. Ethnic cultural influences directly promote social support, socioeconomic status, and illness beliefs. Consequently, the individual’s cultural environment also fosters or permits behaviors and aspects of coping, such as helplessness. Thus, genetic background has direct and indirect contributions to the individual’s quality of life via disease expression, symptom manifestation, and the cultural environment.
Figure 1.
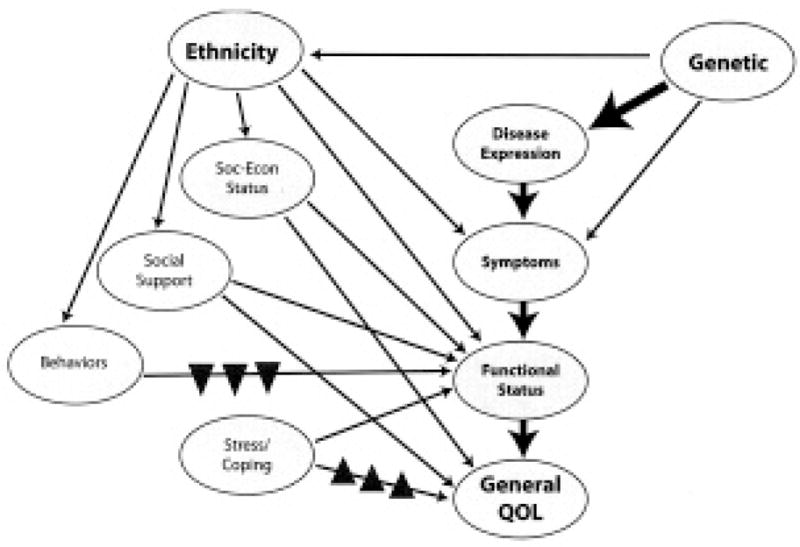
Hypothetical model of variables contributing to quality of life for patients with systemic sclerosis. Strong (block arrows) and weaker (line arrows) proposed contributions of the constructs to general quality of life are illustrated. Disease expression influences symptoms, which influence role-specific functional status. Ultimately, all constructs directly or indirectly contribute to general quality of life. Genetic contributions to central constructs are both direct and/or indirect (ethnic-specific). ▲: May have greater influence in non-Caucasian groups. ▼: May have greater influence in Caucasian groups. Modified from Vidrine DJ, et al7, Qual Life Res 2005;14:923–33, with permission of Springer Science and Business Media.
DISCUSSION
Potentially important ethnic-specific associations were noted between perceived functioning and clinical, laboratory, psychological, behavioral, and immunogenetic variables in patients with SSc. Clinically, ethnic-specific associations with perceived functioning were seen with organ-specific dysfunction and autoantibodies. Higher HAQ-DI and Scleroderma-HAQ scores were previously reported to be associated with greater disease involvement, including organ-specific dysfunction28–31,35–37. Cultural messages and subjective experiences contribute to unique and potentially modifiable psychological and behavioral influences on patient perceptions. Perceived functioning was often robustly associated with behavioral and psychological variables by ethnicity. In Caucasians, fatigue severity, helplessness, and social support scores showed significant associations with PCS, MCS, and Scleroderma-HAQ scores. In African American and Hispanic groups, illness behavior scores were associated with PCS and Scleroderma-HAQ scores, while helplessness scores were associated with MCS. Previous studies also report that disability, pain, and psychosocial adjustment are important contributors to QOL outcomes in SSc37,38. The robust associations of fatigue severity with PCS and Scleroderma-HAQ in Caucasians and PCS in Hispanics might be expected, as many fatigue severity scale items directly assess physical functioning20,21. The association of fatigue with physical functioning has been reported in SSc39,40.
Ours is the first study to report associations between perceived functioning and ethnic-specific HLA genotypes and autoantibodies in SSc. This may indirectly reflect disease severity in the ethnic population expressing a greater frequency of a specific genotype. For example the HLA-DRB*11 genotype was associated with lower MCS scores in African Americans. The HLA-DRB1*11 genotype is also associated with topo-1 autoantibodies. The presence of topo-1 autoantibodies was reported more frequently in African Americans11 (18% vs 10% in Caucasians or Hispanics) and was associated with more severe cutaneous and pulmonary involvement and decreased survival in SSc41. The association of HLA-DRB1*11 genotype with lower MCS may merely reflect the higher frequency of HLA-DRB1*11 in African Americans, although the sample size of the Caucasian group was sufficient to have detected this association if present. Lower MCS scores may reflect more severe disease involvement, also reflected by the association of topo-I with higher Scleroderma-HAQ scores. Or possibly, the association reflects the interactions of gene products with moderating environmental or cultural factors that are more predominant in one ethnicity. Higher Scleroderma-HAQ scores were also significantly associated with the presence of autoantibodies to RNA polymerase III in African Americans.
The presence of HLA-DQA1*0501 was associated with lower MCS scores in Hispanics. HLA-DQA1*0501 was recently identified as a significant predictor of mortality in SSc42. The presence of HLA-DQB1*03 genotype was reported in higher frequency in Hispanic patients with SSc11 and was associated with higher Scleroderma-HAQ scores in the Hispanic group.
Figure 1 incorporates proposed ethnic and genetic contributions to overall patient quality of life and demonstrates 2 major points of this study: (1) Genetic background may significantly affect disease expression (central modifiers), but to a great extent also defines the ethnic experience (peripheral modifiers). (2) Cultural experiences critically affect social support, disease belief systems, and behavior, often independent of demographic, accessibility, or economic factors, and thus influence daily living and ultimately burden of disease.
Our cross-sectional study had limitations that need further elucidation. The ethnic, clinical, and psychological contributors to perceived functioning in SSc identified at study entry may change with disease progression. Longitudinal analyses of our cohort will determine if ethnic-specific associations to functioning persist throughout disease progression and with therapeutic interventions. Improved functioning scores along with clinical improvement following therapeutic intervention have been described in SSc4,30,31.
We demonstrated validation of the ISEL, fatigue severity, IBQ, and helplessness questionnaires, which reflect the individual’s social support and illness beliefs and illness behaviors. A reported strength of these questionnaires is identification of potentially modifiable beliefs and behaviors that affect perceived functioning that may also be dependent on ethnicity. Our study and others have reported differences in the frequency of modifiable behaviors such as smoking tobacco or drinking alcohol by ethnic groups43–46. A possible assumption is to place the primary onus of improving quality of life by behavior modification on the individual and culture, based on coping behavior scores and behaviors, but 2 issues need clarification. First, there is little direct evidence that validates the relative item weights of these questionnaires based on genetically distinct cultures in SSc or other illnesses. Therefore, direct comparisons of scores across ethnic groups may not be valid. Lower scores may not necessarily denote lower functioning, social support, or coping with illness within a defined ethnicity. Moreover these associations may be inconsistent. For example, in Hispanics, ACA was associated with higher PCS but lower MCS scores. Further, genotypes that are reported to be protective in SSc populations may not be associated with higher scores of perceived functioning47. However, we demonstrated that ethnic-specific associations of clinical and laboratory data, social support, illness behaviors, and helplessness and fatigue score variables with perceived functioning were often robust.
Second, our study and others showed no significant differences in access to healthcare across ethnic groups in SSc (or systemic lupus erythematosus11,36,43). However, external to the individual or culture, recent studies also show ethnically defined disparities in potentially modifiable treatment practices of the heathcare system, including primary care providers, independent of income or accessibility issues48,49.
Personalized medicine traditionally emphasizes genetic background in the context of disease manifestations in designing optimal therapeutic interventions for the individual. However, therapeutic interventions in SSc may be suboptimal unless important psychological or behavioral factors are adequately addressed50–53. The patient’s cultural environment may offer beneficially malleable influences on quality of life. For example, will culturally tailored interventions to culturally affected behaviors such as smoking cessation improve success rates and ultimately, clinical outcomes54? Scoring and use of functioning, social support, and coping questionnaires may offer relatively cheap insight to improving individualized therapeutic interventions. Routine and meaningful clinical use of these instruments will require further validation based on cultural background.
Acknowledgments
The authors gratefully acknowledge the assistance of Study Coordinators Rudyard Lanete, Gaston Benavides, Heather Metts, Madonna Berry, Barbara Boyle, and Lena Williams; and technical assistance of Kim Jordan, Julio Charles, and Anthony Thomas. The authors also thank the study nurses at the University Clinical Research Centers of the collaborating institutions and the staff of the South Texas Hospital in Harlingen, Texas, for their assistance during patient study visits. Finally, the authors thank all the physicians who kindly referred their patients to the study.
Supported by NIH Specialized Center of Research (SCOR) Grant in Scleroderma P50AR44888, NIH Centers for Research Translation (CORT) P50AR054144, and University Clinic Research Center Grants M01-RR02558 (UTH-HSC), M01-RR00073 (UTMB) and M01-RR01346 (UT-SA).
References
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nature Clin Pract Rheumatol. 2006;2:134–4. doi: 10.1038/ncprheum0115. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D, Clements PJ, Furst DE, Chon Y, Elashoff R, Roth MD, et al. the Scleroderma Lung Study Group. Correlation of the degree of dyspnea with health-related quality of life, functional abilities, and diffusing capacity for carbon monoxide in patients with systemic sclerosis and active alveolitis: results from the Scleroderma Lung Study. Arthritis Rheum. 2005;52:592–600. doi: 10.1002/art.20787. [DOI] [PubMed] [Google Scholar]
- 4.Khanna D, Furst DE, Clements PJ, Park GS, Hays RD, Yoon J, et al. Scleroderma Clinical Trials Consortium. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32:832–40. [PubMed] [Google Scholar]
- 5.Danieli E, Paolo A, Lorenzo B, Cinquini M, Antonioli CM, Cavazzana I, et al. Health-related quality of life measured by the Short Form 36 (SF-36) in systemic sclerosis: correlations with indexes of disease activity and severity, disability, and depressive symptoms. Clin Rheumatol. 2005;24:48–54. doi: 10.1007/s10067-004-0970-z. [DOI] [PubMed] [Google Scholar]
- 6.Del Rosso A, Boldrini M, D’Agostino D, Placidi GP, Scarpato A, Pignone A, et al. Health-related quality of life in systemic sclerosis as measured by the Short Form 36: Relationship with clinical and biologic markers. Arthritis Rheum. 2004;51:475–81. doi: 10.1002/art.20389. [DOI] [PubMed] [Google Scholar]
- 7.Vidrine DJ, Amick BC, 3rd, Gritz ER, Arduino RC. Assessing a conceptual framework of health-related quality of life in a HIV/AIDS population. Qual Life Res. 2005;14:923–33. doi: 10.1007/s11136-004-2148-1. [DOI] [PubMed] [Google Scholar]
- 8.Alarcon GS, McGwin G, Jr, Uribe A, Friedman AW, Roseman JM, Fessler BJ, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum. 2004;51:465–74. doi: 10.1002/art.20409. [DOI] [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Keller SD, Gandek B, Brazier JE, Sullivan M. Evaluating translations of health status questionnaires. Methods from the IQOLA project. International Quality of Life Assessment. Int J Technol Assess Health Care. 1995;11:525–51. doi: 10.1017/s0266462300008710. [DOI] [PubMed] [Google Scholar]
- 10.Poole JL, Steen VD. The use of the Health Assessment Questionnaire (HAQ) to determine physical disability in systemic sclerosis. Arthritis Care Res. 1991;4:27–31. doi: 10.1002/art.1790040106. [DOI] [PubMed] [Google Scholar]
- 11.Reveille JD, Fischbach M, McNearney T, Friedman AW, Aguilar MB, Lisse J, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–46. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 12.Masi AT, Rodnan GP, Medsger TA, Jr, Altman RD, D’Angelo WA, Fries JF, et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 13.McNearney TA, Reveille JD, Fischbach M, Friedman AW, Lisse JR, Goel N, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic and behavioral factors. Arthritis Rheum. 2007;57:318–26. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 14.Kahaleh MB, Sultany GL, Smith EA, Huffstutter JE, Loadholt CB, LeRoy EC. A modified scleroderma skin scoring method. Clin Exp Rheumatol. 1986;4:367–9. [PubMed] [Google Scholar]
- 15.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 16.Reveille JD, Durban E, MacLeod-St Clair MJ, Goldstein R, Moreda R, Altman RD, et al. Association of amino acid sequences in the HLA-DQB1 first domain with antitopoisomerase I autoantibody response in scleroderma (progressive systemic sclerosis) J Clin Invest. 1992;90:973–80. doi: 10.1172/JCI115974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feghali-Bostwick C, Medsger TA, Jr, Wright TM. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum. 2003;48:1956–63. doi: 10.1002/art.11173. [DOI] [PubMed] [Google Scholar]
- 18.Tan FK, Wang N, Kuwana M, Chakraborty R, Bona CA, Milewicz DM, et al. Association of fibrillin 1 single-nucleotide polymorphism haplotypes with systemic sclerosis in Choctaw and Japanese populations. Arthritis Rheum. 2001;44:893–901. doi: 10.1002/1529-0131(200104)44:4<893::AID-ANR146>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31:1601–7. doi: 10.1093/sleep/31.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 21.Rekand T, Gramstad A, Vedeler CA. Fatigue, pain and muscle weakness are frequent after Guillain-Barre syndrome and poliomyelitis. J Neurol. 2009;256:349–54. doi: 10.1007/s00415-009-0018-z. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13:99–125. [Google Scholar]
- 23.Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: theory, research, and applications. Boston: Martinus Nijhoff; 1985. [Google Scholar]
- 24.Pilowsky L, Spence ND. Illness behavior questionnaire (IBQ) In: Corcoran K, Fischer J, editors. Measures for clinical practice. A source book. New York: The Free Press; 1996. p. 182. [Google Scholar]
- 25.Nicassio PM, Wallston KA, Callahan LF, Herbert M, Pincus T. The measurement of helplessness in rheumatoid arthritis. The development of the Arthritis Helplessness Index. J Rheumatol. 1985;12:462–7. [PubMed] [Google Scholar]
- 26.Engle EW, Callahan LF, Pincus T, Hochberg MC. Learned helplessness in systemic lupus erythematosus: analysis using the Rheumatology Attitudes Index. Arthritis Rheum. 1990;33:281–6. doi: 10.1002/art.1780330220. [DOI] [PubMed] [Google Scholar]
- 27.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. psychometric and clinical test of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Poole JL, Williams CA, Bloch DA, Hollak B, Spitz P. Concurrent validity of the Health Assessment Questionnaire Disability Index in scleroderma. Arthritis Care Res. 1995;8:189–93. doi: 10.1002/art.1790080312. [DOI] [PubMed] [Google Scholar]
- 29.Steen VD, Medsger TA., Jr The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum. 1997;40:1984–91. doi: 10.1002/art.1780401110. [DOI] [PubMed] [Google Scholar]
- 30.Clements PJ, Wong WK, Hurwitz EL, Furst DE, Mayes M, White B, et al. The Disability Index of the Health Assessment Questionnaire is a predictor and correlate of outcome in the high-dose versus low-dose penicillamine in systemic sclerosis trial. Arthritis Rheum. 2001;44:653–61. doi: 10.1002/1529-0131(200103)44:3<653::AID-ANR114>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Khanna D, Furst DE, Clements PJ, Park GS, Hays RD, Yoon J, et al. Relaxin Study Group. Scleroderma Clinical Trials Consortium. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32:832–40. [PubMed] [Google Scholar]
- 32.Beckman HT, Lueger RJ. Readability of self-report clinical outcome measures. J Clin Psychol. 1997;53:785–9. doi: 10.1002/(sici)1097-4679(199712)53:8<785::aid-jclp1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Nunnally JC, Bernstein IH. Psychometric theory. 3. New York: McGraw-Hill; 1994. [Google Scholar]
- 34.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 35.Friedman AW, Alarcon GS, McGwin G, Jr, Straaton KV, Roseman J, Goel N, et al. Systemic lupus erythematosus in three ethnic groups. IV. Factors associated with self-reported functional outcome in a large cohort study. LUMINA Study Group. Lupus in Minority Populations, Nature vs Nurture. Arthritis Care Res. 1999;12:256–66. doi: 10.1002/1529-0131(199908)12:4<256::aid-art4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 36.Hyphantis TN, Tsifetaki N, Siafaka V, Voulgari PV, Pappa C, Bai M, et al. The impact of psychological functioning upon systemic sclerosis patients’ quality of life. Semin Arthritis Rheum. 2007;37:81–92. doi: 10.1016/j.semarthrit.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Malcarne VL, Hansdottir I, McKinney A, Upchurch R, Greenbergs HL, Henstorf GH, et al. Medical signs and symptoms associated with disability, pain, and psychosocial adjustment in systemic sclerosis. J Rheumatol. 2007;34:359–67. [PubMed] [Google Scholar]
- 38.Sandqvist G, Scheja A, Eklund M. Working ability in relation to disease severity, everyday occupations and well-being in women with limited systemic sclerosis. Rheumatology. 2008;47:1708–11. doi: 10.1093/rheumatology/ken359. [DOI] [PubMed] [Google Scholar]
- 39.Thombs BD, Bassel M, McGuire L, Smith MT, Hudson M, Haythornthwaite JA. A systematic comparison of fatigue levels in systemic sclerosis with general population, cancer and rheumatic disease samples. Rheumatology. 2008;47:1559–63. doi: 10.1093/rheumatology/ken331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thombs BD, Hudson M, Bassel M, Taillefer SS, Baron M Canadian Scleroderma Research Group. Sociodemographic, disease, and symptom correlates of fatigue in systemic sclerosis: evidence from a sample of 659 Canadian Scleroderma Research Group Registry patients. Arthritis Rheum. 2009;61:966–73. doi: 10.1002/art.24614. [DOI] [PubMed] [Google Scholar]
- 41.Kuwana M, Kaburaki J, Mimori T, Kawakami Y, Tojo T. Longitudinal analysis of autoantibody response to topoisomerase I in systemic sclerosis. Arthritis Rheum. 2000;43:1074–84. doi: 10.1002/1529-0131(200005)43:5<1074::AID-ANR18>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Assassi S, del Junco D, Sutter K, McNearney TA, Reveille JD, Karnavas A, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Care Res. 2009 doi: 10.1002/art.24734. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alarcon GS, Rodriguez JL, Benavides G, Jr, Brooks K, Kurusz H, Reveille JD. Systemic lupus erythematosus in three ethnic groups. V. Acculturation, health-related attitudes and behaviors and disease activity in Hispanic patients from the LUMINA cohort: LUMINA Study Group. Lupus in minority populations, Nature vs Nurture. Arthritis Care Res. 1999;12:267–76. doi: 10.1002/1529-0131(199908)12:4<267::aid-art5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Alarcon GS, McGwin G, Jr, Uribe A, Friedman AW, Roseman JM, Fessier BJ, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum. 2004;51:465–74. doi: 10.1002/art.20409. [DOI] [PubMed] [Google Scholar]
- 45.Leslie JC, Diehl SJ, Galvin SL. A comparison of birth outcomes among US-born and non-US-born Hispanic women in North Carolina. Matern Child Health J. 2006;10:33–8. doi: 10.1007/s10995-005-0028-0. [DOI] [PubMed] [Google Scholar]
- 46.Trinidad DR, Gilpin EA, Messer K, White MM, Pierce JP. Trends in smoking among Hispanic women in California: relationship to English language use. Am J Prev Med. 2006;10:257–60. doi: 10.1016/j.amepre.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Arnett FC, Gourh P, Shete S, Ahn CW, Honey RE, Agarwal SK, et al. Major histocompatibility complex (MHC) class II alleles, haplotypes, and epitopes which confer susceptibility or protection in the fibrosing autoimmune disease systemic sclerosis: analyses in 1300 Caucasian, African American and Hispanic cases and 1000 controls. Ann Rheum Dis. 2009 July 12; doi: 10.1136/ard.2009.111906. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–24. doi: 10.7326/0003-4819-150-5-200903030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atlas SJ, Grant RW, Ferris TG, Chang Y, Barry MJ. Patient-physician connectedness and quality of primary care. Ann Intern Med. 2009;150:325–35. doi: 10.7326/0003-4819-150-5-200903030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karlson EW, Liang MH, Eaton H, Huang J, Fitzgerald L, Rogers MP, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:1832–41. doi: 10.1002/art.20279. [DOI] [PubMed] [Google Scholar]
- 51.Edworthy SM, Dobkin PL, Clarke AE, Da Costa D, Dritsa M, Fortin PR, et al. Group psychotherapy reduces illness intrusiveness in systemic lupus erythematosus. J Rheumatol. 2003;30:1011–6. [PubMed] [Google Scholar]
- 52.Freedman MK, Saulino MF, Overton EA, Holding MY, Kornbluth ID. Interventions in chronic pain management. 5. Approaches to medication and lifestyle in chronic pain syndromes. Arch Phys Med Rehabil. 2008;89 (Suppl 1):S56–60. doi: 10.1016/j.apmr.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 53.King TK, Borrelli B, Black C, Pinto BM, Marcus BH. Minority women and tobacco: implications for smoking cessation interventions. Ann Behav Med. 1997;19:301–13. doi: 10.1007/BF02892295. [DOI] [PubMed] [Google Scholar]
- 54.Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90:420–8. doi: 10.1016/j.apmr.2008.09.558. [DOI] [PubMed] [Google Scholar]


