Abstract
The occurrence of anemia in older adults has been associated with adverse outcomes including functional decline, disability, morbidity and mortality. It is not clear to what extent these outcomes are the result of the anemia or concurrent illness. We performed a cross-sectional, observational study to determine whether lower hemoglobin concentrations in older adults are associated with reduced health-related quality of life, functional status, depression, disability, and physical strength, independent of chronic disease. Three sites participated in this research; an academic geriatric practice, a hospital based geriatric out patient unit, and a community-based multi-specialty internal medicine group. Health-related quality of life and functional status were measured using the Short Form-36 Health Survey (SF-36) and the Functional Assessment of Chronic Illness Therapy-Anemia (FACIT-An). Disability and depression were assessed using the Instrumental Activities of Daily Living (IADL) and the Geriatric Depression Scale (GDS) questionnaires, respectively. Handgrip strength was used as a physical performance measure. Anemia was defined as hemoglobin < 13 g/dL for men or < 12 g/dL for women. The mean SF-36 physical health component summary scores were 38.9 (with anemia) and 44.1 (without anemia), (P < 0.001). Anemia was associated with greater fatigue (P < 0.001), lower handgrip strength (P = 0.014), increased number of disabilities (P = 0.005) and more depressive symptoms (P = 0.002). Multivariate regression analysis, adjusted for demographic and clinical characteristics, demonstrated strong associations for reduced hemoglobin, even within the “normal” range, and poorer health-related quality of life across multiple domains. Thus, anemia was independently associated with clinically significant impairments in multiple domains of health-related quality of life, especially in measures of functional limitation. Mildly low hemoglobin levels, even when above the WHO anemia threshold, were associated with significant declines in quality of life among the elderly.
INTRODUCTION
An increase in the elderly population is expected due to the high birth rates that occurred in the 1940s and 1950s and an increase in life expectancy (24). In 2000, approximately 35 million people in the United States were aged 65 and older; by 2050, this figure will rise to approximately 80 million. The population of individuals aged 85 and older will grow approximately fivefold by 2050 (13). Thus, the impact of common problems in the elderly, such as chronic illness and disability, will have profound implications for healthcare in the future.
Anemia (hemoglobin ≤ 12g/dL in women and ≤ 13 g/dL in men) is common in people over the age of 65 years, occurring in 10% of those living in the community (20), and in more than 50% of those residing in institutions (4, 30, 38). Although aging alone is not a cause of anemia (9), it has been proposed that either a reduction in hematopoietic reserve or dysregulation of the factors which modulate hematopoiesis predisposes older individuals to anemia during hematopoietic stress.
The explanation for why anemia is so much more common in the elderly is not fully established. Certainly factors such as iron deficiency and concurrent inflammatory disease are more prominent in the elderly, but one must also factor in the presence of age-associated renal impairment, endocrine insufficiency, nutritional inadequacy and myelodysplasia as potential contributors. For one-third to one-half of anemic elderly, a specific cause for the anemia is not readily apparent (unexplained anemia, or UA) (3, 20) and defining the pathogenesis in these cases has been the focus of much current research. Inflammatory pathways, including hepcidin have been implicated under certain circumstances (18, 19). Bone marrow stem cell proliferative capacity also declines somewhat with age (14, 26, 43, 44), but experiments in laboratory animals suggest that this decline alone would be insufficient to result in anemia (21–23). However, myelodysplasia does increase in frequency with advancing age (41), and in some cases this bone marrow disorder will present as anemia without white blood cell or platelet abnormalities apparent on the peripheral blood smear. Thus, some cases of UA may ultimately be attributed to MDS, although it is unclear how large this component is. Thus, it is likely that the one-third or more of elderly anemic subjects for whom a distinct explanation for their anemia is not apparent, are likely to have a composite of several contributing factors.
Over the past 10 years several studies have defined the untoward consequences of anemia in the elderly. These include increased risk of falls (31, 36), weakness (33, 34) and immobility (10). The InCHIANTI study, based on data from a population-base of 1156 individuals aged 65 and older from the Chianti region of Italy, demonstrated that anemia was associated with disability and decreased physical performance using handgrip and knee-extensor strength tests (8, 34). Furthermore, longitudinal studies have demonstrated increased mortality among individuals with even mild anemia (11, 15, 25). A recent retrospective cohort study of VA National Surgical Quality Improvement database, indicated that of 310,311 subjects 65 years and older who underwent non-cardiac surgery, the 30 day mortality and cardiac event rates increased by 1.6% for each 1% change in hematocrit below the level of 39% (54). Thus, although in younger individuals, mild anemia may be well tolerated, in older individuals it is associated with important negative consequences.
Although previous studies focused upon older patients have demonstrated the importance of anemia on these clinical and functional outcomes, health-related quality of life had not been comprehensively evaluated. Accordingly, we embarked on this study to evaluate the association of anemia with measures of health-related quality of life and functional status.
METHODS
Study Design
We performed a multicenter, cross-sectional survey of older patients cared for in an out-patient setting. All study procedures were approved by the institutional review board of each center, and all subjects provided informed consent.
Subjects were enrolled in one of three institutions in the United States; the Institute for Advanced Studies in Aging and Geriatric Medicine, Washington, D.C.; Sarasota Hospital, Sarasota, Florida; and the University of Utah School of Medicine, Salt Lake City, Utah. Total study time was one year from identification of the population to the completion of study of the last subject.
Patient Characteristics
Subjects provided consent to have their medical records reviewed. Data abstracted from charts included: date of birth, sex, eligibility criteria, and medical history pertaining to study criteria. Age was calculated from date of birth to date of enrollment. Height and weight were directly measured. Body mass index (BMI) was estimated by weight (kilograms [kg]) divided by height (meters) squared. Diagnoses and racial category were recorded from the chart history and patient report including prescribed medication for disease management. The presence or absence of specific conditions was also recorded including diabetes mellitus, rheumatoid arthritis, hypertension, or other chronic inflammatory conditions. Chronic inflammatory conditions include cardiovascular (class III or IV New York Heart Association congestive heart failure, myocarditis, pericarditis, vasculitis), any acute or chronic infection, respiratory (hypersensitivity pneumonitis, eosinophilic pneumonitis, bronchiectasis, chronic bronchitis, pleuritis), renal (chronic renal insufficiency [creatinine > 1.5 mg/dL in males, > 1.2 mg/dL in females], glomerulonephritis, acute interstitial nephritis), gastrointestinal (esophagitis, inflammatory bowel disease [Crohn’s disease, ulcerative colitis], diverticulitis, appendicitis, hepatitis, acute pancreatitis, cholecystitis), immune-mediated (lupus, scleroderma, Sjögren’s syndrome, ankylosing spondylitis, Reiter’s disease, psoriatic arthritis, Bechet’s syndrome, dermatomyositis, polymyositis, myositis), or other such conditions. Smoking history was categorized into three groups, current (presently smoking), former (smoking a minimum of 100 cigarettes in a lifetime but not presently), and never (no history of smoking).
Subjects were enrolled if they were 65 years and older, had no previous diagnosis of cancer (other than basal cell carcinoma of the skin), underlying blood disorder (e.g., myelodysplasia), end stage renal failure (or prior renal transplant), or the recent (within 3 months) recipient of blood transfusion or erythropoietin therapy. Subjects were required to be able to read and speak English in order to complete the patient self-assessment questionnaires. In total, 333 participants were enrolled in the study. Of these, five subjects had missing hemoglobin (Hb) data and were therefore excluded from the analysis. We present data on 328 subjects.
Health-related Quality of Life and Functional Status Outcomes
We used performance-based assessments along with patient self-reports to comprehensively evaluate health status and the effects of anemia. The quality of life was based on two instruments: the Short Form-36 Health Survey (SF-36) (48, 50) and the Functional Assessment of Chronic Illness Therapy-Anemia (FACIT-An) (5).
The SF-36 questionnaire is a validated, generic health-related quality of life instrument that consists of eight health concept domains: physical functioning (PF), role limitations due to physical health problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and mental health (MH). From these individual subscales, two overall scores were generated: physical health component (PCS) and mental health component (MCS). The first five subscales (PF, RP, BP, GH, VT) produce the PCS and the last five subscales (GH, VT, SF, RE, MH) produce the MCS; the GH and VT subscales overlap between the two overall components. The scoring for the eight domains and the two overall components are based on a 0 to 100 scale, with a higher value corresponding to a better quality of life. The SF-36 questionnaire has been extensively validated in numerous populations (27, 29, 32).
The FACIT-An questionnaire is specifically designed for anemia of chronic illness (5, 7, 55). The FACIT-An includes two subscales; fatigue and non-fatigue, with 13 and 7 questions, respectively. The overall anemia score is generated by combining the scores from all 20 questions. For the fatigue subscale, the minimum possible score is 0 and the maximum is 52; for the non-fatigue subscale, the range is 0 to 28. Hence, the minimum overall anemia score is 0 and the maximum is 80. A higher score corresponds to a better state of health and functioning. The FACIT scales have been shown to be valid and reliable (55).
Disability, Depression, and Strength Outcomes
Disability in basic activities of daily living was assessed by the Instrumental Activities of Daily Living (IADL) questionnaire (28). The instrument consists of 13 questions; six questions about the respondent's ability to perform basic activities of daily living (eating, bathing, dressing, using the toilet, personal grooming, walking, and getting in and out of bed), and seven questions about instrumental activities of daily living (preparing meals, using the telephone, shopping, doing housework, taking medications, and handling one’s own money) (28). The possible responses to each question are that the activity can be performed without help, with some help, or that the respondent is completely unable to perform the activity (complete help needed). Disability for a particular activity was defined as needing either partial or complete help.
Depression was assessed using the Geriatric Depression Scale (GDS) questionnaire (56, 57). The GDS was designed to measure the symptoms of depression among elderly subjects, and has been validated extensively in psychiatric settings. The questionnaire consists of 15 questions evaluating outlook and satisfaction with life, energy and activity levels, and memory. For each of the questions, the subject is asked how they felt over the past week. The possible responses to each question are 'yes' or 'no' (e.g. Are you basically satisfied with your life?). For each subject, the total number of answers indicating depression was computed to generate the GDS score. Although differing sensitivities and specificities have been obtained across studies, for clinical purposes a score greater than five points suggests a diagnosis of depression (57).
The study also quantified physical performance through handgrip strength with a handheld dynamometer (in kg), using the mean value after performing the task three times. Previous studies demonstrated that handgrip strength has predictive validity for subsequent disability and mortality in older women (37).
Laboratory Analysis
Laboratory analysis from blood included Hb, hematocrit, white blood cell count, platelet count, red blood cell indices, serum iron, total iron binding capacity, erythropoietin, vitamin B12, and serum creatinine. All laboratory tests were performed centrally by LabCorp (Raritan, New Jersey). Questionnaires and measurements were performed within one month of serum Hb measurement.
Anemia was defined by Hb levels below 13 g/dL for men and below 12 g/dL for women, according to World Health Organization (WHO) criteria (53). The WHO definition was previously shown to be suitable for defining anemia in older persons (20, 25).
Statistical Analyses
Statistical analyses were performed using S-PLUS (version 6.2) and SAS (version 8) statistical software.
The primary objective of this analysis was to determine the independent influence of anemia on health related quality of life. For univariate comparisons, t-tests were used for continuous variables and chi-square tests were used for categorical variables. To ascertain the influence of Hb concentration on other health status outcomes, we used analysis of variance to assess the effect of Hb on outcomes variables. Kernel density estimation was used to explore the functional relationship of Hb concentration on outcomes measures (46).
In subsequent analyses, Hb was categorized by increments of 1 g/dL: < 12, 12 to 12.9, 13 to 13.9, 14 to 14.9 and ≥ 15 g/dL. In order to adjust for potential confounding variables we used analysis of covariance (ANCOVA) with Hb category as a main effect while adjusting for age, sex, race, and comorbid conditions (diabetes mellitus, rheumatoid arthritis, hypertension, and chronic inflammatory conditions). Main effects were considered statistically significant if the p-value was less than 0.05. We also investigated whether interaction effects (Hb with age [> 75 years] or sex) were present using a significance level of 0.10. If main effects were statistically significant, pairwise t-tests were calculated using the Hb ≥ 15 g/dL as the reference group. We tested for linear trends using ordinal Hb scores across the Hb categories.
US population norms for the SF-36 are available for gender and age matched cohorts (47). We created a binary variable for each subject that indicated if their quality of life score was above or below the US population norm for their age and gender matched sample. Logistic regression analysis was used to determine the independent effect of low Hb on the likelihood of subjects having quality of life worse than their peers. The multivariate logistic regression model controlled for race, presence of rheumatoid arthritis, diabetes, hypertension, or chronic inflammation.
RESULTS
Subject Characteristics
Table 1 shows the characteristics of study subjects stratified by Hb categories. Sixty-four percent of the 328 subjects were women. The mean (standard deviation [SD]) age was 76.8 (7.1) years, ranging from 65 to 103 years. The mean Hb was 13.2 (1.5) g/dL, with mean values of 12.9 g/dL for women and 13.8 g/dL for men (P < 0.001). Subjects 75 years and older tended to have lower Hb values with a mean of 12.8 g/dL compared to the 13.3 g/dL of the 65 to 74 year old cohort (P < 0.001). Ninety (27%) subjects met the WHO criteria for anemia; 54 (26%) women and 36 (31%) men.
Table 1.
Subject Characteristics by Hb Levels
| Distribution, n (%) by Hb Categories (g/dL) | ||||||
|---|---|---|---|---|---|---|
| < 12 | 12.0–12.9 | 13.0–13.9 | 14.0–14.9 | ≥15 | Total | |
| Characteristic | N=68 (20.7) |
N=75 (22.9) |
N=81 (24.7) |
N=63 (19.2) |
N=41 (12.5) |
N=328 |
| Sex | ||||||
| Female | 54 (79.4) | 53 (70.7) | 56 (69.1) | 37 (58.7) | 11 (26.8) | 211 (64.3) |
| Male | 14 (20.6) | 22 (29.3) | 25 (30.9) | 26 (41.3) | 30 (73.2) | 117 (35.7) |
| Age | ||||||
| < 75 years | 18 (26.5) | 31 (41.3) | 31 (38.3) | 35 (55.6) | 21 (51.2) | 136 (41.5) |
| ≥ 75 years | 50 (73.5) | 44 (58.7) | 50 (61.7) | 28 (44.4) | 20 (48.8) | 192 (58.5) |
| Race, n (%) | ||||||
| African American |
2 (2.9) | 3 (4.0) | 1 (1.2) | 0 (0) | 0 (0) | 6 (1.8) |
| Caucasian | 62 (91.2) | 68 (90.7) | 76 (93.8) | 59 (93.7) | 38 (92.7) | 303 (92.3) |
| Other | 4 (5.9) | 4 (5.3) | 4 (4.9) | 4 (6.4) | 3 (7.3) | 19 (5.8) |
|
Coexisting Conditions |
||||||
| Diabetes | 13 (19.1) | 8 (10.7) | 16 (19.8) | 8 (12.7) | 2 (4.9) | 47 (14.3) |
| Rheumatoid Arthritis |
17 (25.0) | 18 (24.0) | 7 (8.6) | 6 (9.5) | 3 (7.3) | 51 (15.5) |
| Hypertension | 52 (76.5) | 40 (53.3) | 53 (65.4) | 32 (50.8) | 24 (58.5) | 201 (61.2) |
| Chronic Inflammation |
31 (45.6) | 26 (34.7) | 32 (39.5) | 30 (47.6) | 23 (56.1) | 142 (43.3) |
| Smoking | ||||||
| Yes (≥ 100 cigarettes) |
30 (44.1) | 33 (44.0) | 39 (48.2) | 38 (60.3) | 28 (68.3) | 168 (51.2) |
The mean (SD) BMI was 27.6 (6.2) kg/m2. The racial distribution was 92% Caucasian, 2% African-American, and 6% who reported ‘Other.’ Five percent were current smokers, 46% were former smokers, and 49% were never smokers. The following chronic disease states had been diagnosed at the time of enrollment: diabetes (14%), rheumatoid arthritis (16%), hypertension (61%), and chronic inflammatory conditions (43%). One hundred thirty-two subjects (40%) reported only one, 126 (38%) reported two, 15 (4%) reported three, 3 (1%) reported all four disease states.
Compared to the subjects without anemia, subjects with anemia were older on average (P < 0.001), and were more likely to report hypertension (odds ratio [OR] = 2.1; P = 0.006) and twice as likely to report hospitalizations in the past year (OR = 2.1; P = 0.016). Those with anemia reported more chronic health conditions than subjects without anemia (17% increase; P = 0.003).
Quality of Life and Functional Status
Univariate Analysis (Unadjusted)
The unadjusted mean SF-36 physical health component scores was 47.1 for subjects with Hb of 15 g/dL and higher, and 37.3 for subjects with Hb below 12 g/dL (P < 0.01) (Table 2). This decreasing trend in PCS was apparent across the Hb categories (P for trend < 0.001), and was also evident among the five physical function subscales. Trends of the association between Hb level and quality of life scores were also significant for the overall FACIT-An score (P for trend < 0.001) and its fatigue component (P for trend < 0.001), as well as for the GDS (P for trend < 0.001), IADL (P for trend < 0.001), and handgrip strength (P for trend < 0.001).
Table 2.
Unadjusted Means of Quality of Life Assessments by Hb Levels
| Mean (SD) by Hb Categories (g/dL) | ||||||
|---|---|---|---|---|---|---|
| < 12 | 12.0–12.9 | 13.0–13.9 | 14.0–14.9 | ≥15 |
P Value for trend |
|
| Endpoint | N=68 | N=75 | N=81 | N=63 | N=41 | |
|
SF-36‡ Summary Scores |
||||||
| PCS§ | 37.3(1.1)† | 42.2(1.1)† | 43.4(1.0)* | 45.2(1.1) | 47.1(1.4) | < 0.001 |
| MCS∥ | 51.1(1.2)† | 53.9(1.1) | 53.9(1.1) | 52.9(1.2)* | 57.2(1.5) | 0.014 |
|
SF-36‡ Subscales |
||||||
| Physical Functioning |
46.2(3.2)† | 62.2(3.1) | 61.8(2.9) | 69.0(3.3) | 69.8(4.1) | < 0.001 |
| Role Physical |
42.4(4.8)† | 55.5(4.6)† | 63.1(4.4)* | 64.7(5.0)* | 80.7(6.2) | < 0.001 |
| Body Pain | 55.9(2.8)† | 64.8(2.7)† | 67.2(2.6)* | 66.9(2.9)* | 77.7(3.6) | < 0.001 |
| General Health |
56.1(2.4)† | 68.0(2.3)† | 66.9(2.2)† | 71.2(2.5) | 78.9(3.1) | < 0.001 |
| Vitality | 47.9(2.7)† | 58.5(2.6)* | 54.5(2.5)† | 58.1(2.8)* | 69.3(3.5) | < 0.001 |
| Social Functioning |
74.8(2.8)† | 82.8(2.7)* | 84.3(2.5) | 85.7(2.9) | 92.4(3.6) | < 0.001 |
| Role Emotional |
65.2(4.2)† | 70.3(4.0)* | 83.5(3.8) | 83.1(4.3) | 86.2(5.4) | < 0.001 |
| Mental Health |
73.7(2.1)† | 81.3(2.1) | 78.2(2.0)* | 75.7(2.2)† | 86.7(2.8) | 0.026 |
|
FACIT¶ Scores |
||||||
| Anemia | 45.2(1.1)† | 48.1(1.1)* | 47.8(1.0)† | 49.1(1.1) | 52.4(1.4) | < 0.001 |
| Fatigue | 34.6(1.2)† | 38.2(1.1)* | 38.2(1.1)* | 39.1(1.2) | 42.3(1.5) | < 0.001 |
| Non-fatigue | 22.3(0.4) | 22.6(0.4) | 21.9(0.4) | 22.3(0.4) | 23.0(0.5) | 0.622 |
| GDS# | 11.4(0.3)† | 12.9(0.3) | 12.6(0.3)* | 12.8(0.4) | 13.8(0.4) | < 0.001 |
| IADL** | 2.3(0.3)† | 1.1(0.3) | 1.1(0.2) | 1.2(0.3) | 0.3(0.3) | < 0.001 |
|
Handgrip strength,kg |
19.4(1.1)† | 19.9(1.1)† | 22.8(1.0)† | 26.6(1.2)† | 32.4(1.5) | < 0.001 |
P < 0.05, comparison versus Hb ≥ 15 g/dL ;
P < 0.01, comparison versus Hb ≥ 15 g/dL ;
Short Form 36 Health Survey ;
Physical Health Component ;
Mental Health Component ;
Functional Assessment of Chronic Illness Therapy ;
Geriatric Depression Scale ;
Instrumental Activities of Daily Living
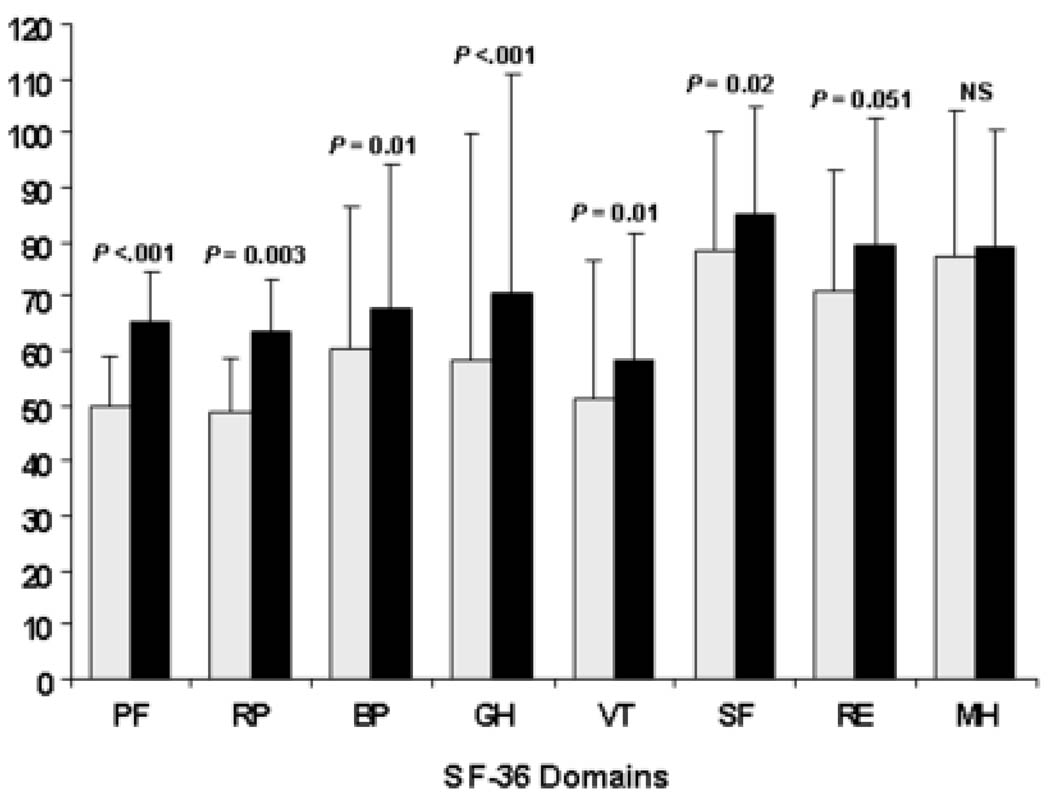
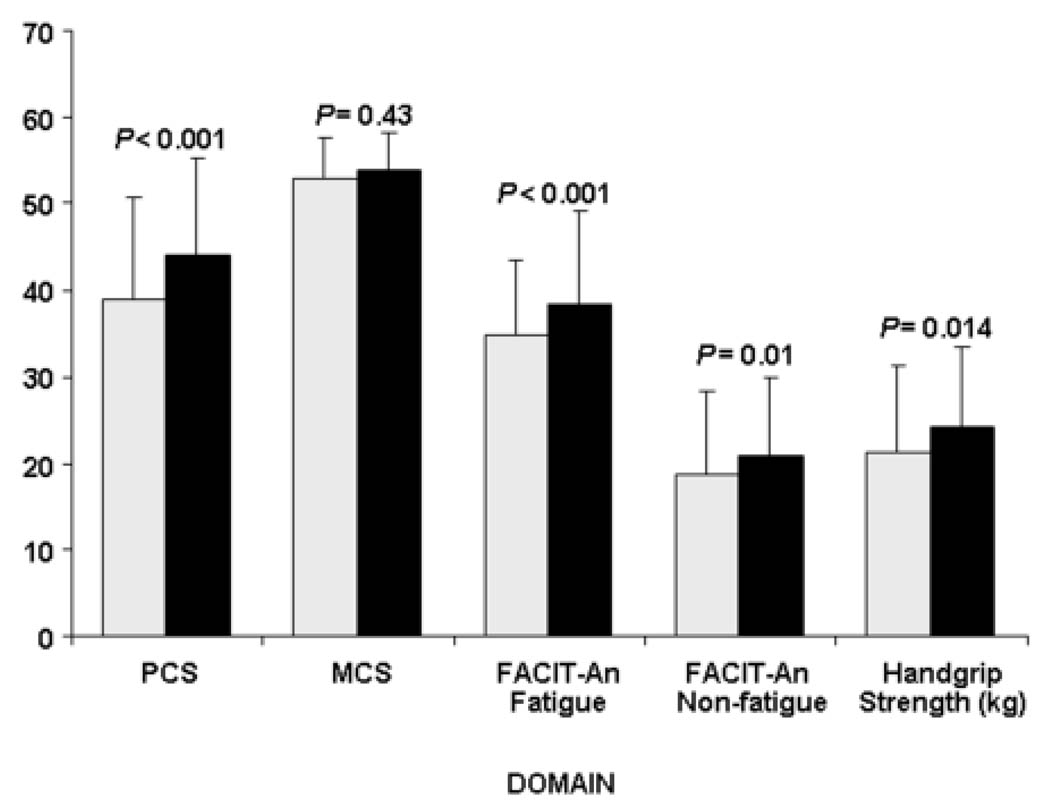
The unadjusted mean SF-36 PCS was 38.9 and 44.1 (P < 0.001) for subjects with and without anemia. Significant differences were also observed for the five underlying subscales (Figure 1). Significant differences between subjects with anemia and subjects without anemia were found for all the FACIT scales: the overall (P = 0.004), non-fatigue (P = 0.01), and fatigue (P < 0.001) scales (Figure 2). The mean (SD) number of disabilities was 2.0 (2.9) activities for subjects with anemia and 1.0 (1.9) for subjects without anemia (P = 0.005). Fifty-seven (63%) subjects with anemia and 101 (43%) subjects without anemia indicated at least one disability (P < 0.001). Fifty-three (59%) subjects with anemia and 103 (44%) of the subjects without anemia indicated depression in at least two symptoms by GDS (P = 0.002). The mean (SD) number of symptoms indicating depression was 3.1 (3.1) for subjects with anemia and 2.0 (2.7) for subjects without anemia (P = 0.002). Subjects with anemia had a significantly lower handgrip strength than subjects without anemia (21.3 vs. 24.1 kg; P = 0.014) (Figure 2).
Figure 1.
Comparison of individual Short Form 36 Health Survey (SF-36) scores by anemia status. White bars represent mean SF-36 domain scores for subjects with anemia, filled-in bars represent mean the SF-36 domain scores for subjects without anemia. Physical functioning (PF), role limitations due to physical health problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and mental health (MH) domains are shown. Lines above bars represent one positive standard deviation. Statistical significance (P values) based on t-tests are shown above each domain comparison (NS = not significant).
Figure 2.
Comparison of Short Form 36 Health Survey (SF-36) summary scores, Functional Assessment of Chronic Illness Therapy – Anemia (FACIT-An) scores and handgrip strength by anemia status. White bars represent SF-36, FACIT-An scores and handgrip strength for subjects with anemia, filled-in bars represent SF-36, FACIT-An scores and handgrip strength for subjects without anemia. The SF-36 physical component (PCS) and mental health component (MCS) scores are shown. FACIT fatigue and non-fatigue scores are shown. The overall FACIT-An score is the sum of the fatigue and non-fatigue scores. Mean values are shown in the bars. Lines above bars represent one positive standard deviation. Statistical significance (P values) based on t-tests are shown above each comparison between anemia and no anemia groups.
Multivariate Analysis (Adjusted)
Multivariate regression analysis adjusted for age, sex, weight (for handgrip strength only) and presence of diabetes, hypertension, chronic inflammation, or rheumatoid arthritis, confirmed strong associations with declining Hb and poorer health-related quality of life (Table 3). The adjusted mean loss of handgrip strength was 4.0 kg from the highest Hb category (Hb > 15 g/dL) to the lowest (Hb < 12 g/dL) (P < 0.01). Hb effects on handgrip strength were evident for Hb levels below 14 g/dL (P < 0.05). Some SF-36 measures (GH, VT, and MH) showed significantly lower adjusted scores for Hb below 15 g/dL, when compared to subjects with Hb above 15 g/dL; for example, the mental health scores dropped approximately ten points for subjects with mean Hb below 15 g/dL (P < 0.01). Lower Hb levels were associated with an adjusted decrease of more than six points in the PCS (P for trend = 0.002).
Table 3.
Adjusted Means‡ of Quality of Life Assessments by Hb Levels
| Mean (SD) by Hb Categories (g/dL) | ||||||
|---|---|---|---|---|---|---|
| < 12 | 12.0–12.9 | 13.0–13.9 | 14.0–14.9 | ≥15 |
P Value for trend |
|
| Endpoint | N=68 | N=75 | N=81 | N=63 | N=41 | |
|
SF-36§ Summary Scores |
||||||
| PCS∥ | 39.2(1.1)† | 42.3(1.0) | 43.7(1.0) | 44.3(1.1) | 45.6(1.4) | 0.002 |
| MCS¶ | 51.6(1.2)* | 53.4(1.1) | 54.1(1.1) | 52.8(1.2) | 56.1(1.5) | 0.077 |
|
SF-36§ Subscales |
||||||
| Physical Functioning |
51.4(3.3)† | 62.2(3.0) | 63.2(2.9) | 66.9(3.2) | 66.6(4.2) | 0.002 |
| Role Physical |
48.9(5.0)† | 56.2(4.6)† | 64.2(4.4) | 61.7(5.0) | 77.2(6.4) | 0.001 |
| Body Pain | 59.3(2.9)† | 64.9(2.7) | 67.2(2.5) | 65.1(2.8) | 73.4(3.7) | 0.011 |
| General Health |
58.3(2.4)† | 66.6(2.3)† | 67.0(2.1)† | 70.1(2.4)* | 78.7(3.1) | < 0.001 |
| Vitality | 50.6(2.8)† | 57.1(2.6)* | 55.2(2.5)† | 57.1(2.8)* | 66.7(3.6) | 0.005 |
| Social Functioning |
76.5(2.9)† | 82.2(2.7) | 84.5(2.6) | 84.9(2.9) | 90.5(3.7) | 0.005 |
| Role Emotional |
70.1(4.4) | 70.6(4.0) | 85.3(3.8) | 81.2(4.3) | 80.2(5.5) | 0.022 |
| Mental Health |
74.1(2.2)† | 80.0(2.1) | 78.5(2.0) | 75.7(2.2)† | 85.3(2.8) | 0.070 |
|
FACIT# Scores |
||||||
| Anemia | 46.4(1.1)† | 47.8(1.0)* | 48.0(1.0) | 48.5(1.1) | 51.3(1.4) | 0.017 |
| Fatigue | 35.8(1.2)† | 37.9(1.1) | 38.4(1.1) | 38.5(1.2) | 41.1(1.5) | 0.015 |
| Non-fatigue | 22.5(0.4) | 22.3(0.4) | 21.9(0.4) | 22.3(0.4) | 23.0(0.5) | 0.699 |
| GDS** | 11.6(0.4)† | 12.9(0.3) | 12.6(0.3) | 12.7(0.4) | 13.6(0.5) | 0.005 |
| IADL†† | 2.0(0.3)† | 1.1(0.3) | 1.0(0.2) | 1.3(0.3) | 0.6(0.4) | 0.012 |
|
Handgrip strength, kg |
23.0(0.8)† | 21.4(0.8)† | 23.7(0.7)* | 25.5(0.8) | 27.0(1.1) | < 0.001 |
P < 0.05, comparison versus Hb ≥ 15 g/dL ;
P < 0.01, comparison versus Hb ≥ 15 g/dL ;
ANCOVA models included age, sex, race and comorbid conditions (diabetes mellitus, rheumatoid arthritis, hypertension,and chronic inflammatory conditions);
Short Form 36 Health Survey ;
Physical Health Component;
Mental Health Component;
Functional Assessment of Chronic Illness Therapy;
Geriatric Depression Scale;
Instrumental Activities of Daily Living
Population-Based Comparisons
The independent association of lower Hb levels with lower quality of life measures is supported by comparison of PCS and MCS using population norms (30) (data not shown). Subjects with Hb below 13 g/dL were between 2.5 to 3.5 times (depending on their Hb category) more likely to report a PCS lower than their age and gender matched peers, as compared to subjects with Hb above 15 g/dL Subjects with Hb below 13 g/dL were approximately 3.5 times more likely to report a lower MCS for the SF-36 than their age and sex matched peers.
DISCUSSION
This observational study examined the correlation of hemoglobin and quality of life among community based elderly subjects. We determined not only that anemia was associated with a lower quality of life, but that a trend for reduced quality of life began for Hb levels as they fell below 15 g/dL. Our analyses showed that anemia, independent of chronic disease, represents a risk factor for depression and disability. Furthermore, we observed a significant association of anemia with declines in health-related quality of life, functional status, and physical strength.
Anemia is commonly defined according to medical discipline. For example, the National Kidney Foundation defines anemia as Hb levels below 12 g/dL in adult males and post-menopausal females (1), whereas cancer and chemotherapy-associated anemia is defined by the National Comprehensive Cancer Network by a Hb level of less than 11 g/dL (40). In this study, we defined anemia in accordance with WHO criteria of Hb levels less than 13 g/dL for men and less than 12 g/dL for women (53). This definition establishes a high threshold for the anemia diagnosis, and therefore could result in the inclusion of asymptomatic individuals in the anemia group. This concern notwithstanding, we defined two groups (anemic and not anemic) with an overall difference in mean Hb of 2.3 g/dL and found quality of life was significantly lower for those in the anemia group.
In an attempt to provide a more comprehensive description of the effects of anemia on quality of life, data were stratified by Hb levels. Further analysis disclosed that quality of life measures progressively declined in groups with lower Hb concentrations, even for Hb levels higher than the WHO definition for anemia. Even after adjustment for demographic differences and comorbid conditions, SF-36 measures of vitality, general health, and mental health showed a significant decline for subjects with mean Hb levels below 15 g/dL. The handgrip strength assessment revealed that significant reductions in grip strength were evident even for Hb concentrations below 14 g/dL. These findings, combined with prior data indicating inferior survival for reduced hemoglobin concentration even in the non-anemic range, suggest that Hb concentrations traditionally considered normal, may have untoward consequences for the elderly with respect to health-related quality of life and functional performance (11, 25).
The physical symptoms associated with anemia are possibly explained by reduced exercise tolerance (35). Affected individuals often experience fatigue and may be consequently less active, with a sedentary lifestyle resulting in muscle wasting (sarcopenia), falls and diminished cardiovascular fitness that further reduce exercise tolerance. Poor muscle oxygenation and reduced physical strength associated with lower Hb levels may also affect quality of life and functional outcomes. In this study, handgrip strength was used a measure of physical strength. After adjusting for differences in weight, the anemia-related decline in handgrip strength of 1.7 kg was equivalent to aging approximately four years according to the multivariate linear regression model (data not shown). The effect of anemia on physical strength was particularly significant for men. Anemia has been shown to be more prevalent in men than in women beyond the age of 70 (20). This may be the result of declining testosterone levels (andropause) in this age group, as hypogonadal levels are associated with modest (0.5 to 0.8 g/dL) declines in Hb level (16, 52).
The SF-36 is perhaps the most widely used instrument for measuring health-related quality of life. The decrease in physical component scores associated with anemia is consistent with individuals who have greater limitations in self-care, physical, social, and role activities, body pain, frequent tiredness, and who are more likely to rate their overall health as “poor” (49). In our study, anemia and lower Hb levels were associated with clinically meaningful differences in SF-36 scores. The SF-36 physical component score effect size for individuals with anemia is similar to the PCS measured for individuals with myocardial infarction in the past 12 months or chronic obstructive pulmonary disease in other studies (12, 32, 45, 49, 51). Subjects with Hb levels below 12 g/dL were 3.5 times more likely to have a PCS measured lower than their age and gender matched peers in the US population (47), when compared with individuals with Hb above 15 g/dL.
Penninx and colleagues reported on the impact of anemia on physical function in two distinct cohorts of elderly individuals (33–35). In these studies, they found that anemia was associated with negative effects on physical performance and strength. Anemia is associated with a three-fold increase in the risk of falls and the risk was comparable to a fall risk for those with arthritis.
Studies have also demonstrated diminished physical function with lower Hb levels that are above the WHO anemic threshold (10, 33). Our study extends those findings by demonstrating a significant decline in physical strength as well as significant levels of disability and diminished quality of life as reported by the elderly.
The present study is limited in a number of ways. Since it is a cross-sectional survey, we were unable to measure longitudinal changes in Hb or long-term implications of persistently low Hb, and because the study population does not adequately include sufficient numbers of minority subjects, the results should not be generalized to all racial groups. Additionally, due to the observational nature of the study, we cannot exclude the possibility of unmeasured confounding influences (i.e. that other factors associated with anemia that we did not measure may have caused the poor clinical outcomes). However, the association of lower Hb levels persisted even after multivariate analysis adjusted for differences in demographic and disease characteristics. While interventional trials may be required to confirm the independent influence of anemia (or anemia correction) in this population, significant data exists in other cohorts demonstrating improved quality of life with pharmacologic interventions aimed at reversing anemia (6, 17, 42), including in geriatric patients (2). In the present study, we utilized validated patient self-administered questionnaires to reduce the possibility of introducing questionnaire-based bias. By using these standardized measures it was also possible to compare the effects of anemia in the elderly with other well-known diseases, and with age and gender matched norms, again confirming the association of lower Hb values and reduced quality of life.
Our findings indicate that anemia is associated with poor health-related quality of life, functional decline, low muscle strength, more disability and more depression. Men may experience greater physical effects of lower Hb, and higher age and the presence of comorbidities corresponded with poorer quality of life and functional performance. Moreover, we demonstrated that adverse outcomes in the elderly occur at higher Hb levels than the anemia thresholds established by the WHO. The downward spiral of reduced exercise capacity resulting from anemia may ultimately contribute to both dependency and depression, both of which are components of the frailty syndrome (39).
Acknowledgments
The authors wish to thank Miriam Beattie, RN, GNP for her contributions to the project. This work was supported in part by Amgen Inc. William Ershler is supported by the Intramural Research Program, National Institute on Aging. Gerald Rothstein received support from the Val A. Browning Foundation.
This work was supported in part by a grant from Amgen, Inc. William Ershler is supported by the Intramural Research Program, National Institute on Aging. Gerald Rothstein received support from the Val A. Browning Foundation.
Abbreviations
- SF-36
Short Form-36 Health Survey
- FACIT-AN
Functional Assessment of Chronic Illness Therapy-Anemia
- GDS
Geriatric Depression Scale
- IADL
Instrumental Activities of Daily Living
- UA
Unexplained Anemia
- Hb
hemoglobin
- SF-36
Domains
- PF
physical functioning
- RP
role limitations due to physical health problems
- BP
bodily pain
- GH
general health
- VT
vitality
- SF
social functioning
- RE
role limitations due to emotional problems
- MH
mental health
- PCS
physical health component score
- MCS
mental health component score.
References
- 1.IV. NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: update 2000. Am J Kidney Dis. 2001;37(1 Suppl 1):S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 2.Agnihotri P, Telfer M, Butt Z, Jella A, Cella D, Kozma CM, Ahuja M, Riaz S, Akamah J. Chronic anemia and fatigue in elderly patients: results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc. 2007;55(10):1557–1565. doi: 10.1111/j.1532-5415.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 3.Artz AS, Fergusson D, Drinka PJ, Gerald M, Bidenbender R, Lechich A, Silverstone F, McCamish MA, Dai J, Keller E, Ershler WB. Mechanisms of unexplained anemia in the nursing home. J Am Geriatr Soc. 2004;52(3):423–427. doi: 10.1111/j.1532-5415.2004.52116.x. [DOI] [PubMed] [Google Scholar]
- 4.Artz AS, Fergusson D, Drinka PJ, Gerald M, Gravenstein S, Lechich A, Silverstone F, Finnigan S, Janowski MC, McCamish MA, Ershler WB. Prevalence of anemia in skilled-nursing home residents. Arch Gerontol Geriatr. 2004;39(3):201–206. doi: 10.1016/j.archger.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–19. [PubMed] [Google Scholar]
- 6.Cella D, Dobrez D, Glaspy J. Control of cancer-related anemia with erythropoietic agents: a review of evidence for improved quality of life and clinical outcomes. Ann Oncol. 2003;14(4):511–519. doi: 10.1093/annonc/mdg167. [DOI] [PubMed] [Google Scholar]
- 7.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 8.Cesari M, Penninx BW, Lauretani F, Russo CR, Carter C, Bandinelli S, Atkinson H, Onder G, Pahor M, Ferrucci L. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):249–254. doi: 10.1093/gerona/59.3.m249. [DOI] [PubMed] [Google Scholar]
- 9.Chatta GS, Lipschitz D. Anemia. In: Hazzard WR, Blass JP, Ettinger WH, Halter JB, Ouslander JG, editors. Principles of Geriatric Medicine and Gerontology. 4th ed. Vol. New York: McGraw-Hill; 1999. pp. 899–906. [Google Scholar]
- 10.Chaves PH, Ashar B, Guralnik JM, Fried LP. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc. 2002;50(7):1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 11.Chaves PH, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52(11):1811–1816. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 12.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 13.Day JC. Population projections of the United States by age, sex, race and Hispanic origin: 1995–2050. Washington D.C: Current Population Reports: U.S. Government Printing Office; U.S. Bureau of the Census. 1996:25–1130.
- 14.Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, Donehower LA. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109(4):1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107(2):223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 16.Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, Valenti G, Ershler WB, Guralnik JM, Longo DL. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166(13):1380–1388. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fishbane S. Anemia treatment in chronic renal insufficiency. Semin Nephrol. 2002;22(6):474–478. doi: 10.1053/snep.2002.35963. [DOI] [PubMed] [Google Scholar]
- 18.Ganz T. Hepcidin--a peptide hormone at the interface of innate immunity and iron metabolism. Curr Top Microbiol Immunol. 2006;306:183–198. doi: 10.1007/3-540-29916-5_7. [DOI] [PubMed] [Google Scholar]
- 19.Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G199–G203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 21.Harrison DE. Long-term erythropoietic repopulating ability of old, young, and fetal stem cells. J Exp Med. 1983;157(5):1496–1504. doi: 10.1084/jem.157.5.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison DE, Astle CM, Delaittre JA. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med. 1978;147(5):1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison DE, Astle CM, Stone M. Numbers and functions of transplantable primitive immunohematopoietic stem cells. Effects of age. J Immunol. 1989;142(11):3833–3840. [PubMed] [Google Scholar]
- 24.Hetzel L, Smith A. The 65 years and over population: 2000. Census 2000 Brief. 2001:1–7. [Google Scholar]
- 25.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. Jama. 1999;281(18):1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 26.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443(7110):421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 27.Kantz ME, Harris WJ, Levitsky K, Ware JE, Jr, Davies AR. Methods for assessing condition-specific and generic functional status outcomes after total knee replacement. Med Care. 1992;30(5 Suppl):MS240–MS252. doi: 10.1097/00005650-199205001-00024. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. the Index of Adl: a Standardized Measure of Biological and Psychosocial Function. Jama. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Krousel-Wood MA, Re RN. Health status assessment in a hypertension section of an internal medicine clinic. Am J Med Sci. 1994;308(4):211–217. doi: 10.1097/00000441-199430840-00001. [DOI] [PubMed] [Google Scholar]
- 30.Landi F, Russo A, Danese P, Liperoti R, Barillaro C, Bernabei R, Onder G. Anemia status, hemoglobin concentration, and mortality in nursing home older residents. J Am Med Dir Assoc. 2007;8(5):322–327. doi: 10.1016/j.jamda.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. Bmj. 2003;327(7417):712–717. doi: 10.1136/bmj.327.7417.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115(2):104–110. doi: 10.1016/s0002-9343(03)00263-8. [DOI] [PubMed] [Google Scholar]
- 34.Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, Nevitt M, Visser M, Harris T, Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52(7):1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 35.Penninx BW, Pahor M, Cesari M, Corsi AM, Woodman RC, Bandinelli S, Guralnik JM, Ferrucci L. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52(5):719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 36.Penninx BW, Pluijm SM, Lips P, Woodman R, Miedema K, Guralnik JM, Deeg DJ. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005;53(12):2106–2111. doi: 10.1111/j.1532-5415.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 37.Rantanen T, Guralnik JM, Ferrucci L, Penninx BW, Leveille S, Sipila S, Fried LP. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc. 2001;49(1):21–27. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- 38.Robinson B, Artz AS, Culleton B, Critchlow C, Sciarra A, Audhya P. Prevalence of anemia in the nursing home: contribution of chronic kidney disease. J Am Geriatr Soc. 2007;55(10):1566–1570. doi: 10.1111/j.1532-5415.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- 39.Rockwood K, Mitnitski A. Frailty in Relation to the Accumulation of Deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers GM. Cancer- and chemotherapy-induced anemia. V.I. NCCN. Vol. 2009 http://www.nccn.org/professionals/physician_gls/PDF/anemia.pdf.
- 41.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, Edwards BK, List AF. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 42.Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clin Ther. 2003;25(6):1786–1805. doi: 10.1016/s0149-2918(03)80170-4. [DOI] [PubMed] [Google Scholar]
- 43.Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42(5):385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 45.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15(2):141–155. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 46.Silverman BW. Density Estimation for Statistics and Data Analysis. London: Chapman & Hill; 1986. [Google Scholar]
- 47.Ware JE., Jr U.S. Population Norms. 1998 http://www.SF-36.org/research/sf98norms.pdf.
- 48.Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51(11):903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 49.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–AS279. [PubMed] [Google Scholar]
- 50.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 51.Ware JE, Jr, Snow KK, Kosinski M. Health Survey Manual and Interpretation Guide. Boston: 1993. [Google Scholar]
- 52.Weber JP, Walsh PC, Peters CA, Spivak JL. Effect of reversible androgen deprivation on hemoglobin and serum immunoreactive erythropoietin in men. Am J Hematol. 1991;36(3):190–194. doi: 10.1002/ajh.2830360306. [DOI] [PubMed] [Google Scholar]
- 53.WHO Scientific Group. Nutritional anemias. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed]
- 54.Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297(22):2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 55.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 56.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 57.Yesavge JA. The Geriatric Depression Scale (GDS) Short Form http://www.stanford.edu/~yesavage/GDS.html.