Abstract
To evaluate the epidemiology and to investigate the impact of respiratory viral infections (RVI) on chronic allograft rejection after pediatric lung transplantation, a retrospective study of pediatric lung transplant recipients from 2002 to 2007 was conducted. Association between RVI and continuous and categorical risk factors was assessed using Wilcoxon rank-sum tests and Fisher’s exact tests, respectively. Association between risk factors and outcomes were assessed using Cox proportional hazards models.
Results
Fifty-five subjects were followed for a mean of 674 days (range 14–1790). Twenty-eight (51%) developed 51 RVI at a median of 144 days posttransplant (mean 246; range 1–1276); 41% of infections were diagnosed within 90 days. 25 subjects developed 39 lower respiratory infections, and eight subjects had 11 upper respiratory infections (URI). Organisms recovered included rhinovirus(n=14), adenovirus(n=10), parainfluenza(n=10), influenza(n=5) and RSV(n=4). Three subjects expired secondary to their RVI (2 adenovirus, 1 RSV). Younger age and prior CMV infection were risks for RVI (HR 2.4 95% CI 1.1–5.3 and 17.0; 3.0–96.2, respectively). RVI was not associated with the development of chronic allograft rejection (P=0.25) or death during the study period.
Conclusions
RVI occur in the majority of pediatric lung transplant recipients, but were not associated with mortality or chronic allograft rejection.
Keywords: Lung transplantation, Respiratory virus infection, Pediatrics
INTRODUCTION
End-stage pulmonary disease in children can be treated with lung transplantation; however, despite the therapeutic potential of lung transplantation, the 1-year and 5-year post-LTX survival rates remain poor at only 80% and 50%, respectively (1). Most lung transplant recipients develop an infectious episode and infection accounts for nearly 40% of all posttransplant mortality (1–4). Respiratory viral infections (RVI) have been reported in 9–66% of evaluated lung transplant recipients in the literature include a rate of 14% in a large retrospective cohort of pediatric lung transplant recipients (5–11).
Aside from the direct effects of infection, RVI has been epidemiologically associated with chronic graft rejection in lung transplant recipients. Several centers have reported that viruses such as influenza, parainfluenza, adenovirus, and respiratory syncytial virus (RSV) are associated with the development of bronchiolitis obliterans (BO) and bronchiolitis obliterans syndrome (BOS)(7–9, 12–17). In animal models, introduction of respiratory viruses increased allograft rejection(18, 19). Although the underlying mechanisms remain to be elucidated, it is hypothesized that respiratory viral infections injure the graft and activate a cascade of events to culminate in chronic graft rejection. However, in a recent review of RVI within the first year after transplantation, RVI was associated with death but not BOS in a cohort of nearly 600 pediatric lung transplant recipients (11). To further assess the association between RVI and chronic allograft failure, a longitudinal single-center evaluation of pediatric lung transplant recipients evaluated the hypothesis that an RVI was associated with BOS or mortality in primary pediatric lung transplant recipients beyond the first year following transplantation.
MATERIALS & METHODS
A longitudinal retrospective review of respiratory viral infection (RVI) in pediatric lung transplant recipients was performed. After Institutional Review Board approval, a comprehensive evaluation of clinical, microbiologic and pathology records was completed. Subjects transplanted at Texas Children’s Hospital from the inception of the program in 2002 until 2007 were eligible for enrollment. Data was collected from the date of transplant until September 2007 unless the subject expired or transferred care.
Immunosuppressive regimen
All subjects received induction therapy with basiliximab, an IL-2 receptor antagonist on day 0 and 4 posttransplant. In addition, triple-drug immunosuppressive therapy including a calcineurin inhibitor, mycophenolate mofetil and prednisone was initiated at transplantation. Subjects received cytomegalovirus prophylaxis for 5.5 months with a combination of intravenous ganciclovir transitioned to valganciclovir when tolerating oral medications.
Definitions
Respiratory Viral Infection (RVI)
Diagnosis was based upon positive viral identification in bronchoalveolar lavage, bronchial wash, tracheal aspirate, sputum, or nasopharyngeal swab specimens in addition to clinical evidence of infection. Clinical evidence of viral infection was indicated by rhinorrhea, cough, and fever and supported by radiographic and/or transbronchial biopsy findings. Distinction between upper respiratory tract infection (URI) and lower respiratory tract infection (LRI) was based on the presence of clinicopathological changes on physical examination, chest imaging and site of viral recovery. Subjects without respiratory distress, hypoxia, new wheezing or crackles on exam, or new abnormal chest imaging were considered to have URI. Viral cultures, rapid detection with enzyme immunoassay (EIA) and polymerase chain reaction (PCR) were the principal diagnostic methods used. The application and specific methodology varied over the duration of the study. Episodes with clinical evidence of viral infection but without identification of specific viral pathogens were not included. Posttransplant fungal infection (PFI) definitions are adapted from those proposed by the European Organization for Research and Treatment of Cancer/Mycoses Study Group (20) and previously reported studies of fungal infection after transplantation (21, 22).
Posttransplant outcome measures including acute rejection (AR) and bronchiolitis obliterans/bronchiolitis obliterans syndrome (BO/BOS) were based on definitions proposed by working groups from the International Society for Heart and Lung Transplantation (ISHLT) (23–25).
Statistical methods
Comparisons of patients with and without RVI on demographic data and outcomes were performed using Wilcoxon rank-sum tests for continuous variables and Chi-square tests or Fisher’s exact tests for categorical variables as appropriate. Associations between risk factors and time to RVI and other posttransplant outcomes were assessed using univariable and multivariable Cox proportional hazards models. Events that occurred during post-transplant follow-up, such as CMV infection and rejection, were modeled as time-dependent covariates. The functional forms for age and era of transplant were chosen by modeling the quartiles of these variables as categorical variables and assessing the resulting parameter estimates, and final models were chosen using backwards selection with a significance criterion of 0.05. The proportional hazards assumption for the multivariable models was assessed by entering risk-factor-by-time interactions into the model; this assumption was also assessed graphically using log-log-survival plots. All tests were two-tailed and performed at a significance level of 0.05. SAS version 9.2 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
Demographics
Of the fifty-five subjects evaluated, the majority (95%) received bilateral cadaveric lung transplantation and was Caucasian (75%). Transplantation was performed secondary to cystic fibrosis (62%), pulmonary hypertension (9%), bronchiolitis obliterans (5%), interstitial pneumonitis (5%) and other etiologies (19%).
Transplant related outcomes
Subjects were followed for an average of 22.2 month (median 21.8 months) after transplantation, with 45% being followed for more than 2 year after transplant. Of the 55 subjects, histopathologic diagnosis of bronchiolitis obliterans (BO) occurred in six (11%) while clinical diagnosis of BOS (of any grade including 0-p) developed in twelve (22%). BO and BOS both occurred at a mean of 20.6 months after transplant (median 16.5 and 21.8 months, respectively). Acute rejection occurred commonly with 65% of subjects having at least one episode of AR at a mean of 3.5 and median of 1.4 months after transplant. A2 or greater AR occurred in 27 subjects (49.1%) at a mean of 5.3 months and a median of 2.8 months posttransplant.
RVI episodes
Twenty-eight subjects (51%) developed 46 RVI during the study period (range 1–6 episodes). RVI occurred at a median of 144 days posttransplant (range 1–1276 days; mean 246 days). Organisms included rhinovirus (14), adenovirus (10), parainfluenza (10), influenza (3) and RSV (4). Of the RVI, thirty-eight (82.6%) met the criteria for lower respiratory tract infection (LRI) while eight (17.4%) were recovered from the upper respiratory tract (URI). All episodes of adenovirus infection were LRI as were the majority of rhinovirus infections (11/14) and parainfluenza infections (9/10), Table 2. Viral detection was predominantly from culture (38) in addition to PCR (2), and enzyme immunoassay (3). Infections occurred during the seasons when the viruses are known to circulate, Figure 1. Rhinovirus showed no seasonal predilection compared to influenza and RSV which occurred during the winter months. Of the 15 subjects who expired during the study period, three died from RVI (adenovirus = 2, RSV = 1). Of the 28 with RVI, eleven subsequently developed BOS/BO while seven of 18 without RVI developed BOS/BO.
Table 2.
Viral Recovery
| Virus | LRI | URI | |
|---|---|---|---|
| Adenovirus | 10 | ||
| Rhinovirus | 11 | 3 | |
| Influenza (any) | 4 | 1 | |
| Influenza A | 3 | 1 | |
| Influenza B | 1 | ||
| Parainfluenza (any) | 9 | 1 | |
| Parainfluenza 1 | 3 | ||
| Parainfluenza 2 | 1 | ||
| Parainfluenza 3 | 4 | 1 | |
| Respiratory syncytial virus | 3 | 1 | |
| Enterovirus | 1 | ||
| Herpes simplex virus | 1 | 1 | |
| TOTAL | 38 | 8 | |
LRI – lower respiratory tract infection; URI – upper respiratory tract infection
Figure 1. Viral Recovery by Calendar Month.
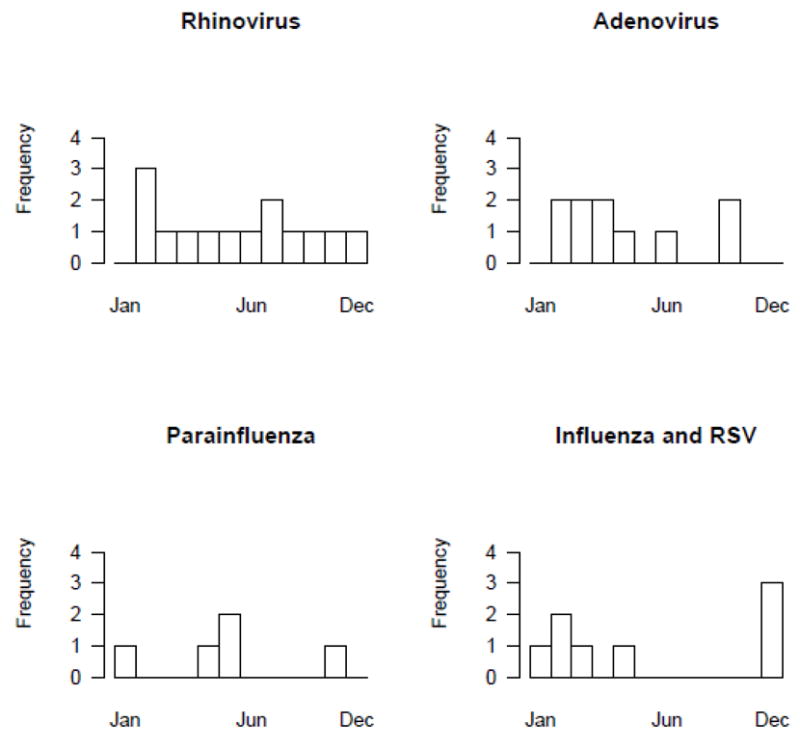
The figure demonstrates the recovery of the five most common viruses divided by month of recovery. Rhinovirus recovery was steady throughout the year. Adenovirus occurred primarily in the spring while influenza and RSV predominated in the winter months.
Risk for RVI
Univariable analyses evaluated potential risks for RVI, only history of a treated rejection episode or an episode of CMV infection prior to RVI were statistically significant. We explored age in greater detail as those with RVI were younger, although not statistically significantly as a continuous variable, Table 3.
Table 3.
Univariable risks for RVI
| Univariable hazards for RVI Risk factor | N | Hazard ratio (95% CI) | P-value | |
|---|---|---|---|---|
| D/R CMV status verses D-/R- | 43 | 0.97 | ||
| 1.Donor +/Recipient + | 1.1 (0.36, 3.5) | |||
| 2.Donor +/Recipient − | 1.3 (0.44, 3.9) | |||
| 3.Donor −/Recipient + | 1.3 (0.26, 6.4) | |||
| CMV prior to RVI | 55 | 15.0 (2.9, 77.4) | 0.001 | |
| Age at transplant verses 16.5–20.9 | 55 | 0.27 | ||
| 0.2–6.6 yrs | 2.9 (0.92, 8.9) | |||
| 6.6–13.6 yrs | 1.3 (0.41, 4.1) | |||
| 13.6–16.5 yrs | 1.5 (0.50, 4.7) | |||
| Year of transplant verses 2006 | 55 | 0.62 | ||
| 2002–2004 | 1.8 (0.58, 5.7) | |||
| 2004–2005 | 1.09 (0.35, 3.4) | |||
| 2005–2006 | 1.7 (0.61, 4.6) | |||
| Any Rejection prior to RVI | 55 | 1.5 (0.63, 3.4) | 0.38 | |
| Treated Rejection prior to RVI | 55 | 2.5 (1.08, 5.8) | 0.031 | |
| Pulmonary fungal infection prior to RVI | 55 | 0.60 (0.08, 4.6) | 0.62 |
In the multivariable model, age was evaluated as a discrete variable comparing the risk for subject less than 10 years compared to those over 10 years at transplantation. Increased risk of RVI included patients younger than 10 years of age at transplant (HR 2.4; 95% CI 1.1–5.3, p = 0.028) and those with a history of CMV infection prior to RVI (HR 17.0; 95% CI 3.0–96.2, p=0.001). In the multivariable model, treated acute rejection was not an independent predictor of subsequent RVI.
Risk after RVI
RVI was assessed as a risk factor for outcomes. RVI trended toward a statistically significant two-fold increase in the risk ensuing A2 or greater acute rejection (HR 2.2, 95% CI 0.99–4.9; p=0.053). However, RVI was not a significant risk factor for subsequent pulmonary fungal infection, CMV infection, post-transplant lymphoproliferative disease or BO/BOS (p>0.20). RVI was not associated with increased risk for death/retransplantation. In addition, LRI was not a risk factor for subsequent BOS or death/retransplantation when the subjects with LRI were evaluated (p >0.2).
DISCUSSION
Infection remains a significant complication after lung transplantation and mortality has only minimally improved in the past decade for pediatric lung transplant recipients. Few studies in pediatric lung transplant recipients have evaluated the impact of RVI on outcome. In a small cohort of pediatric subjects, adenovirus infection was associated with graft failure and death, while a larger cohort followed for only one year posttransplant showed RVI was associated with death but not BOS (7, 11).
RVI occurred in 51% of the pediatric subjects in this cohort, compared to prior reports ranging from 9–66%(7–9, 26). As in prior reports, viral infections occurred in typical seasonal patterns and clinicians should maintain vigilance regarding circulating community viral infections (11). Rhinovirus, adenovirus and parainfluenza were the most common viruses recovered (8, 9). Rhinovirus was detected by conventional viral culture in this cohort; however, new molecular methods for viral detection will likely increase the recovery of this and other viruses, including human metapneumovirus, coronaviruses and bocaviruses (9, 26, 27).
Younger age at transplant was again a risk factor for RVI similar to previous reports (LDI, RVI, Txp ID). CMV infection prior to RVI was associated with a 17-fold increased risk for subsequent RVI although CMV donor/recipient serostatus was not associated with an increased risk. CMV infection may be a marker for over-immunosuppression of the transplant recipient. Alternatively, the immunomodulatory effects of CMV may allow for viral infection after exposure compared to subjects without CMV infection and similar exposure profiles.
RVI was not associated with increased risk of BOS after transplant in this cohort which is similar to the larger pediatric cohort followed for one-year after transplantation but is distinct from the adult lung transplant literature (7–9, 12–17). However, the multi-factorial nature of BOS and the number of events in the cohort, limit the extent to which BOS could be explored. Preceding RVI did trend toward increased statistically significant risk of ensuing A2 acute rejection indicating that the inflammatory cascade or reduction in immunosuppression related to an episode of RVI may not be inconsequential in the pediatric lung transplant population.
As with any retrospective study, there are limitations related to the study design, availability of data, and selection bias. This study was performed at a single center with routine induction and immunosuppressive strategies and routine bronchoscopy for surveillance including viral studies. As the only pediatric lung transplant center in the region, the follow-up and retention of patients was high. Additional limitation include the number of available subjects and the duration of follow-up which may impact the results. However, a significant proportion of subjects were followed for at least two years after transplantation (mean follow-up 22 months).
Another limitation of this study involves RVI diagnosis and diagnostic testing. Most episodes of cold symptoms were routinely evaluated and included in this report decreasing the potential to underestimate the number of episodes of RVI that existed in prior studies (11). Fifty-one percent of subjects experienced an RVI compared to only 13% in the previous pediatric lung transplant cohort. Still, specimens were not banked or evaluated with advanced molecular diagnostics routinely. Therefore, evaluation specifically for recently identified viruses like human metapneumovirus, human coronaviruses and bocaviruses was not performed which could affect the number of RVI recovered.
Unlike previous studies in pediatric patients, differentiation between upper and lower RVI was assessed in this cohort. RVI of the lower tract have been reported to carry more serious risks for subsequent mortality and rejection post-transplant (8); however, LRI in our cohort was not associated with increased risk of BOS or death.
This study provides a longitudinal evaluation of RVI in the pediatric lung transplant population. The data indicates that younger age and CMV infection are independent risk factors for the development of RVI after pediatric lung transplantation. In addition, a single episode of RVI trends toward increased subsequent A2 acute rejection but is not associated with BOS or death. Future studies must prospectively evaluate the incidence of RVI in this population, including potential consequences to RVI related to graft injury.
Table 1.
Demographics of Pediatric Lung Transplant Recipients
| n=55 (%) | With RVI n = 28 | Without RVI n = 27 | P-value | ||
|---|---|---|---|---|---|
| Age at Transplant | Mean (Median) in years | 10.6 (11.3) | 12.3 (13.9) | 0.36* | |
| Female | 28(51%) | 16 (57%) | 12 (44%) | 0.35** | |
| Transplant | Bilateral cadaveric | 52 (95%) | 27 (96%) | 25 (93%) | 0.74*** |
| Heart-Lung | 2(4%) | 1 (4%) | 1 (4%) | ||
| Living Donor | 1(2%) | 1 (4%) | |||
| Ethnicity | Caucasian | 39 (71%) | 17 (68%) | 22 (82%) | 0.67*** |
| African-American | 2(4%) | 1 (4%) | 1 (4%) | ||
| Hispanic | 9 (16%) | 6 (24%) | 3 (11%) | ||
| Others | 2 (4%) | 1 (4%) | 1 (4%) | ||
| Etiology | Cystic Fibrosis | 34 (62%) | 17 (61%) | 17 (63%) | 0.91*** |
| Pulmonary Hypertension (Idiopathic/Secondary) | 3 (5%)/2(4%) | 1 (4%)/2 (7%) | 2 (7%)/0 | ||
| Bronchiolitis obliterans | 3 (5%) | 1 (4%) | 2 (7%) | ||
| Interstitial pneumonitis | 3 (5%) | 1 (4%) | 2 (7%) | ||
| Others | 10 (18%) | 6 (21%) | 4 (15%) | ||
| Cyclosporine | 49 (91%) | 27 (96%) | 22 (85%) | 0.18*** |
Wilcoxon rank-sum test
Chi-square test
Fisher’s exact test
Table 4.
Multivariable model for RVI after Pediatric Lung Transplantation (55 subjects with 28 events)
| Label | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Age <= 10 vs > 10 | 2.4 (1.10, 5.3) | 0.028 |
| CMV (time dependent) | 17.0 (3.0, 96.2) | 0.001 |
Acknowledgments
Supported by National Institutes of Health/K23 RR022956 (LDI), Infectious Diseases Society of America Summer Scholarship (ML)
Footnotes
Presented: International Society of Heart and Lung Transplantation Annual Meeting, Boston, MA 2008
References
- 1.Waltz DA, Boucek MM, Edwards LB, Keck BM, Trulock EP, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: ninth official pediatric lung and heart-lung transplantation report--2006. J Heart Lung Transplant. 2006;25(8):904–911. doi: 10.1016/j.healun.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Kanj SS, Tapson V, Davis DR, Madden J, Browning I. Infections in Patients with Cystic Fibrosis following Lung Transplantation. Chest. 1997;112(4):924–930. doi: 10.1378/chest.112.4.924. [DOI] [PubMed] [Google Scholar]
- 3.Kramer MR, Marshall SE, Starnes VA, Gamberg P, Amitai Z, Theodore J. Infectious complications in heart-lung transplant. Archives of Internal Medicine. 1993;153:2010–2016. [PubMed] [Google Scholar]
- 4.Maurer JR, Tullis DE, Grossman RF, Vellend H, Winton Tl, Patterson GA. Infectious complications following isolated lung transplant. Chest. 1992;101:1056–1059. doi: 10.1378/chest.101.4.1056. [DOI] [PubMed] [Google Scholar]
- 5.Milstone AP, Brumble LM, Barnes J, Estes W, Loyd JE, Pierson RN, 3rd, et al. A single-season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J. 2006;28(1):131–137. doi: 10.1183/09031936.06.00105505. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins P, McNeil K, Kermeen F, Musk M, McQueen E, Mackay I, et al. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am J Respir Crit Care Med. 2008;178(8):876–881. doi: 10.1164/rccm.200711-1657OC. [DOI] [PubMed] [Google Scholar]
- 7.Bridges ND, Spray TL, Collins MH, Bowles NE, Towbin JA. Adenovirus infection in the lung results in graft failure after lung transplantation. J Thorac Cardiovasc Surg. 1998;116(4):617–623. doi: 10.1016/S0022-5223(98)70168-0. [DOI] [PubMed] [Google Scholar]
- 8.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 9.Kumar D, Erdman D, Keshavjee S, Peret T, Tellier R, Hadjiliadis D, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5(8):2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerna G, Vitulo P, Rovida F, Lilleri D, Pellegrini C, Oggionni T, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78(3):408–416. doi: 10.1002/jmv.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Worley S, Arrigain S, Aurora P, Ballmann M, Boyer D, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis. 2009 doi: 10.1111/j.1399-3062.2009.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilchez RA, Dauber J, Kusne S. Infectious etiology of bronchiolitis obliterans: the respiratory viruses connection - myth or reality? Am J Transplant. 2003;3(3):245–249. doi: 10.1034/j.1600-6143.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 13.Vilchez RA, McCurry K, Dauber J, Lacono A, Griffith B, Fung J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2(3):287–291. doi: 10.1034/j.1600-6143.2002.20315.x. [DOI] [PubMed] [Google Scholar]
- 14.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest. 2001;119(4):1277–1280. doi: 10.1378/chest.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 15.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75(12):2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 16.Vilchez R, McCurry K, Dauber J, Iacono A, Keenan R, Griffith B, et al. Influenza and parainfluenza respiratory viral infection requiring admission in adult lung transplant recipients. Transplantation. 2002;73(7):1075–1078. doi: 10.1097/00007890-200204150-00010. [DOI] [PubMed] [Google Scholar]
- 17.Bando K, Paradis I, Komatsu K, Konishi H, Matsushima M, Keenan RJ, et al. Analysis of time-dependent risks for infection, rejection, and death after pulmonary transplantation. Journal of Thoracic and Cardiovascular Surgery. 1995;109:49–59. doi: 10.1016/s0022-5223(95)70419-1. [DOI] [PubMed] [Google Scholar]
- 18.Winter JB, Gouw AS, Groen M, Wildevuur C, Prop J. Respiratory viral infections aggravate airway damage caused by chronic rejection in rat lung allografts. Transplantation. 1994;57(3):418–422. doi: 10.1097/00007890-199402150-00018. [DOI] [PubMed] [Google Scholar]
- 19.Kuo E, Bharat A, Goers T, Chapman W, Yan L, Street T, et al. Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann Thorac Surg. 2006;82(3):1043–1050. doi: 10.1016/j.athoracsur.2006.03.120. [DOI] [PubMed] [Google Scholar]
- 20.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34(1):7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 21.Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, et al. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45(8):2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danziger-Isakov LA, Worley S, Arrigain S, Aurora P, Ballmann M, Boyer D, et al. Increased mortality after pulmonary fungal infection within the first year after pediatric lung transplantation. J Heart Lung Transplant. 2008;27(6):655–661. doi: 10.1016/j.healun.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, et al. A working formulation for the standardization of the nomenclature and for clinical staging of chronic dysfunction in lung allografts. Journal of Heart and Lung Transplantation. 1993;12:713–716. [PubMed] [Google Scholar]
- 24.Yousem SA, Berry G, Brunt E, Chamberlain D, Hruban R, Sibley R, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection. Journal of Heart Transplantation. 1990;9:593–601. [PubMed] [Google Scholar]
- 25.Yousem SA, et al. Revision of the 1990 working formulation of the classification of pulmonary allograft rejection: Lung rejection study group. Journal of Heart and Lung Transplantation. 1996;15:1–15. [PubMed] [Google Scholar]
- 26.Larcher C, Geltner C, Fischer H, Nachbaur D, Muller LC, Huemer HP. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant. 2005;24(11):1891–1901. doi: 10.1016/j.healun.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Dare R, Sanghavi S, Bullotta A, Keightley MC, George KS, Wadowsky RM, et al. Diagnosis of human metapneumovirus infection in immunosuppressed lung transplant recipients and children evaluated for pertussis. J Clin Microbiol. 2007;45(2):548–552. doi: 10.1128/JCM.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


