Abstract
Graft-versus-host disease (GVHD) is a major complication of allogeneic bone marrow or hematopoietic stem cell transplantation. GVHD is thought to be primarily due to the response of mature T cells transferred along with the bone marrow graft to foreign histocompatibility antigens expressed on host tissues. Recent studies, however, have challenged this paradigm set forth in the 1960s and have suggested that self-MHC class II antigens can be recognized in GVHD. Many questions still remain unanswered particularly in regard to the role of immune reconstitution, the ability to recognize and discriminate self and the re-establishment of self-tolerance. In fact, the failure to re-establish tolerance to self can lead to systemic autoimmunity that may exacerbate or even mimic GVHD. The present review summarizes our studies in autologous GVHD characterizing the underlying immune mechanisms and their potential impact in allogeneic hematopoietic stem cell transplantation.
Keywords: Regulatory T cells, Autologous graft-versus-host disease, Allogeneic graft-versus-host disease, Hematopoietic stem cell transplantation
Allogeneic bone marrow or hematopoietic stem cell transplantation (HSCT) is a viable therapeutic option for the treatment of many haematological malignancies [1–4]. A major complication of HSCT is acute graft-versus-host disease (GVHD), which is thought to be primarily due to the response of mature T cells transferred along with the bone marrow graft to foreign histocompatibility antigens expressed on host tissues. Despite the development of superior post-grafting immunosuppressive regimens, GVHD continues to be a significant clinical problem particularly for patients receiving hematopoietic stem cell grafts from unrelated donors [5]. Although recent studies have provided new and exciting insights into the immune mechanisms that underlie GVHD, many questions remain unanswered particularly in regard to the role of immune reconstitution, the ability to recognize and discriminate self (donor anti-donor) and the re-establishment of self-tolerance. In fact, the failure to re-establish tolerance to self can lead to systemic autoimmunity that may exacerbate or even mimic GVHD.
Considerable progress has been made during the past several years elucidating the pathophysiology of acute GVHD that occurs after allogeneic bone marrow (BM) or hematopoietic stem cell transplantation (HSCT). Although many factors contribute to the pathophysiology of GVHD, the preparative regimens utilized for HSCT was found to set the initial stage for the initiation of GVHD [6–9]. These preparative regimens (i.e., radiation, cytotoxic drugs) are routinely selected for their anti-tumor activity as well as their ability to eliminate all lymphohaematopoietic elements. This provides space allowing for the engraftment of the new lymphohaematopoietic system. However, the preparative regimens by damaging normal host tissues also have an unintended consequence leading to both the direct and indirect release of immunologically active cytokines. For example, damage to gastrointestinal epithelium allows for the release of endotoxin into the peripheral circulation triggering the production of a complex milieu of immune stimulatory cytokines, in essence, a “cytokine storm” [8, 9]. In this environment, the activated donor anti-host T cells expand in the milieu of cytokines ultimately targeting and damaging the skin, liver and gastrointestinal tract. In addition to the direct lymphocyte-mediated destruction of cells within the target organs, these biologically active cytokines can also indirectly potentiate the clinical complexity of GVHD [8, 9].
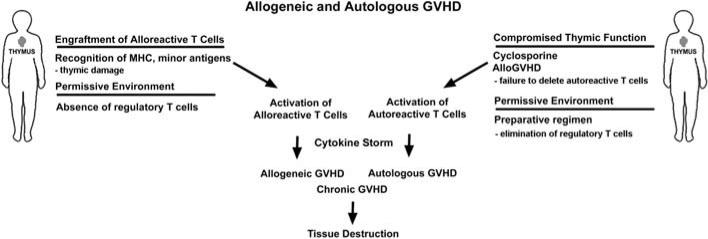
Acute GVHD was classically thought to be due to histocompatibility differences between donor and host initiating a donor anti-host response as postulated by Billingham in the late 1960s [10]. Recent studies in man and in a number of animal models, however, have revealed that an autoimmune syndrome can occur after marrow transplants performed between identical twins (syngeneic) or even after autologous BMT [11–15]. The pathology of this autoimmune syndrome, termed autologous or syngeneic GVHD was primarily limited to the skin and was virtually identical to the pathology of GVHD that occurs after allogeneic BMT. In a number of instances, the liver and gastrointestinal tract were also targeted but with identical pathology as observed in allogeneic GVHD [11–15]. The occurrence of GVHD after autologous or syngeneic HSCT was initially met with great skepticism since it directly challenged the dogma that histo‘in’compatibility between donor and host is an absolute requirement for the induction of graft-versus-host reaction (GVHR) [10]. Recent studies, however, have provided some insight into the complex immunobiology of autologous GVHD. Moreover, this unique autoaggression syndrome may even play a major role in the exacerbation of GVHD after allogeneic BMT as schematically illustrated in Fig. 1. The initial studies indicated that there are two major factors necessary for the induction of an autologous GVHD, the disruption of thymic-dependent immune reconstitution and the failure to re-establish peripheral self-tolerance underlie the development of autologous GVHD. Hence, there is dysregulation of the central and peripheral mechanisms that control reactivity to self [15].
Fig. 1.
Potential interactions between allogeneic and autologous GVHD. Alloreactive T cells recognize and respond to histocompatibility antigen differences in the host and can damage the thymic epithelium. In addition, administration of CsA alters T cell differentiation in the thymus. Both processes inhibit thymic-dependent clonal deletion inducing the release of autoreactive T cells that can potentiate allogeneic GVHD and induce an autoaggression that is virtually identical to allogeneic GVHD
Acquisition of tolerance to self-MHC antigens is not limited to the developing immune system in the neonate but must also be re-established after autologous, syngeneic and allogeneic HCST. One of the principle mechanisms thought to underlie autologous GVHD is the failure of thymic-dependent clonal deletion mechanisms [15–25]. Clonal deletion of autoreactive T cells is thought to be the central mechanism in preventing autoaggression. Damage to the thymic epithelium by the HSCT or BMT preparative regimen may compromise the ability of the thymus to negatively select or delete the autoreactive T cells [15]. Similarly, thymic epithelial cells can be targeted and destroyed by alloreactive T cells compromising thymic function [16]. The administration of certain immunosuppressive drugs after HSCT can also alter normal T cell differentiation. In fact, the role of the thymus in autologous GVHD was clearly elucidated in studies with cyclosporine (CsA). This immunosuppressive drug directly inhibits thymic-dependent clonal deletion [21]. Administration of CsA after autologous or syngeneic (and allogeneic) HSCT facilitates the induction of autologous or autoimmune GVHD [15, 21–25]. Animal studies revealed that the presence of a thymus was a critical requirement for the induction of autologous GVHD since this autoimmune disorder could not be induced if the HSCT recipients were thymectomized prior to transplant [25]. Many factors can affect thymic function resulting in the failure to delete autoreactive T cells. Subsequently, the autoreactive T lymphocytes are exported to the periphery where they can manifest autoaggression.
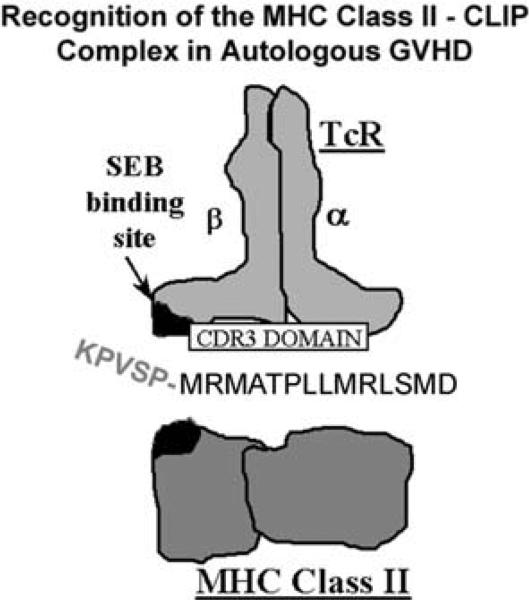
Studies in man and in animal models revealed that autologous GVHD is primarily mediated by a highly restricted repertoire of autoreactive T cells that recognize MHC class II antigens [26–31]. Interestingly, both allo- and self-MHC class II antigens were recognized by the autologous GVHD effector T cells. Additional studies revealed that the promiscuous recognition of MHC class II antigens was dependent on the presentation of a peptide from the invariant chain, termed CLIP (class II invariant chain peptide) [26–29]. The invariant chain shepherds the biosynthesis of MHC class II. As the MHC class II molecule is transported to the cell surface, the invariant chain is proteolyzed leaving CLIP in the peptide-binding domain. The broad or promiscuous specificity of the effector T cells appeared to be governed by a functional and agonistic interaction between the N-terminal flanking domain of CLIP and the Vβ chain of the T cell receptor at or near the binding site for the super antigen, Staphylococcal enterotoxin B (SEB) (Fig. 2). Recent studies suggest that an autoimmune GVHD may also emerge during acute GVHD after allogeneic HSCT [17–20]. The thymic epithelium can be damaged by donor anti-host reactive T cells impairing negative selection in the thymus subsequently leading to the export of autoreactive T lymphocytes into the periphery [16]. The autoreactive T cells can recognize both self (donor) and host MHC class II antigens [20]. The emergence of these autoreactive T exacerbates acute allogeneic GVHD and is thought to underlie the pathophysiology of chronic allogeneic GVHD with its clinical autoimmune manifestations including scleroderma and fibrosis [17–20].
Fig. 2.
Recognition of the MHC class II–CLIP complex in autologous GVHD. Autologous GVHD effector T cells recognize MHC class II antigens presenting a peptide from the invariant chain, termed CLIP. In addition to recognition of the MHC class II binding domain of CLIP, the N-terminal flanking domain appears to interact with the Vβ chain of the T cell receptor at or near the binding site for the SEB super antigen
The inhibition of clonal deletion with the export of autoreactive T cells to the periphery, by itself, is insufficient for the induction of an autologous GVHD [22, 23]. A permissive environment must be provided for the autoreactive T cells to manifest autoaggression. In normal animals (and in man), there is a peripheral regulatory mechanism that controls the development of autologous GVHD. This T cell-dependent regulatory system is a peripheral safeguard to control the activity of T cells with autoreactive potential that were not centrally deleted in the thymus [22, 23, 32, 33]. The elimination of this T cell-dependent regulatory system by the lymphoablative preparative regimen, in fact, provides a permissive environment for the autoreactive effector T lymphocytes to manifest tissue destruction. Moreover, the resolution of autologous GVHD is also dependent on the reconstitution and establishment of a peripheral regulatory system. Once the peripheral regulatory T cell system is activated, the pathogenic effector lymphocytes can be effectively down regulated. It is of interest that a similar process occurs in allogeneic GVHD [34–37]. The regulatory T cell compartment must be reconstituted and activated in order to suppress the pathogenic effects of the alloreactive effector T cells. This leads to the establishment of donor anti-host tolerance. Moreover, self-tolerance (donor anti-donor) must also be re-established in the periphery.
Several studies in animal models clearly indicate that peripheral regulatory mechanisms play a critical role in preventing the development of autologous GVHD [15, 23, 33]. Mature T cells from naïve animals were able to suppress both the induction and the adoptive transfer of autologous GVHD. In this setting, effective suppression of autologous GVHD required both CD4+ and CD8+ T cells. Moreover, resolution of autologous GVHD and the re-establishment of self-tolerance were critically dependent on the reconstitution of the peripheral immunoregulatory system [33, 38, 39]. Interestingly, the recovery of the regulatory T cell system can only occur following the discontinuation of CsA treatment [33]. These results suggested that CsA with its ability to inhibit thymic-dependent T cell development also prevents the de novo reconstitution of the regulatory T cell compartment. In addition, the regulatory system is adaptive [33, 38, 39]. The presence of autoreactive lymphocytes or the autoantigen presented on antigen-presenting cells (APCs) induces dynamic changes in the functional behavior of the peripheral regulatory system including the activation (or expansion) of antigen-specific CD4+ CD25+ regulatory T cells. Primed by the presence of autoreactive T cells or the antigen, the regulatory T cells can suppress the adoptive transfer of autologous GVHD independent of CD8+ T cells [33, 38]. Of interest, recent studies indicate that both the antigen-specific regulatory and pathogenic effector T cells in autologous GVHD recognize the MHC class II–CLIP complex [28, 38]. In this setting, an activation signal conveyed either by the autoreactive T cells (perhaps mediated by the cell-surface expression of the MHC class II–CLIP complex on activated T cells) or by specific antigen presented on APCs appears to be required for the development of functional regulatory activity. It is important to note that activated T cells in the rat model directly recognize this antigenic complex on the activated effector lymphocytes [28, 33]. On the other hand, the apparent initial requirement for CD8+ T regulatory cells from naive animals and their functional role in suppressing the adoptive transfer of autologous GVHD remain unclear. Perhaps, CD4+ CD25+ regulatory T cells once activated can interact with and direct CD8+ regulatory T cells newly formed that arise through normal T cell differentiation in the thymus within the host. Nevertheless, the CD4+ CD25+ regulatory T cell subset plays a crucial role in preventing autoaggression and promoting the development of immune tolerance to self-MHC class II antigens after autologous BMT.
A very similar process appears to occur in GVHD after allogeneic HSCT. Observations in man and in animals clearly indicate that a number of individuals who develop acute GVHD following allogeneic BMT eventually recover. Studies in animal models suggested that the down regulation of acute allogeneic GVHD and the establishment of donor to host tolerance were mediated by antigen-specific regulatory T cells [34–37, 40–46]. Initial characterization of this peripheral regulatory system suggested that both CD8+ and CD4+ T cells were required for the establishment of donor to host tolerance [17, 18]. Further studies suggested that the CD8+ regulatory T cells do not express the CD28 co-stimulatory cell-surface accessory molecule [43]. The immune tolerance that developed in the allogeneic BMT recipient was antigen-specific and could be adoptively transferred into naïve hosts [34–37]. Of interest, donor to host tolerance mediated by regulatory T cells failed to develop in thymectomized animals [34–37]. These results suggested that the thymus was critically important for the de novo development of regulatory T cells after allogeneic BMT. Moreover, maintenance of donor to host tolerance by donor anti-host-specific regulatory T cells was dependent on the continued presence of host MHC antigens, particularly MHC class II antigens [36]. In the absence of specific antigen, regulatory T cell activity waned rapidly.
Although distinct subsets of T cells play an important role in peripheral regulation of the immune response, recent studies suggest that CD4+ T cells that innately express CD25 (interleukin 2 receptor α-chain) orchestrate the regulation of GVHD and the development of donor to host tolerance [34, 44, 45]. This population of regulatory lymphocytes may work directly or in concert with CD8+ CD28– T cells that have regulatory function to effectively down regulate the immune response. The CD4+ CD25+ regulatory population arises naturally in the thymus and is positively selected by dendritic cells. Moreover, MHC class II expression was critical for the development of CD4+ CD25+ T cells with regulatory potential [45, 46]. Of interest, recent studies indicate that Hassal's corpuscles in the thymus instruct dendritic cells to positively select the regulatory T cell population [47]. Development of functional activity within the CD4+ CD25+ regulatory compartment and the ability to control responses to alloantigens appears to require an antigenic signal [42, 48–50]. Interestingly, a subset of CD4+ CD25– T cells, once activated, may also acquire regulatory function. Following activation, the regulatory T cells capable of modulating GVHD are induced to express high levels of L-selectin [51]. Moreover, recent studies suggest that subsets of antigen-presenting cells (APCs) provide specific signals allowing for the activation and maturation of regulatory T cells [52].
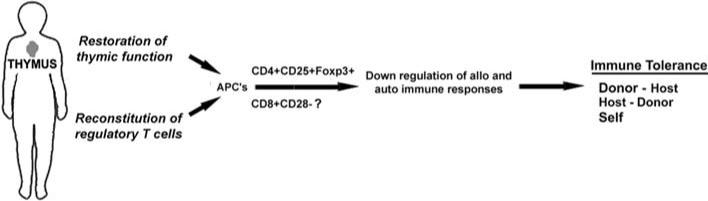
The regulatory control of a graft-versus-host response mediated either by alloreactive or autoreactive T cells is complex. Although multiple populations of cells appear to participate in this process, recent pre-clinical studies suggest that naturally occurring CD4+ CD25+ regulatory T cells are vital for the regulation of the immune response to both allo- and self-MHC antigens as illustrated in Fig. 3 [44, 48–50]. Moreover, this population of regulatory T cells must be reconstituted following allogeneic or autologous BMT. Interestingly, the regulatory compartments controlling both alloreactivity and autoreactivity are, in fact, remarkably parallel, perhaps differing only in the antigen specificity of the regulatory cells. The underlying molecular mechanisms accounting for regulation of the immune response, however, remain controversial. A number of mechanisms have been proposed including the release of regulatory cytokines (i.e., IL-10, TGFβ) that can dampen the immune response, the induction of unresponsiveness to antigen (T cell anergy) or induce the apoptotic death of the pathogenic autoreactive effector T cells [44, 48–50]. The general consensus is that cell-to-cell contact is required for effective regulation of the immune response. One of the limiting factors that hinders the in depth characterization of the CD4+ CD25+ regulatory T cell compartment is the lack of suitable markers unique to regulatory lymphocytes. Since activated CD4+ and CD8+ effector T cells can also express CD25, this marker is not unique to regulatory T cells. Recent studies, however, suggest that the forkhead-winged helix family, nuclear transcription factor Foxp3 is specifically expressed by naturally occurring CD4+ CD25+ regulatory T cells [53, 54]. Foxp3 appears to be a “master gene” governing the development and suppressive function of CD4+ CD25+ regulatory T cells [54]. Animals deficient in Foxp3 develop widespread autoimmune disorders due to the lack of regulatory T cells [53–55]. A number of studies in animal systems clearly confirm that CD4+ CD25+ Foxp3+ T cells participate in the regulatory control of both allogeneic and autologous GVHD [56]. In addition, CD8+ CD28– regulatory T cells may also express Foxp3 [57].
Fig. 3.
Development of donor to host and self-tolerance. Reconstitution of the regulatory compartment allows for the down regulation of alloreactive and autoreactive T cells. CD4+ CD25+ Foxp3+ T cells activated by APCs may orchestrate the establishment of immune tolerance. CD8+ CD28– regulatory T cells may also interact to facilitate this state of unresponsiveness
Evidence for the regulatory control of GVHD and the development of tolerance (donor to host, self) in clinical bone marrow transplantation is quite limited. Functional assays to detect regulatory activity in clinical BMT recipients were often hindered because patients and donors were matched for both MHC class I and II antigens. Assays that can detect the regulation of donor anti-host reactivity by regulatory T cells were absent. In addition, cells that suppressed in an antigen non-specific manner were often detected in the BMT recipient. The role of these non-specific regulatory cells remains ill defined. Apart from these rudimentary functional assays, the lack of specific markers for regulatory T cells also precluded the quantitative analysis of cell-mediated immune regulatory mechanisms following clinical BMT. The findings that naturally occurring CD4+ regulatory T cells innately express the CD25 cell-surface molecule and the Foxp3 nuclear transcription factor has provided a unique opportunity to quantify and correlate regulatory T cell function in human bone marrow transplant recipients. Several studies indicate that naturally occurring CD4+ CD25+ T cells that can suppress in vitro immune responses can be detected in human peripheral blood [47–50]. Moreover, the Foxp3 nuclear transcription factor appears to be preferentially expressed by the CD4+ CD25+ regulatory T cell compartment. Interestingly, CD4+ CD25+ regulatory T cells also innately express high levels of CTLA-4 (cytotoxic lymphocyte-associated antigen 4), the regulatory cell-surface accessory molecule [47–50, 56]. Comparatively, expression of other markers including PD-1 (programmed death receptor 1), GITR (glucocorticoid-induced tumor necrosis factor receptor) could not effectively distinguish between CD4+ CD25+ T cells from CD4+ CD25–, activated CD4+ CD25+ effector and CD8+ effector T lymphocytes [56]. Thus, the results from these studies clearly suggest that Foxp3 might be a useful marker to assess and quantify CD4+ CD25+ regulatory T cells in clinical bone marrow transplant recipients.
Initial studies by Miura et al. in a series of patients undergoing allogeneic BMT or autologous BMT with the experimental induction of autologous GVHD provide substantial evidence that CD4+ CD25+ Foxp3+ T cells play a central role in regulating both alloimmune and autoimmune reactivity [56]. Foxp3 gene expression in this study was quantified by evaluating mRNA transcript levels for this nuclear transcription factor as assessed by quantitative polymerase chain reaction (qPCR). Analysis of peripheral blood lymphocytes revealed that Foxp3 mRNA transcript levels were significantly reduced in patients with either allogeneic or autologous GVHD compared to patients who did not develop any clinical evidence of GVHD (either allogeneic or autologous). Moreover, there was an inverse correlation of Foxp3 expression with the grade of acute allogeneic GVHD. Levels of Foxp3 mRNA transcripts in peripheral blood lymphocytes were almost undetectable in patients with grade III or grade IV acute GVHD. Foxp3 mRNA transcript levels were also significantly reduced (~twofold) in patients with grade I or grade II acute allogeneic GVHD compared to patients who did not develop any evidence of this post-transplant complication following allogeneic BMT. Analysis of a limited number of patients with chronic allogeneic GVHD indicated that Foxp3 levels were also significantly reduced, particularly for patients who had extensive disease. Interestingly, sequential analysis of the lymphocytes from patients with acute GVHD that evolved into a chronic GVHD revealed that levels of Foxp3 mRNA transcripts were consistently reduced while there was active disease. Following resolution of GVHD, levels of Foxp3 mRNA transcripts returned to normal. The return to normal levels of Foxp3 gene expression also correlated with de novo T cell development as revealed by the assessment of T cell recombination excision circles (TRECs). These results suggest that the regulatory T cells that mediate the down regulation of GVHD are recent thymic emigrants. The emergence of this regulatory population, in fact, coincides with the resolution of GVHD. In addition, studies by Miura et al. reveal that regulatory T cell function can also be heightened by antigen-specific stimulation [56]. These initial studies were confirmed flow cytometrically indicating that the recovery of CD4+ CD25+ Foxp3+ T cells correlated with the resolution of acute allogeneic GVHD. In addition, this T cell compartment did suppress pre-transplant donor anti-host responses in patients who received haploidentical bone marrow grafts [58]. Recent studies by Zorn et al. confirm and extend the findings in patients with chronic allogeneic GVHD. Foxp3 gene expression was significantly reduced in patients who develop chronic GVHD after allogeneic BMT [58]. Phenotypic studies also revealed that there was a reduced frequency of CD4+ CD25+ T cells in patients with chronic GVHD. Of interest, the regulatory T cells derived from patients with chronic GVHD were capable of suppressing in vitro immune reactivity.
Strategies to isolate and expand this population may provide a novel therapeutic approach to control or prevent the development of allogeneic GVHD. A number of clinical trials are ongoing adoptively transferring CD4+ CD25+ Foxp3+ T cells expanded ex vivo to prevent acute allogeneic GVHD. However, transfer of ex vivo expanded T cells may disrupt normal immune homeostasis. In this regard, strategies to adoptively transfer ex vivo expanded T cells ex vivo by anti-CD3 and anti-CD28 stimulation to augment anti-tumor immunity after autologous HSCT resulted in the development of autologous GVHD [59]. These results suggest that quiescent autoreactive T cells are in the peripheral circulation and that a broad stimulus such as anti-CD3 and anti-CD28 activates this population and in a permissive environment can lead to tissue destruction. Nevertheless, strategies to expand this population by ex vivo culture or in vivo by specific stimulation (i.e., antigen/APCs, infusion of apoptotic cells) will help to devise novel therapeutic strategies to control untoward immune responses.
Although many questions remain, studies in a variety of animal models have clearly defined the importance of regulatory T cells are critically important in controlling alloreactivity and autoreactivity. Evidence for regulatory T cells in clinical settings still remains rudimentary. The recent identification of CD4+ T cells that innately express CD25 and the Foxp3 nuclear transcription factor has provided unique opportunities to explore immunoregulation in man, particularly after allogeneic and autologous HSCT. Of great importance will be the question of specificity of these regulatory T cells. Is the development of donor to host and self (donor anti-donor)-tolerance mediated by separate populations of regulatory cells or by one population recognizing a common determinant? Perhaps, the regulatory T cells promiscuously recognize MHC class II determinants in a similar fashion as the effector and regulatory T cells involved in autologous GVHD!
Acknowledgments
This work was supported by grants (CA 15396, CA 82583 and AI 24319) from the National Institutes of Health.
References
- 1.Kersey JH, Weisdorf D, Nesbit ME, LeBien TW, Woods WG, McGlave PB, et al. Comparison of autologous and allogeneic bone marrow transplantation for treatment of high-risk refractory acute lymphoblastic leukemia. N Engl J Med. 1987;317:461–7. doi: 10.1056/NEJM198708203170801. [DOI] [PubMed] [Google Scholar]
- 2.Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Anti-leukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–73. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 3.Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991;77:649–53. [PubMed] [Google Scholar]
- 4.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb H-J, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. [PubMed] [Google Scholar]
- 5.Petersdorf EW. Hematopoietic cell transplantation from unrelated donors. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ hematopoietic cell transplantation. Blackwell; Malden: 2004. pp. 1132–49. [Google Scholar]
- 6.Shlomchik WD, Coizens MS, Tang CB, et al. Prevention of graft-versus-host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 7.Matte CC, Liu J, Anderson BE, et al. Donor APC's are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–92. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 8.Reddy PP. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–61. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara LM, Antin J. The pathophysiology of graft-versus-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ hematopoietic cell transplantation. Blackwell; Malden: 2004. pp. 353–68. [Google Scholar]
- 10.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966-67;62:21–35. [PubMed] [Google Scholar]
- 11.Gluckman J, Devergie A, Sohier J, Sauret JH. Graft-versus-host reactions in recipients of syngeneic bone marrow. Lancet. 1980;1:253–6. [PubMed] [Google Scholar]
- 12.Rappeport J, Reinherz E, Mihm M, et al. Acute graft-versus-host reactions in recipients of bone marrow transplantation from identical twin donors. Lancet. 1979;2:717–20. doi: 10.1016/s0140-6736(79)90644-5. [DOI] [PubMed] [Google Scholar]
- 13.Hood AF, Vogelsang GB, Black LP, et al. Acute graft-versus-host disease: development following autologous and syngeneic bone marrow transplantation. Arch Derm. 1987;123:745–51. doi: 10.1001/archderm.123.6.745. [DOI] [PubMed] [Google Scholar]
- 14.Thien SW, Goldman JM, Galton DG. Acute “graft-versus-host disease” after autografting for chronic granulocytic leukemia in transplantation. Ann Intern Med. 1981;94:210–6. doi: 10.7326/0003-4819-94-2-210. [DOI] [PubMed] [Google Scholar]
- 15.Hess AD, Thoburn CJ. Immunobiology and immunotherapeutic implications of syngeneic/autologous graft-vs-host disease. Immunol Rev. 1997;157:111–23. doi: 10.1111/j.1600-065x.1997.tb00977.x. [DOI] [PubMed] [Google Scholar]
- 16.Hollander GA, Widmer B, Burakoff SJ. Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft-versus-host disease. J Immunol. 1994;152:1609–17. [PubMed] [Google Scholar]
- 17.Tivol E, Komorowkski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105:4885–91. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teshima T, Reddy P, Liu C, Williams D, Cooke KR, Ferrara JL. Impaired negative selection causes autoimmune graft-versus-host disease. Blood. 2003;102:429–35. doi: 10.1182/blood-2003-01-0266. [DOI] [PubMed] [Google Scholar]
- 19.Parkman R. Is chronic graft-versus-host disease an autoimmune disease? Curr Opin Immunol. 1993;5:800–3. doi: 10.1016/0952-7915(93)90140-n. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Sugihara K, Ohtsuki F, et al. Generation of self HLA-DR specific CD3+ CD4-CD8+ cytotoxic T cells in chronic graft-versus-host disease. Bone Marrow Transpl. 1994;14:525–33. [PubMed] [Google Scholar]
- 21.Jenkins MK, Schwartz RH, Pardoll DM. Effects of CsA on T cell development and clonal deletion. Science. 1988;241:1655–9. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- 22.Glazier A, Tutschka PJ, Farmer ER, Santos GW. GVHD in CsA treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med. 1983;158:1–12. doi: 10.1084/jem.158.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer AC, Beschorner WE, Hess AD. Requirements for the induction and adoptive transfer of syngeneic GVHD. J Exp Med. 1989;169:1031–8. doi: 10.1084/jem.169.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RJ, Hess AD, Mann RB, et al. Induction of graft-versus-host disease after autologous bone marrow transplantation. Lancet. 1989;1:754–7. doi: 10.1016/s0140-6736(89)92575-0. [DOI] [PubMed] [Google Scholar]
- 25.Sorokin R, Kimura H, Schroeder K, Wilson DB. Cyclosporine-induced autoimmunity: conditions for expressing disease, requirement for an intact thymus, and potency estimates of autoimmune lymphocytes in drug-treated rats. J Exp Med. 1986;164:1615–26. doi: 10.1084/jem.164.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hess AD, Thoburn CJ, Horwitz L. Promiscuous recognition of major histocompatibility complex class II determinants in Cyclosporine-induced syngeneic graft-vs-host disease. Transplantation. 1998;65:785–92. doi: 10.1097/00007890-199803270-00004. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Thoburn C, Hess AD. Characterization of the pathogenic autoreactive T cells in Cyclosporine-induced syngeneic graft-vs-host disease. J Immunol. 1998;161:7040–6. [PubMed] [Google Scholar]
- 28.Hess AD, Thoburn CJ, Chen W, Bright EC. Unexpected T-cell diversity in syngeneic graft-versus-host disease revealed by interaction with peptide-loaded soluble MHC class II molecules. Transplantation. 2003;75:1361–7. doi: 10.1097/01.TP.0000063691.54928.CF. [DOI] [PubMed] [Google Scholar]
- 29.Hess AD, Bright EC, Thoburn C, et al. Specificity of effector T lymphocytes in autologous graft-vs-host disease: role of the major histocompatibility complex class II invariant chain peptide. Blood. 1997;89:2203–9. [PubMed] [Google Scholar]
- 30.Thoburn CJ, Miura Y, Bright EC, Hess AD. Functional divergence of antigen-specific T-lymphocyte responses in syngeneic graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:591–603. doi: 10.1016/j.bbmt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Hess AD, Thoburn CJ, Chen W, Horwitz LR. Complexity of effector mechanisms in syngeneic graft-vs-host disease. Biol Blood Marrow Transplant. 2000;6:13–24. doi: 10.1016/s1083-8791(00)70048-6. [DOI] [PubMed] [Google Scholar]
- 32.Sykes M. Mechanisms of tolerance. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ hematopoietic cell transplantation. Blackwell; Malden: 2004. pp. 300–23. [Google Scholar]
- 33.Hess AD, Fischer AC, Horwitz L, Bright EC, Laulis MK. Characterization of peripheral autoregulatory mechanisms that prevent development of Cyclosporine-induced syngeneic graft-vs-host disease. J Immunol. 1994;153:400–11. [PubMed] [Google Scholar]
- 34.Taylor PA, Noelle RJ, Blazar BR. CD4+ CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via co-stimulatory blockade. J Exp Med. 2001;193:1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tutschka PJ, Hess AD, Beschorner WE, Santos GW. Suppressor cells in the transplantation tolerance. I. Suppressor cells in the mechanism of tolerance in radiation chimeras. Transplantation. 1981;32:203–9. doi: 10.1097/00007890-198109000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Tutschka PJ, Hess AD, Beschorner WE, Santos GW. Suppressor cells in transplantation tolerance. III. The role of antigen in the maintenance of transplantation tolerance. Transplantation. 1982;33:510–4. doi: 10.1097/00007890-198205000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Tutschka PJ, Ki P, Beschorner WE, et al. Suppressor cells in transplantation tolerance. II. Maturation of suppressor cells in the bone marrow chimera. Transplantation. 1985;32:321–9. doi: 10.1097/00007890-198110000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Hess AD, Thoburn CJ. Immune tolerance to self-MHC class II antigens after bone marrow transplantation: regulatory role of CD4+ CD25+ Foxp3+ T cells. Biol Blood Marrow Transplant. 2006;12:518–29. doi: 10.1016/j.bbmt.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Wu DY, Goldschneider I. Cyclosporin-induced autologous graft-vs-host disease: a prototypical model of autoimmunity and active (dominant) tolerance coordinately expressed induced by recent thymic emigrants. J Immunol. 1999;162:6926–33. [PubMed] [Google Scholar]
- 40.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nature Rev. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann P, Ermann J, Edinger J, et al. Donor type CD4+ CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joffre O, Gorssee N, Romagnoli P, et al. Induction of antigen-specific tolerance to bone marrow allografts with CD4+ CD25+ T lymphocytes. Blood. 2004;103:4216–21. doi: 10.1182/blood-2004-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang S, Lechler RI. Regulatory T cells in the control of transplantation tolerance and autoimmunity. Am J Transpl. 2003;3:516–24. doi: 10.1034/j.1600-6143.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 44.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Ann Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 45.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonistic self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 46.Bensinger SJ, Bandeira A, Jordan MS, et al. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+ CD25+ immunoregulatory T cells. J Exp Med. 2001;194:427–38. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe N, Hong-Wang Y, Lee HK, et al. Hassal's corpuscles instruct dendritic cells to induce CD4+ CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 48.Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+ CD25+ regulatory T cells. Trends Immunol. 2004;25:374–80. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Read S, Powrie F. CD4+ regulatory T cells. Curr Opin Immunol. 2001;13:644–9. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- 50.Shevach E. Regulatory T cells in autoimmunity. Ann Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 51.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-selectin-hi but not the L-selectin-lo CD4+ 25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–12. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 52.Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+ CD4+ regulatory cell activation and function by dendritic cells. Inter Immunol. 2004;16:1769–80. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 53.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 54.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 55.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family of transcription factor Foxp3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 56.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–93. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 57.Liu JW, Liu ZR, Witkowski P, et al. Rat CD8+ Foxp3+ T suppressor cells mediate tolerance to allogeneic heart transplants, inducing PIR-B in APC and rendering the graft invulnerable to rejection. Transpl Immunol. 2004;13:239–47. doi: 10.1016/j.trim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of Foxp3+ CD4+ CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005 doi: 10.1182/blood-2005-03-1257. Online 21 June 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapoport AP, Stadtmauer EA, Aqui N, Vogl D, Chew A, et al. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res. 2009;15:4499–507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]