The causes and mechanisms leading to loss of visual function in Jeune syndrome have not been extensively explored. The authors show that zebrafish lacking ift80, a gene responsible for a subset of Jeune syndrome, undergo photoreceptor degeneration in a mechanism consistent with defects in cilia maintenance.
Abstract
Purpose.
Jeune's asphyxiating thoracic dystrophy (JATD) is an autosomal recessive disorder with symptoms of retinal degeneration, kidney cysts, and chondrodysplasia and results from mutations in the ift80 gene. This study was conducted to characterize zebrafish lacking ift80 function for photoreceptor degeneration and defects in ciliogenesis to establish zebrafish as a vertebrate model for visual dysfunction in JATD and to determine whether ift80 interacts genetically with Bardet-Biedl syndrome (BBS) genes.
Methods.
Zebrafish were injected with morpholinos (MOs) targeted to the ift80 gene. Retinas were analyzed by histology, transmission electron microscopy, and immunohistochemistry. Ear and kidney cilia were analyzed by whole-mount immunostaining. Intraflagellar transport (IFT) particle composition was subjected to Western blot analysis. Genetic interactions were tested by coinjection of MOs against ift80 and bbs4 or bbs8 followed by in situ hybridization.
Results.
Zebrafish lacking ift80 function exhibited defects in photoreceptor outer segment formation and photoreceptor death. Staining with opsin antibodies revealed opsin mislocalization in both rods and cones. Ultrastructural analysis showed abnormal disc stacking and shortened photoreceptor outer segments. The kinocilia of the ear and motile cilia in the kidney were shorter and reduced in number. Western blot analysis revealed a slight increase in the stability of other IFT proteins. Coinjection of MOs against ift80 and BBS genes led to convergent-extension defects.
Conclusions.
Zebrafish lacking ift80 exhibited defects characteristic of JATD. Because the developing outer segments degenerated, Ift80 could possibly act as a maintenance factor for the IFT particle.
Cilia are microtubule-based structures that protrude from almost all eukaryotic cells, including photoreceptors.1 As sensory antennae,2 vertebrate photoreceptors rely on a modified sensory cilia (i.e., the outer segment) for function. Mutations affecting cilia biogenesis or function cause pleiotropic symptoms frequently observed in a spectrum of hereditary diseases known as ciliopathies.3 Such diseases include Bardet-Biedl syndrome (BBS), Senior-Løken syndrome, Meckel-Gruber syndrome, and Jeune asphyxiating thoracic dystrophy (JATD). Defects in the motile cilia that generate fluid flow within the respiratory epithelia and move cerebrospinal fluid result in fluid accumulation within the lungs, brain, and spine. Situs inversus stems from loss of cilia, which help establish left-right asymmetry, in the embryonic node. In the nonmotile sensory cilia, receptor molecules and ion channels decorate the ciliary membrane to detect signaling ligands and changes to the extracellular environment. Thus, ciliopathies often manifest with retinal degeneration, situs inversus, sensorineural hearing loss, mental impairment, and disorders of the kidney, liver, and pancreas.4
Cilia biogenesis requires intraflagellar transport (IFT) to build and maintain the microtubule axoneme.5 IFT refers to the bidirectional movement of IFT particles along the axoneme. IFT particles are composed of at least 17 distinct IFT proteins. The molecular motors kinesin-II and cytoplasmic dynein 2 control anterograde and retrograde movement, respectively. IFT transports proteins necessary for cilia assembly and for specific cargos, such as membrane-bound receptors or ion channels. In photoreceptors, IFT is essential for outer segment formation and maintenance.6–8
JATD, also known as Jeune syndrome, is a rare, multisystem, autosomal recessive disorder that often results in neonatal lethality.9,10 Mutations in the gene for IFT80, a component of the IFT particle, were recently found in a subset of patients with JATD.11 JATD is principally characterized by abnormal skeletal development resulting in a long, narrow thorax, short ribs, shortened long bones, and occasional polydactyly.12 Of the 20% of patients who survive beyond the neonatal period, many develop kidney cysts, hepatic fibrosis, and pancreatic abnormalities.13 In milder cases, patients also exhibit reduced scotopic and photopic ERG amplitudes and night blindness as early as 5 years of age.14–18 Postmortem pathology findings of an 8-year-old JATD patient revealed rod and cone degeneration and gliosis in the inner retina.17 Because JATD shares features with other ciliopathies,4 it would be useful to examine the loss-of-function phenotype in a genetically tractable model organism to understand the basis of retinal degeneration and to examine other ciliated cell types for dysfunction. Furthermore, genes associated with ciliopathies show epistatic effects with each other,19,20 and some alleles can act as modifiers toward pathogenesis in ciliopathies.21 Thus, exploring genetic interactions between IFT80 and other disease-causing loci may provide insight into the molecular basis of JATD and identify other loci that contribute to JATD symptoms.
Null mutations in mouse IFT genes cause embryonic lethality between embryonic day (E)10.5 and E13.5, thus preventing examination of cell types such as photoreceptors that differentiate near birth or during postnatal periods.22–25 In contrast, zebrafish develop rapidly, with photoreceptor differentiation starting at 50 to 52 hours postfertilization (hpf); robust visual behaviors are present by 5 days postfertilization (dpf).26,27 Although zebrafish IFT mutants die at 8 to 9 dpf, the effects of these mutations on photoreceptor structure and function can be investigated. We report that zebrafish deficient for ift80 function exhibit phenotypes consistent with those of previously described IFT mutants and with symptoms associated with JATD. Morpholino knockdown of ift80 disrupted photoreceptor outer segment structure and caused opsin mislocalization. In embryos lacking ift80 function, cilia were disrupted in the kidney and otic vesicle. Finally, we show that ift80 genetically interacts with bbs4 and bbs8 to regulate cell movement during gastrulation. Our results show that loss of ift80 results in photoreceptor degeneration and that zebrafish may serve as a useful model for retinal dysfunction in JATD.
Materials and Methods
Fish Maintenance and Breeding
Wild-type zebrafish of the AB strain were housed, bred, and staged according to standard procedures.28 Zebrafish were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Morpholino Knockdown
ift80 knockdown was carried out using a morpholino targeted to the splice site at the 3′ end of the second exon. Morpholinos were injected into wild-type embryos at the 1- to 2-cell stage according to standard procedures.29 Morpholino sequences were as follows: ift80, AGGTGTATGTGGAACCTGTGATAAG; bbs4, GAAAAAGATCACTACTGTAAAGCAT; bbs8, AGCTGTATACTCACGAGCCACCTGA. The ift80 morpholino was described previously,11 and the bbs4 and bbs8 morpholinos were described by Gerdes et al.30 and Yen et al.,31 respectively.
Transmission Electron Microscopy
Animals were fixed at 96 hpf in 1% paraformaldehyde, 2.5% glutaraldehyde, and 1% tannic acid. Embryos were processed with osmium tetroxide as secondary fixative, followed by a dehydration series in ethanol, and were infiltrated with epoxy resin as previously described.32 Transverse sections (0.1 μm in thickness) obtained at the optic nerve region were poststained with 2% uranyl acetate and Reynolds lead citrate. Images were collected on a transmission electron microscope (1200EX; JEOL 1200EX, Tokyo, Japan) and were processed with image editing software (Photoshop; Adobe, Mountain View, CA).
In Situ Hybridization and Reverse Transcription–Polymerase Chain Reaction
Digoxigenin-labeled (DIG) antisense riboprobes were synthesized from partial or full-length cDNAs using standard protocols. The pax2.1 and myoD full-length cDNAs were gifts from Arne Lekven. In situ hybridizations were performed as previously described.33 RT-PCR was performed using standard protocols.
Image Analysis of Whole-Mount and Flat-Mount Embryos
Whole-mounted embryos were imaged with a stereomicroscope (Lumar; Carl Zeiss, Jena, Germany) and photographed with a digital camera (AxioCam; Carl Zeiss). Embryos were prepared for flat-mounting by dissecting the yolk away from the embryo proper with a small needle and were mounted in glycerol on glass slides. Commercial software (Axio Imager; Carl Zeiss) was used to measure embryo lengths from the anterior-most region of pax2a staining to the tip of the paraxial mesoderm myoD staining. Statistical analysis with appropriate software (Excel; Microsoft, Redmond, WA).
Immunohistochemistry
Immunohistochemistry was performed as previously described.34 Images were obtained on a fluorescence microscope (ImagerZ1; Carl Zeiss) fitted with a fluorescence microscope slider module (ApoTome; Carl Zeiss) using a 63× oil objective. Images were prepared using image editing software (Photoshop; Adobe). The monoclonal antibody 1D1 (Fadool JM, et al. IOVS. 1999;40:ARVO Abstract 1251) and the monoclonal antibody zpr-1 (Zebrafish International Resource Center) were both used at 1:200. Affinity-purified polyclonal rabbit antibodies against zebrafish IFT88 (1:5000) and zebrafish IFT52 (1:3000) were used as previously described.32 Affinity-purified polyclonal rabbit antibodies against the blue cone opsin antibody (1:200), a gift from David Hyde, were used as previously described.35 Affinity-purified polyclonal rabbit antibodies against mouse Centrin-3 were used at 1:200.36 The appropriate fluorescently conjugated Alexa secondary antibodies (Invitrogen) were used at 1:500. Slides were counterstained with DAPI (Invitrogen, Carlsbad, CA) to label DNA.
TUNEL Assays
Embryos were fixed at various time points and prepared for cryosectioning as described. TUNEL assays were performed on 10-μm–thick cryosections with an FITC label (In situ Cell Death Detection Kit; Roche Applied Sciences, Basel, Switzerland) according to the manufacturer's instructions. Images were obtained on a fluorescence microscope (ImagerZ1; Carl Zeiss) fitted with a fluorescence microscope slider module (ApoTome; Carl Zeiss) using a 20× objective. Five to 10 animals were imaged for each time point, and multiple distinct cryosections surrounding the optic nerve were analyzed for each retina. Between 26 and 81 data points were averaged for wild-type and ift80 morphant embryos at each age and were compared using a two-tailed Student's t-test with unequal variance.
SDS-PAGE and Immunoblot Analysis
Western blot analysis was performed as previously described.32 The following dilutions of primary antibodies were used: monoclonal K2.4 (KIF3A, 1:10,000; Covance, Madison, WI); affinity-purified polyclonal rabbit antibodies against zebrafish IFT88 (1:5000), zebrafish IFT52 (1:5000), zebrafish IFT20 (1:5000), and zebrafish IFT57 (1:5000); and monoclonal anti–acetylated tubulin (1:10,000; Sigma).
Results
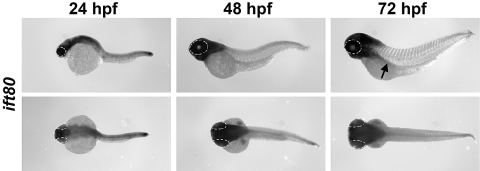
We first examined ift80 gene expression at multiple time points during development by in situ hybridization. Like other IFT genes, ift80 was expressed maternally (data not shown). At 24 hpf, ift80 was broadly expressed throughout the central nervous system (CNS) and the notochord (Fig. 1). CNS expression persisted through 72 hpf, though notochord expression diminished. Consistent with a role in cilia formation and function, ift80 was expressed in the pronephric ducts (Fig. 1, arrow). We also observed expression in the pectoral fins, which are analogous to the mammalian limb bud. Noticeably, ift80 expression was strongly expressed in the eye but not in the lens/cornea.
Figure 1.
Expression of ift80 during zebrafish development. Whole-mount in situ hybridization with an ift80 riboprobe on 24 hpf, 48 hpf, and 72 hpf. Top row: lateral views. Bottom row: dorsal view. Dashed lines: eyes are outlined. Arrow: pronephric duct in the lateral view of the 72-hpf embryo.
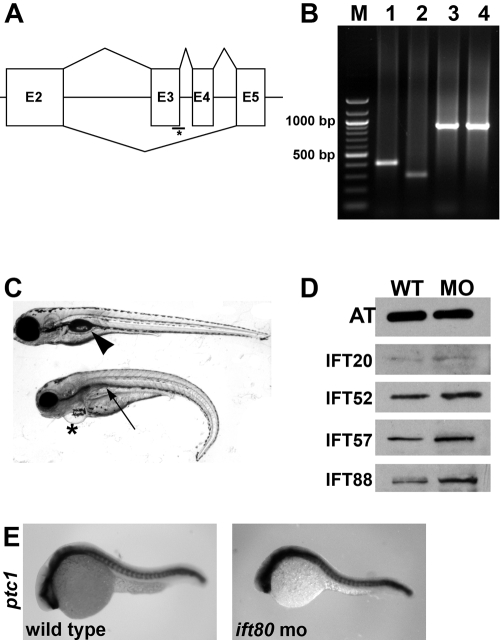
To test the function of ift80, we injected 1.5 ng morpholino oligonucleotide (MO) targeted to disrupt normal splicing of the ift80 mRNA.11 Morpholinos were targeted to the splice site at the 3′ end of the second exon (Fig. 2A) and were predicted to cause the loss of exon 3. Sequence analysis of cDNA from injected embryos revealed that 1.5 ng morpholino blocked the inclusion of exons 3 and 4 until at least 5 dpf (Fig. 2B; data not shown). To test whether the loss of ift80 affected other components of the IFT particle, we examined extracts of whole embryos 5 dpf by Western blot analysis (Fig. 2D). The IFT particle consisted of two multi-subunit complexes, complex A (five proteins) and complex B (Ift80 and at least 10 other polypeptides).37 Although not quantitative, Western blots of protein extracts from ift80 morphants appeared to contain similar amounts of the four other complex B proteins—Ift20, Ift52, Ift57, and Ift88. Because ift80 is not predicted to be involved in transcriptional regulation, the qualitative increase of these proteins may reflect posttranslational stabilization. Interestingly, we previously found that loss of ift57 in zebrafish reduced the level of complex B proteins.32 It is unclear how different IFT components affect the stability of other IFT polypeptides.
Figure 2.
Loss of ift80 function by morpholino knockdown. (A, asterisk) Diagram of splicing defect caused by the ift80 morpholino targeted to the splice donor site on exon 3. After morpholino injection, both exons 3 and 4 are skipped (bottom line), resulting in a 180-bp deletion. (B) The DNA ladder marker (M) is shown at the far left. Lane 1, wild-type PCR product; lane 2, ift80 morphant PCR product that is lower than the wild type product and consistent in size with loss of exons 3 and 4; lanes 3 and 4, β-actin PCR product from the wild-type and morphant cDNA samples, respectively, as a control. (C, top) 5-dpf wild-type control animal; (bottom) 5-dpf ift80 morphant animal. Asterisk: heart edema in ift80 morphant. Kidney cysts (arrow) are distinct from the swim bladder (arrowhead) seen in the wild-type animal. (D) Western blot analysis of IFT proteins from an ift80 morphant. Protein extracts from 5-dpf wild-type and morphant animals were blotted with antibodies against IFT20, IFT52, IFT57, and IFT88. Acetylated tubulin (AT) was included as a loading control. (E) Whole-mount in situ hybridization of 24 hpf wild-type and ift80 morphant with ptc1 riboprobe.
Embryos injected with an ift80-MO had a ventral tail curvature, slightly smaller eyes, pericardial edema, and kidney cysts (Fig. 2C) at 5 dpf. The phenotype of MO-injected animals was similar to that of previously described IFT mutants7,32 and consistent with defects in cilia function. Whole-mount immunostaining on embryos injected with ift80-MO with an antibody for acetylated tubulin revealed subtle defects of cilia in the ear and pronephric duct at 30 hpf (Supplementary Material, http://www.iovs.org/cgi/content/full/51/7/3792/DC1). Importantly, ift80 morphants did not exhibit a noticeable optokinetic response under normal lighting conditions at 5 dpf (data not shown). IFT has been implicated in mammalian Hedgehog signaling,22 and knockdown of ift80 was previously reported to reduce the expression of ptc1, a downstream target of Hedgehog signaling.11 As was shown, however, for previously described zebrafish IFT mutants,38 ptc1 expression was unchanged at 24 hpf after injection of ift80 morpholino (Fig. 2E). One possible explanation for the difference between our result and the effects on Hedgehog signaling previously reported for this morpholino11 is that we injected less morpholino to achieve full knockdown (1.5 ng vs. 4.0 ng). When injecting higher doses of morpholino, we observed necrosis in the brain and somites (data not shown).
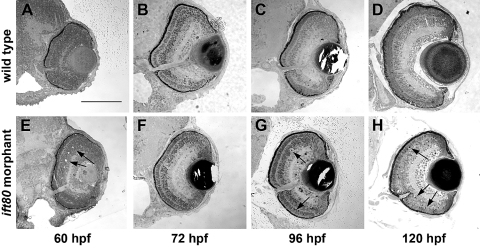
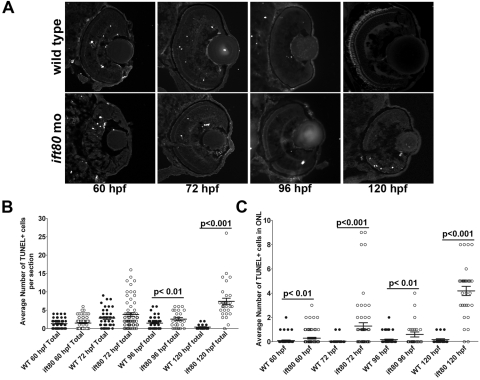
Because ift80 morphants did not exhibit visual function by optokinetic response assays, we performed histologic analysis to further examine the underlying visual defect. Retinal development proceeded normally in the absence of ift80 function, but eye size and photoreceptor survival were disrupted (Fig. 3). At 60 hpf, wild-type animals exhibited optic nerves and distinct nuclear layers (Fig. 3A). For the next 2 to 3 days, the eye continued to grow, and photoreceptor outer segments became apparent, particularly in the ventral retina (Figs. 3B–D). At 60 hpf, ift80 morphants displayed optic nerves and retinal lamination, as did wild-type embryos, though some disorganization was apparent (Fig. 3E). By 5 dpf, the eyes of ift80-MO–injected embryos were smaller than those wild-type controls, and very few outer segments were seen (Figs. 3F–H), similar to phenotypes observed in zebrafish IFT mutants.7,32,39 Pyknotic nuclei and acellular holes were seen in all cellular layers in the ift80-MO retinas but were rarely observed in wild-type retinas (Figs. 3E, 3G, 3H; arrows). These results suggested that the small eye phenotype observed in ift80-MO–injected animals likely reflected the combination of cell death throughout the retinal and loss of volume normally occupied by photoreceptor outer segments. To test the hypothesis of cell death, TUNEL staining was performed on wild-type and ift80 morphant retinas at 60 hpf, 72 hpf, 96 hpf, and 5 dpf, and the number of apoptotic nuclei was quantified (Fig. 4). In wild-type animals, the number of TUNEL+ cells was 1.3 ± 0.1 (mean ± SEM) at 60 hpf, 2.8 ± 0.4 at 72 hpf, 1.5 ± 0.2 at 96 hpf, and 0.4 ± 0.1 at 5 dpf. This is similar to a previous report showing cell death is highest at 72 hpf and declines by 5 dpf.40 The total number of TUNEL+ cells in ift80 morphant retinas was not statistically different from that in controls at 60 hpf and 72 hpf (1.5 ± 0.2 and 3.8 ± 0.5, respectively), though considerable variation was observed in both wild-type and morphant analyses (Fig. 4B). The total number of TUNEL+ cells was statistically higher in the ift80 morphant retinas than in controls at both 96 hpf (2.6 ± 0.4 vs. 1.5 ± 0.2) and 5 dpf (7.4 ± 0.9 vs. 0.4 ± 0.1). Apoptosis within the photoreceptor layer of ift80 morphants was elevated at 72 hpf, 96 hpf, and 5 dpf; most photoreceptors died at 5 dpf (Fig. 4C).
Figure 3.
ift80 morphants exhibit retinal degeneration. Transverse histologic sections through wild-type and ift80 morphant retinas at several stages of development are shown. Wild-type zebrafish retina at (A) 60 hpf, (B) 72 hpf, (C) 96 hpf, and (D) 120 hpf. Retinas from ift80 morphants at (E) 60 hpf, (F) 72 hpf, (G) 96 hpf, and (H) 120 hpf. Arrows: in ift80 morphants, few outer segments are observed, and several pyknotic nuclei and acellular holes are seen. Scale bar, 100 μm.
Figure 4.
ift80 morphants exhibited retinal degeneration. (A) TUNEL+ cells were observed in wild-type and ift80 morphants at 60 hpf, 72 hpf, 96 hpf, and 5 dpf. (B) Total number of TUNEL+ cells and (C) number of TUNEL+ cells in the outer nuclear layer (ONL) were quantified at each developmental stage for wild-type (closed circles) and ift80 morphants (open circles), as shown in dot blots. Mean ± SEM for each time point is shown. Student's t-test was used to compare values for statistical significance.
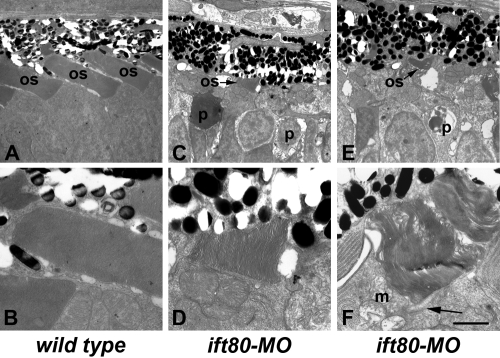
Disrupted photoreceptor outer segments were easily observed in ift80-MO animals by transmission electron microscopy. In control animals, rod and cone photoreceptor outer segments extended toward the retinal pigment epithelium at 5 dpf (Figs. 5A, 5B). In contrast, the few existing outer segments were shorter and highly disorganized after injection of ift80-MO (Figs. 5C–F). Cellular debris and pyknotic nuclei were seen in ift80 morphants. Connecting cilia were seen in those ift80 morphant photoreceptors with an outer segment but whose disc membranes were occasionally disordered. These results indicate that loss of ift80 does not completely block outer segment formation but that ift80 is necessary for proper organization of disc membranes and photoreceptor survival.
Figure 5.
ift80 morphants make cilia and disorganized outer segments. Transmission electron microscopy of 4-dpf wild-type and dynein morphant retinas are shown. (A) Wild-type photoreceptor outer segments (OS) are extending toward the RPE and are regularly spaced. (B) Higher-magnification image highlights the regular spacing of the disc membranes within the wild-type outer segments. (C–F) ift80 morphants had very short outer segments (OS; arrow) and numerous pyknotic nuclei (p) in the photoreceptor layer. Disc membranes were disorganized, and mitochondria (m) were abnormal. (F, arrow) Connecting cilia were present. Scale bars: (A, C, E) 5 μm; (B, D, F) 2 μm.
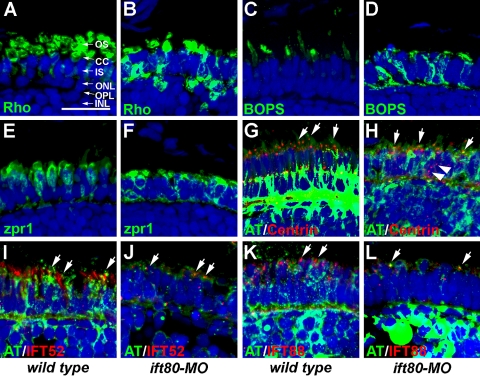
Given that outer segment assembly requires rhodopsin transport and defects in other IFT genes lead to rhodopsin mislocalization in zebrafish,2,32 we hypothesized that the morphant photoreceptor phenotypes reflected defects in the transport of outer segment membrane proteins. A monoclonal antibody against rhodopsin, 1D1, labeled outer segments of wild-type zebrafish at 5 dpf (Fig. 6A). The ift80 morphants exhibited significant mislocalization of rhodopsin, though some staining was observed in the outer segments (Fig. 6B). Blue opsin was expressed in the long single cones of zebrafish41 and localized to the outer segment at 5 dpf (Fig. 6C). In contrast, injection of ift80-MO resulted in considerable mislocalization to the inner segment and a reduction in outer segment staining (Fig. 6D). Because opsin mislocalization can result in photoreceptor degeneration, cone morphology was examined using the zpr-1 antibody, which recognizes a cell-surface epitope on red-green double cones. Staining with zpr-1 showed wild-type cones with a tightly packed columnar morphology in the photoreceptor layer (Fig. 6E). The ift80 morphants exhibited significant alterations in photoreceptor morphology indicative of degeneration and consistent with electron microscopy results (Fig. 6F).
Figure 6.
Immunohistochemical analysis of wild-type and ift80 morphants at 5 dpf. (A) 1D1 (green), a marker for rhodopsin (Rho), localized to the outer segment region in wild-type fish. (B) Strong mislocalization to the inner segment was observed in ift80 morphants. (C, D) Blue opsin (BOPS) was localized to the outer segments of wild-type photoreceptors. Strong mislocalization was seen in the ift80 morphants. (E, F) Zpr1 (green), a label for red/green double cones, gave an elongated, columnar morphology in wild-type animals. Cone morphology was disheveled in the ift80 morphants. (G, H) Centrin (red) localized to the apical surface of the inner segment (short arrows). Acetylated tubulin (AT; green) denotes microtubules labeled by anti–acetylated tubulin. Centrin was occasionally observed to be mislocalized (H; white arrowheads). (I, J) Ift52 (red) colocalized with acetylated tubulin (green) in the connecting cilia of wild-type and ift80 morphants (arrows). (K, L) Ift88 (red) also showed strong apical localization in wild-type and ift80 morphants. The number of Ift52- and Ift88-stained foci was significantly reduced in the ift80 morphant retinas. Staining overlapped with AT (green). In all images, the tissues were counterstained with DAPI (blue). Outer segments (OS), connecting cilia (CC), inner segment (IS), outer nuclear layer (ONL), outer plexiform layer (OPL), and inner nuclear layer (INL) are shown for orientation purposes. Scale bar, 20 μm.
We next examined whether components of the IFT machinery and axoneme were properly positioned. Centrin is a calcium-binding protein and a component of the basal body.36 Centrin localized to the base of the connecting cilium of wild-type photoreceptors (Figs. 6G, 6H; arrows). Centrin immunoreactivity was also detected near the outer plexiform layer. We did not observe significant colocalization between centrin and acetylated tubulin in the cilium, which we attributed to the low signal from acetylated tubulin in the basal body region. Injection of ift80-MO did not eliminate centrin from the apical inner segment in most cells (Fig. 6H; arrows), but we did observe some centrin mislocalization throughout the inner segment (Fig. 6H; arrowheads) that was not observed in controls. IFT particle components Ift52 and Ift88 also localized to the connecting cilium of wild-type photoreceptors (Figs. 6I, 6K; arrows). Fewer connecting cilia were present in embryos after ift80-MO injection; however, both IFT52 and IFT88 remained colocalized with cilia (Figs. 6J, 6L; arrows). These results indicated that loss of ift80 disrupts opsin trafficking to the outer segment and leads to photoreceptor degeneration but does not significantly affect the localization of proteins required for ciliogenesis.
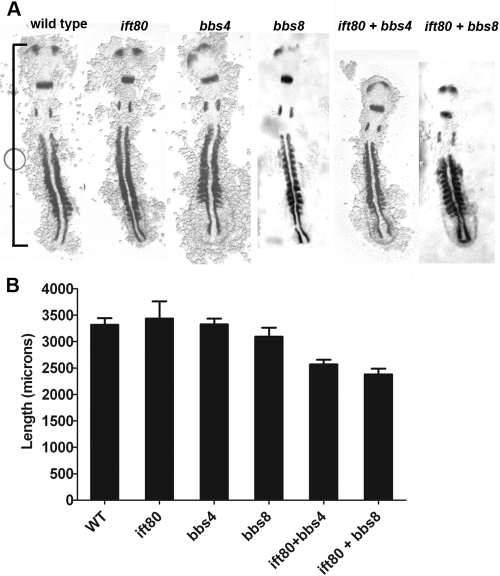
JATD exhibits traits similar to those in BBS, and reduction of BBS gene function compromises Wnt/planar cell polarity (PCP) signaling in tissue culture and zebrafish embryos.21,30,42 The basal body is proposed to serve as a key organelle for intracellular signaling,43 and depletion of basal body proteins disrupts Wnt/PCP signaling during gastrulation. Injection of morpholinos targeted against zebrafish bbs genes leads to convergence-extension phenotypes, similar to those seen in the zebrafish mutants trilobite (the zebrafish vangl2 ortholog) and pipetail (wnt5).30 Given the similarity between JATD and BBS and given that basal body components were occasionally mislocalized in ift80 morphant photoreceptors, we looked for genetic interactions between ift80 and the bbs genes. In embryos injected with morpholinos against ift80 (1.5 ng) or a modest dose of bbs4 (1.0 ng) or bbs8 (1.0 ng), the expression of myoD (somite) and pax2a (otic vesicle and forebrain) was similar to that of wild-type embryos at the 12- to 14-somite stage during gastrulation and early somitogenesis (Fig. 7A). However, embryos injected with a combination of morpholinos against ift80 (1.5 ng) and bbs4 (1.0 ng) or ift80 and bbs8 exhibited significant shortening of the body axis (Figs. 7A, 7B), indicating a synthetic genetic interaction between ift80 and bbs genes.
Figure 7.
ift80 interacts genetically with BBS genes. (A) Whole-mount in situ hybridization of embryos injected with 1.5 ng ift80 morpholino (ift80), 1.0 ng bbs8 morpholino (bbs8) or 1.0 ng bbs4 morpholino (bbs4), either alone or in combination. Embryos were fixed at the 10- to 12-somite stage and stained with myoD and pax2a riboprobes. All embryos were flat-mounted and photographed from the dorsal side, with the anterior portion at the top. (B) Body axis lengths were measured from the anteriormost region of pax2a staining to the tip of the paraxial mesoderm myoD staining (A, bracket). Bars represent the mean for each set of embryos (n ≥ 4), and error bars are SEM. No statistical difference (t-test) from wild-type was found for any morpholino treatment alone. Injection of ift80 morpholino in combination with either bbs morpholino decreased body length (P < 0.005).
Discussion
Zebrafish depleted of ift80 exhibited photoreceptor degeneration and surviving photoreceptors had shortened outer segments with mislocalized rod and cone opsin proteins. Given the importance of IFT in cilia biogenesis and maintenance, it was not surprising that loss of ift80 resulted in phenotypes similar to those reported for other zebrafish IFT mutants or morphants.7,32,44 Although photoreceptors degenerated in the ift80 morphants, outer segment formation was not blocked, implying that ift80 is required for outer segment maintenance but not for ciliogenesis. Thus, the vision loss described in some patients with JADT likely reflects photoreceptor degeneration rather than a morphogenesis failure or a nonphotoreceptor defect. Although vision loss has been described in some patients with JATD,14,15 the three alleles of IFT80 that cause JATD were not present in renal or retinal phenotypes.11 In that study, two alleles were missense mutations, and one was an in-frame deletion. These mutations may be hypomorphic, or, in distinct domains of the Ift80 protein, they may result in retinal degeneration. Future identification and analysis of IFT80 alleles in patients with JATD and vision loss will help explain the range of phenotypes observed by IFT80 mutation.
Previous studies of ift80 in invertebrate systems suggested a role in the biogenesis of cilia more pronounced than that seen in zebrafish. Knockdown of Ift80 in Tetrahymena abolished cilia formation,11 whereas nonsense alleles of ift80 (che-2) in Caenorhabditis elegans resulted in very short cilia in sensory neurons.45 Nevertheless, the ift80/che-2 mutant phenotype reflected ciliogenesis failure rather than cilia degeneration.45 Although ciliogenesis occurs in zebrafish ift80 morphants, it is unlikely that photoreceptors and other cilia complete normal morphogenesis before degeneration. No examples of such phenomena have been reported for null IFT mutants. Mutations in ift80 have not been described in Chlamydomonas or mouse, and no direct binding partners are known. It is unclear why loss of ift80 does not completely abolish ciliogenesis and block outer segment formation in zebrafish, but the trafficking phenotypes we observed indicated the IFT particle remained sufficiently intact to bind some ciliary cargo and underwent limited IFT. Although several Chlamydomonas IFT mutants show destabilization of the IFT particles and reductions in IFT proteins, our results show that components of the IFT complex B are more abundant in ift80 morphant zebrafish. As a component of IFT complex B, the Ift80 protein readily dissociated from the core complex under high salt conditions, suggesting Ift80 may reside on the surface of complex B.46 The Ift57 protein also dissociated from complex B under high salt conditions, and mutation of ift57 in zebrafish resulted in short outer segments, similar to those seen in ift80 morphants.32 Thus, though loss of Ift80 may not dramatically destabilize the remaining particle, it clearly disrupts overall IFT function.
We show that ift80 genetically interacts and produces synthetic phenotypes with bbs genes. BBS proteins are components of the basal body that also mediate cilia biogenesis.47,48 Recent studies suggest the cilia and basal body are distinct organelles and the basal body mediates Wnt signaling, as evidenced by genetic interactions between bbs genes and the noncanonical Wnt pathway, also known as the PCP pathway.30 We show that morpholino knockdown of ift80 with bbs4 or bbs8 reduced the body axis and led to mild convergence-extension defects, a phenotype associated with defective Wnt/PCP signaling. Given that proper cell polarity and basal body positioning are essential for photoreceptor morphogenesis, it is intriguing to consider a role for Wnt/PCP signaling in outer segment formation. The role of IFT proteins in Wnt/PCP signaling, however, is controversial.49–51 Null alleles of several mouse and zebrafish IFT genes show no defects in Wnt signaling during gastrulation.50,51 However, a conditional knockout allele of Ift88 can disrupt cell polarity within the inner ear, as can mutations in Bbs6 and Bbs4.42,49 Perhaps ift80 has dual roles in IFT and basal body function. It is interesting that zebrafish bbs genes showed genetic interactions with ift80. When analyzing IFT movement in sensory neurons of C. elegans, mutation of BBS8 had little effect on the movement of Ift80 protein, suggesting there was no direct contact, whereas mutation of either BBS7 or BBS8 severely compromised the movement of OSM-5/Ift88.52 Future research will be necessary to determine whether the BBS proteins directly interact with Ift80 in zebrafish and whether such interactions regulate distinct functions in ciliary transport and photoreceptor cell polarity.
Supplementary Material
Footnotes
Supported by National Institutes of Health Grant EY017037 (BDP).
Disclosure: L.M. Hudak, None; S. Lunt, None; C.-H. Chang, None; E. Winkler, None; H. Flammer, None; M. Lindsey, None; B.D. Perkins, None
References
- 1.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol 2007;69:377–400 [DOI] [PubMed] [Google Scholar]
- 2.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn 2008;237:1982–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 2006;7:125–148 [DOI] [PubMed] [Google Scholar]
- 4.Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet 2007;28:113–125 [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum J. Intraflagellar transport. Curr Biol 2002;12:R125. [DOI] [PubMed] [Google Scholar]
- 6.Pazour GJ, Baker SA, Deane JA, et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 2002;157:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 2004;42:703–716 [DOI] [PubMed] [Google Scholar]
- 8.Sukumaran S, Perkins BD. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 intraflagellar transport mutants. Vision Res 2009;49:479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeune M, Beraud C, Carron R. Asphyxiating thoracic dystrophy with familial characteristics. Arch Fr Pediatr 1955;12:886–891 [PubMed] [Google Scholar]
- 10.Jeune M, Carron R, Beraud C, Loaec Y. Polychondrodystrophie avec blocage thoracique d'evolution fatale. Pediatrie 1954;9:390–392 [PubMed] [Google Scholar]
- 11.Beales PL, Bland E, Tobin JL, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet 2007;39:727–729 [DOI] [PubMed] [Google Scholar]
- 12.Oberklaid F, Danks DM, Mayne V, Campbell P. Asphyxiating thoracic dysplasia: clinical, radiological, and pathological information on 10 patients. Arch Dis Child 1977;52:758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson CA, Gissen P, Sergi C. Molecular pathology and genetics of congenital hepatorenal fibrocystic syndromes. J Med Genet 2003;40:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casteels I, Demandt E, Legius E. Visual loss as the presenting sign of Jeune syndrome. Eur J Paediatr Neurol 2000;4:243–247 [DOI] [PubMed] [Google Scholar]
- 15.Phillips CI, Stokoe NL, Bartholomew RS. Asphyxiating thoracic dystrophy (Jeune's disease) with retinal aplasia: a sibship of two. J Pediatr Ophthalmol Strabismus 1979;16:279–283 [DOI] [PubMed] [Google Scholar]
- 16.Bard LA, Bard PA, Owens GW, Hall BD. Retinal involvement in thoracic-pelvic-phalangeal dystrophy. Arch Ophthalmol 1978;96:278–281 [DOI] [PubMed] [Google Scholar]
- 17.Allen AW, Jr, Moon JB, Hovland KR, Minckler DS. Ocular findings in thoracic-pelvic-phalangeal dystrophy. Arch Ophthalmol 1979;97:489–492 [DOI] [PubMed] [Google Scholar]
- 18.Wilson DJ, Weleber RG, Beals RK. Retinal dystrophy in Jeune's syndrome. Arch Ophthalmol 1987;105:651–657 [DOI] [PubMed] [Google Scholar]
- 19.Leitch CC, Zaghloul NA, Davis EE, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet 2008;40:443–448 [DOI] [PubMed] [Google Scholar]
- 20.Badano JL, Leitch CC, Ansley SJ, et al. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature 2006;439:326–330 [DOI] [PubMed] [Google Scholar]
- 21.Khanna H, Davis EE, Murga-Zamalloa CA, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet 2009;41:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A 2005;102:11325–11330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003;426:83–87 [DOI] [PubMed] [Google Scholar]
- 24.Houde C, Dickinson RJ, Houtzager VM, et al. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol 2006;300:523–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 2005;132:3103–3111 [DOI] [PubMed] [Google Scholar]
- 26.Brockerhoff SE, Hurley JB, Janssen-Bienhold U, Neuhauss SC, Driever W, Dowling JE. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc Natl Acad Sci U S A 1995;92:10545–10549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol 1999;404:515–536 [PubMed] [Google Scholar]
- 28.Westerfield M. The Zebrafish Book 3rd ed Eugene, OR: University of Oregon Press; 1995 [Google Scholar]
- 29.Detrich HW, III, Zon LI, Westerfield M. Methods in Cell Biology 2nd ed New York: Academic Press; 2004 [Google Scholar]
- 30.Gerdes JM, Liu Y, Zaghloul NA, et al. Disruption of the basal body compromises proteosomal function and perturbs intracellular Wnt response. Nat Genet 2007;39:1350–1360 [DOI] [PubMed] [Google Scholar]
- 31.Yen HJ, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet 2006;15:667–677 [DOI] [PubMed] [Google Scholar]
- 32.Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci 2008;121:1907–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jowett T, Lettice L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet 1994;10:73–74 [DOI] [PubMed] [Google Scholar]
- 34.Perkins BD, Nicholas CS, Baye LM, Link BA, Dowling JE. dazed gene is necessary for late cell type development and retinal cell maintenance in the zebrafish retina. Dev Dyn 2005;233:680–694 [DOI] [PubMed] [Google Scholar]
- 35.Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci 1999;16:571–585 [DOI] [PubMed] [Google Scholar]
- 36.Giessl A, Pulvermuller A, Trojan P, et al. Differential expression and interaction with the visual G-protein transducin of centrin isoforms in mammalian photoreceptor cells. J Biol Chem 2004;279:51472–51481 [DOI] [PubMed] [Google Scholar]
- 37.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol 2002;3:813–825 [DOI] [PubMed] [Google Scholar]
- 38.Lunt S, Haynes T, Perkins BD. Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect Hedgehog signaling. Dev Dyn 2009;238:1744–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross JM, Perkins BD, Amsterdam A, et al. Identification of zebrafish insertional mutants with defects in visual system development and function. Genetics 2005;170:245–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biehlmaier O, Neuhauss SC, Kohler K. Onset and time course of apoptosis in the developing zebrafish retina. Cell Tissue Res 2001;306:199–207 [DOI] [PubMed] [Google Scholar]
- 41.Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK. Expression of rod and cone visual pigments in goldfish and zebrafish: a rhodopsin-like gene is expressed in cones. Neuron 1993;10:1161–1174 [DOI] [PubMed] [Google Scholar]
- 42.Ross AJ, May-Simera H, Eichers ER, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 2005;37:1135–1140 [DOI] [PubMed] [Google Scholar]
- 43.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol 2009;19:R526–R535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krock B, Mills-Henry I, Perkins B. Retrograde intraflagellar transport by cytoplasmic dynein-2 is required for outer segment extension in vertebrate photoreceptors but not arrestin translocation. Invest Ophthalmol Vis Sci 2009;50:5463–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiwara M, Ishihara T, Katsura I. A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of C. elegans sensory cilia. Development 1999;126:4839–4848 [DOI] [PubMed] [Google Scholar]
- 46.Lucker BF, Behal RH, Qin H, et al. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem 2005;280:27688–27696 [DOI] [PubMed] [Google Scholar]
- 47.Ansley SJ, Badano JL, Blacque OE, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 2003;425:628–633 [DOI] [PubMed] [Google Scholar]
- 48.Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007;129:1201–1213 [DOI] [PubMed] [Google Scholar]
- 49.Jones C, Roper VC, Foucher I, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet 2008;40:69–77 [DOI] [PubMed] [Google Scholar]
- 50.Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 2009;136:3089–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn 2008;237:2030–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blacque OE, Reardon MJ, Li C, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev 2004;18:1630–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 2005;132:1907–1921 [DOI] [PubMed] [Google Scholar]
- 54.Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev Biol 1997;191:191–201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.