Abstract
Magnetic nanoparticles (MNPs) have attracted enormous research attention due to their unique magnetic properties that enable the detection by the non-invasive medical imaging modality—magnetic resonance imaging (MRI). By incorporating advanced features, such as specific targeting, multimodality, therapeutic delivery, the detectability and applicability of MNPs have been dramatically expanded. A delicate design on structure, composition and surface chemistry is essential to achieving desired properties in MNP systems, such as high imaging contrast and chemical stability, non-fouling surface, target specificity and/or multimodality. This article presents the design fundamentals on the development of MNP systems, from discussion of material selection for nanoparticle cores and coatings, strategies for chemical synthesis and surface modification and their merits and limitations, to conjugation of special biomolecules for intended functions, and reviews the recent advances in the field.
1. Introduction
The development of magnetic nanoparticles (MNPs) with advanced features has been a major focus of research in nanomedicine.1, 2 The unique magnetic properties of MNPs enable their detectability by magnetic resonance imaging (MRI).3, 4 MRI can provide both morphological and anatomical information with high spatial resolutions and virtually no limit on penetration depth,5, 6 and its detectability can be significantly expanded by using molecular imaging contrast agents such as MNPs.7 The large surface-to-volume ratio of MNPs provides abundant chemically active sites for biomolecule conjugation,8 allowing delicate design and engineering of these MNPs for intended functions such as long-circulating in the bloodstream,2, 9 target-specificity to lesion tissue,9–11 optical detectability,12 and therapeutic delivery.10, 13–15 Combining the merits of these advanced medical imaging modalities with the breakthrough of molecular and cell biology, molecular imaging can visualize, characterize, and quantify biological processes at cellular and molecular levels in a non-invasive manner,16 enabling timely and accurate diagnosis and individualized treatment of devastating disease, such as cancer and cardiovascular disease.17, 18 The operation of MRI is based on the mechanism of nuclear magnetic resonance (NMR) and the relaxation of hydrogen proton spins in an applied magnetic field.5 Two independent processes, longitudinal relaxation (T1-recovery) and transverse relaxation (T2-decay), can be acquired to generate an MR image. The contrast in MR images, which refers to the signal differences between adjacent regions, arises from variations in relaxation time among protons associated with local environment in the tissue. The clinical application of MRI is often hampered by the low contrast between lesion and surrounding healthy tissue in acquired images. MRI contrast agents, with high magnetic moments, shorten the longitudinal and transverse relaxation time of their surrounding protons,19 thus amplifying the signal difference between lesion and healthy tissues. Small molecule contrast agents, such as gadolinium diethylenetriaminepentaacetic acid complex (Gd-DTPA), were the first generation clinical contrast agents,19 followed by iron oxide nanoparticle-based contrast agents initially developed for a liver and spleen imaging.20 Recently, a number of nanoparticle-based contrast agents have been approved for clinical use, including Lumiren® for bowel imaging, Combidex® for lymph node metastases imaging.21–24
A typical MNP system (Fig. 1A) is comprised of a magnetic core that provides contrast enhancement, a polymer shell that renders the stability and biocompatibility, and, frequently, surface-bound or embedded molecules that endows target-specific capability and/or multifunctionality.3, 4, 22 In a multifunctional system, MNPs may be further incorporated with therapeutic payloads (chemotherapeutics or biotherapeutics), serving as both an imaging agent and a therapeutic carrier. Depending on the magnetic property of the core, the MNP can either serve as either T1 or T2 contrast agent. The effectiveness of an MNP contrast agent can be described by its longitudinal relaxivity r1 and transverse relaxivity r2, the rate of relaxation, or the slope of R1 (1/T1) or R2 (1/T2) versus the contrast agent concentration. Superparamagnetic nanoparticles has very large magnetization under applied magnetic field, which can cause large susceptibility difference between the particles and surrounding medium resulting in microscopic magnetic field gradients.2 The microscopic field gradients due to the presence of MNPs will cause pronounced T2-shortening effect, and hence superparamagnetic nanoparticles are typically used to provide negative contrast enhancement using T2-weighted imaging pulse sequences. The T2-shortening ability is dependent directly on the magnet moment of nanoparticles,25 that is determined by the composition, size, shape and surface chemistry of nanoparticles.26–28 However, the negative contrast agents have their drawbacks on T2-weighted MRI. The resulting dark signal from the nanoparticles is often confused with the signals from pathogenic conditions, and the susceptibility artifacts distort the background image around lesions.29, 30 Alternatively, T1 contrast agents are predominantly paramagnetic nanoparticles and generate positive contrast enhancement via T1-weighted sequences. Despite the fact that T1-shortening processes require a close interaction between protons and contrast agents, which can be hindered by the thickness of the coating on the MNPs, several nanoparticle-based T1 contrast agents have been reported recently.13, 29, 31–36
Figure 1.
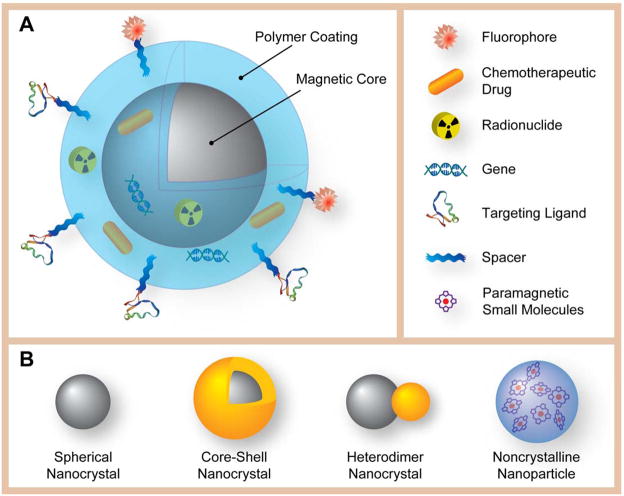
Graphic illustration of the structure of a multifunctional/multimodality MNP and different types of magnetic cores. (A) The structure of a multifunctional/multimodality MNP, with a magnetic core, a polymeric coating, and targeting ligands extended from the surface of MNP with the aid of polymeric spacers. Therapeutic payloads (drugs and genes) and imaging reporters (fluorophores and radionuclides) can be either embedded in the coating, or conjugated on the surface. (B) Schematic representation of different types of magnetic cores: Spherical nanocrystal, core-shell nanocrystal, heterodimer nanocrystal, and non-crystalline nanoparticle.
In the following sections, we will review the recent advances on the development of MNP systems, including the design and fabrication of magnetic cores and coatings, and the further derivations for imaging applications.
2. Building magnetic cores
The majority of MNP systems utilize inorganic nanocrystals as their magnetic cores. The composition of those inorganic nanocrystals ranges from metal, alloy to metal oxides. Noncrystalline nanoparticles, such as polymeric nanoparticles,37, 38 self-assembled micelles,13 can also serve as magnetic cores. Fig. 1B listed the most commonly used magnetic cores for building MNP systems. The design of the magnetic cores for MNPs requires careful and balanced consideration on materials properties, synthesis feasibility and application requirement.
Magnetic property, chemical stability and toxicity are the primary focus in the design of a MNP system. Materials that have high magnetizations (Ms) are ideal candidates for MRI contrast agents. Common magnetic materials contain certain transitional elements with unpaired electrons, such as Fe, Co, Ni, Mn, Cr, Gd, and can be categorized as metals, metal alloys, and metal oxides. Metals and alloys, such Fe, FeCo, are among magnetic materials with the highest magnetizations. However, most of them are susceptible of oxidation and corrosion. Metal oxides materials are generally stable, and have acceptable magnetizations. Therefore, most MNP-based contrast agents that have been thoroughly investigated are metal oxides. Toxicity consideration should also be integrated into the materials selection, since elements with an inoffensive toxicity profile are most likely accepted for clinical applications. Therefore, iron-based MNPs are most investigated since iron has an innocuous toxicity profile, is a basic element in human bodies, and can be added to the body’s iron store after particle degredation.39 Manganese is another essential trace element in human bodies, but its tolerable limit is much lower than iron’s. Other elements, such as Co, Ni, Cr, Gd, are highly toxic, which necessitates proper coatings or chelation when they are used in vivo.
Metal oxide nanocrystals were the most investigated in search of MNP-based MRI contrast agents. Among those metal oxide compounds, superparamagnetic iron oxide nanoparticles (SPIONs), including magnetite (Fe3O4) and maghemite (γ-Fe2O3), have been a major research focus during the past decade.22 These nanocrystalline iron oxides belong to ferrite family, and have spinel structures, where the oxygen atoms form fcc lattices and iron atoms occupy tetrahedral (Td) and octahedral (Oh) interstitial sites.40 Particularly, magnetite has an inverse spinel structure, where the Fe2+ ions occupy Td sites and the Fe3+ ions occupy both Oh and Td sites. The spins of those ions in the Td sites and Oh sites align antiparallel under an external magnetic field, yielding large total net magnetization and a ferrimagnetic spin structure.11 Owing to their large surface-to-volume ratio, SPIONs exhibit superparamagnetism at room temperature. The major advantage regarding to clinical applications is that iron oxides are biocompatible and biodegradable, and have good chemical stability against solvent leaching. There are a number of clinically approved SPIONs that serve as T2 contrast agents for various MRI applications.41, 42
Other types of ferrites, in which Fe2+ ions are fully or partially replaced by other transitional metals (e.g. Mn, Co, Ni and Zn), were also studied.11, 43–45 Lee et al reported characterization and in vivo evaluation of a series of ferrite nanocrystals (MFe2O4, M = Mn, Fe, Co, Ni).11 Among those ferrite nanocrystals, manganese ferrite (MnFe2O4) nanocrystals demonstrated the largest magnetization and T2 contrast enhancement. Recently, Bárcenet et al reported the successful synthesis of zinc ferrite nanocrystals with a mixed spinel structure.45 Those zinc ferrite nanocrystals demonstrated better T2 contrast enhancement than comparable magnetite nanocrystals.
In addition to the ferrites family, other transitional metal compounds have also been evaluated as potential MRI contrast agents. Nanocrystals of those compounds display various magnetic properties, such as paramagnetism and antiferromagnetism, and some of them could serve as T1 contrast agents. Rare earth elements, represented by gadolinium, have generated great research interest because they have very high magnetic moments and interesting optical properties for integration of other imaging modalities.46 Synthesis of size- and shape-controlled rare earth oxide nanocrystals has also been demonstrated.47–49 Recently, contrast agents based on rare earth fluoride50 and phosphate36 have also been synthesized. Despite the fact that some of those nanocrystals show promising T1 contrast enhancement, few in vivo applications have been reported. Bridot et al reported the synthesis and in vivo applications of a multimodal contrast agents based on gadolinium (III) oxide (Gd2O3) nanocrystals.35 The nanocrystals were synthesized by nonaqueous co-precipitation method, and coated with a polysiloxane shell. In addition to rare earth elements, manganese-based compounds have also been investigated. Na et al synthesized manganese (II) oxide (MnO) nanocrystals of different sizes,29 for tumor detection and cell tracking.29, 51 Future clinical applications of those MNPs require rigorous surface engineering and careful toxicity evaluation.
Metal nanocrystals, including metal alloy nanocrystals, are promising candidates for high performance contrast agents. Since the net magnetization of metals is generally much higher than those of metal oxides, the clinical dosages of metal-based MNPs required for sufficient contrast enhancement would be much lower than those of metal oxides-based MNPs. However, chemical instability exhibited by most of metal nanocrystals is the major hurdle limiting their application in medical imaging. Metal nanocrystals are readily oxidized upon exposure to oxygen or moisture, and transformed to metal oxides or hydroxides with inferior magnetic property,52 compromising their contrast enhancement ability. Furthermore, a significant amount of free metal ions can be released during the oxidation process, resulting in severe toxicity.
To prevent the metallic cores from oxidization and corrosion, compact and chemically stable shells are grown on the cores. Noble metals53 (e.g. Au, Pt, etc.) and graphitic carbon32 have been used as shell materials. Alternatively, crystalline oxide shells can be created by controlled oxidation of as-synthesized metal nanocrystals. For example, Peng et al reported the synthesis of Fe nanocrystals and subsequent creation of a crystalline oxide shell utilizing an oxygen transferring agent.54 They also revealed that amorphous oxide coating could not protect the iron core from complete oxidation.
In some metal alloy nanocrystals, such as FePt, the interaction between two metal elements may lead to greater chemical stability in comparison to the single-element metallic nanocrystals. A more detailed coverage on the synthesis and surface modification of FePt nanocrystals has been presented in a recent review by Sun et al.55 Hydrophilic, biocompatible FePt nanocrystals have been produced,56 and their application as bifunctional imaging and therapeutic agents has also been reported.57
The FeCo nanocrystal is another type of metal alloy nanocrystals that have drawn the interest of researchers. FeCo nanocrystals have extremely high magnetization, but are highly susceptible to oxidation and corrosion. To solve this problem, Seo et al synthesized graphite shell-coated FeCo nanocrystals with 4 nm and 7 nm core sizes.32 While the 7 nm nanocrystals were proven to be excellent T2 contrast agents, the 4 nm nanocrystals are suitable for both T1 and T2 contrast enhancement. The graphitic shells are chemically inert, and their near-infrared absorbance property allows the potential use of the nanocrystals in photothermal ablation therapeutics.
Recently, heterostructured magnetic nanocrystals have attracted tremendous research attention. Multiple components with distinct functionalities, such as surface plasmon resonance, fluorescence, can be integrated in to a single heterostructured magnetic core. The typical heterostructures include above-mentioned core-shell structure, as well as heterodimers,58–60 and hollow nanoparticles.34 The formation of core-shell and heterodimer nanoparticles is typically via the seed-induced nucleation process,58 and a comprehensive review on the synthetic mechanism of those heterostructures has been reviewed by Zeng et al.61 Noble metal-based heterostructured MNPs can serve as MR and optical reflection dual-imaging probes,62 while semiconductor-based MNPs can serve as MR and fluorescence dual-imaging probes.57, 63 Hollow nanoparticles are derived from colloidal solid nanocrystals via acid etching.34, 64 Those hollow nanocrystals can serve as both an imaging contrast agent and a drug carrier where therapeutic payloads can be easily incorporated in the center vacancy of MNPs.64 Additionally, hollow MnO nanoparticles were found to improve T1 contrast as compared to the comparable solid MnO nanoparticles.34, 64
For most inorganic magnetic nanocrystals serving for medical imaging, the chemical synthesis methods can be divided into two categories: co-precipitation and thermal decomposition.52
Co-precipitation methods are predominately performed in aqueous environment, and the process includes dissolving precursors (metal salts) in acidified water optionally with surface coating materials, and then titrating the solution with alkaline compounds.52 For example, aqueous synthesis of SPIONs can be achieved by titrating base solutions into Fe2+/Fe3+ salts solutions.65 During the co-precipitation process, the nucleation stage and growth stage of nanocrystals can not be easily separated, resulting in polydisperse nanocrystals.66 The low reaction temperature of co-precipitation method leads to poor crystallinity of products, resulting in low saturated magnetization.52 Nevertheless, large-scale production of nanocrystals is feasible via co-precipitation methods. Since functional coating materials can be added during the synthesis step, it is possible to integrate core synthesis and coating in a single reaction (so-called “one-pot synthesis” or in situ coating process, which will be discussed in next section) without post-synthetic surface coating procedures.67 SPIONs coated with other hydrophilic materials, such as dextran68, poly(ethylene glycol) (PEG),69 chitosan,70 have also been synthesized in such “one-pot” method. Alternatively, co-precipitation reaction could be accomplished in a constrained environment. For example, some surfactants can form water-in-oil reverse micelles, and co-precipitation reaction can take place in those so-called “nanoreactors”.71 The reverse micelle method can be used to achieve size control of nanocrystals, but it has drawbacks of low yield and poor crystallinity.72
The thermal decomposition processes include dissolving metal organic precursors in organic solvents with the assistance of surfactants, and decomposing those precursors at elevated temperature.52 The organic precursors are either organometallics (e.g. metal carbonyl compounds73) or metal organic acid salts (e.g. acetate,49 acetonacetate,44, 49, 74, 75 oleate,43, 76 etc), and the surfactants typically contain polar capping groups and long hydrocarbon chains. Nanocrystals produced by thermal decomposition methods have excellent monodispersity and crystallinity.52 As a result, those nanocrystals exhibit superior magnetic properties over nanocrystals of similar compositions synthesized by co-precipitation methods. The size and shape control of nanocrystals can be easily achieved by changing the reaction temperature, precursor concentration, precursor to surfactant ratio, and other factors.43, 73, 76 Additionally, large-scale production of nanocrystals can also be achieved with thermal decomposition methods.43 The drawbacks of thermal decomposition methods include: highly toxic precursors in some cases,73 difficulty to achieve one-pot synthesis despite limited success.77, 78 In most cases, nanocrystals produced by the thermal decomposition method are stabilized by surfactants (e.g. oleic acid), and are only dispersible in nonpolar solvents (e.g. hexane, toluene) or weak polar solvents (e.g. chloroform, tetrahydrofuran). Therefore, post-synthetic processes are required to introduce hydrophilic and biocompatible coatings on those nanocrystals, which will be discussed in detail at next section.
In addition to those two categories, other methods, such as sol-gel reactions, flow injection syntheses, electrochemical methods, aerosol/vapor methods and sonolysis have also been employed to produce inorganic nanocrystals. A detailed coverage on those methods was provided by Laurent et al.22
In addition to crystalline nanoparticles, noncrystalline nanoparticles can also serve as the magnetic cores of MNPs. Those noncrystalline nanoparticles comprise myriads of small paramagnetic molecules such as Gd3+, Mn2+ or other metal ions with unpaired electrons. Those metal ions are always chelated by small molecule ligands, which can prevent the release of free metal ions, minimizing potential toxicity.79, 80 Other imaging reporters or therapeutic drugs can be easily incorporated during the production of those nanoparticles, which allows them to serve as multifunctional platforms.13
Those nanoparticles can be fabricated via hydrophobic self-assembly13 or polymerization methods.37, 80 Among those metal ions, Gd3+ is the best candidates since it has high magnetic moment and seven unpaired electrons, and small molecule ligands for chelating Gd3+ have been thoroughly investigated.81 Taylor et al reported the fabrication of Gd3+-based nanoparticles by a sol-gel synthesis method.80 Another element being investigated is manganese. Nanoparticles based on manganese complexes has been reported which shows promising T1 relaxivity.13, 37 Chemotherapeutic drugs can also be embedded inside the cores so that MNP systems can also serve as drug carriers.13 Based on those examples of success, noncrystalline nanoparticles have great potential to achieve multimodality and multifunctionality.
3. Constructing biocompatible surface coatings
Biomedical applications require a robust MNP system to be properly coated by hydrophilic polymers. First, surface coatings are important to prevent MNPs from agglomeration in physiological environment. Second, coatings act as a barrier, effectively shielding the magnetic core against the attack of chemical species in the aqueous solution. Third, coatings provide functional groups (e.g. amine, carboxyl) that can serve as anchor points for further attachment of functional moieties, such as targeting ligands, fluorescent molecules. For in vivo applications, the coatings of MNPs strongly influence the longevity of MNPs in the blood. Upon entering the blood circulation, MNPs are subjected to opsonization, the non-specific fouling of plasma protein on the surface of MNPs, and subsequent uptake by reticuloendothelial system (RES). Therefore, proper coatings are required to prevent the opsonization of MNPs, and to increase the ability to evade RES. As a result, MNPs with long blood-circulation time would maximize the possibility to reach target tissue. In addition, coatings can also be tailored to improve MNPs’ intracellular behaviors, therapeutic loading and release properties.
The surface coatings also affect the relaxivity of MNPs. Laconte et al reported that the increased coating thickness would dramatically decrease the R2 relaxivity of monocrystalline iron oxide nanoparticles.82 Studies also indicated that the hydrophobicity of surface coating, and the coordination chemistry of inner capping ligands, strongly influenced the relaxation properties of MNPs.83, 84
A diverse variety of materials have been developed and employed as the coatings for MNPs, and most of them are organic macromolecules, i.e., polymers. Based on different criteria, those macromolecules can be categorized as hydrophilic or amphiphilic polymers, neutral or charged polymers, naturally derived or synthetic polymers, and homopolymers or copolymers. Neutral polymers contain abundant neutral, hydrophilic groups (e.g. hydroxyl, ether) which offer excellent resistance against opsonization. The major drawback of neutral polymers is the lack of functional groups (e.g. amine, carboxyl) for convenient bioconjugation, and chemical derivations are often required. On the contrary, functional groups for bioconjugation are abundant in charged polymers. However, MNPs coated with charged polymers are prone to opsonization due to strong electrostatic interactions between MNP surface and plasma proteins. Typical neutral polymers include dextran and PEG. Dextran, a branched polysaccharide comprised of glucose subunits, is one of the most investigated coating materials for MNPs due to its proven biocompatibility.68 Most of clinically approved iron oxide nanoparticles are coated with dextran or its derivatives.42, 85–87 PEG is a neutral, linear synthetic polyether that can be prepared with a wide range of terminal functional groups. PEG is nontoxic, nonimmunogenic and resistant to protein fouling, and MNPs coated with PEG exhibit long blood circulation time.88 PEG has been widely accepted as nanoparticle coatings for biomedical applications.89–92 Examples of charged polymers include natural polymers, such as chitosan, hyaluronic acid, and synthetic polymers, such as poly(acrylic acid) (PAA), polyethyleneimine (PEI). Chitosan is a cationic polysaccharide that is nontoxic, hydrophilic, biocompatible, bioabsorbable, and biodegradable. Chitosan and its derivatives have long been used as carriers for nucleic acids and other pharmaceutical formulations.93 PEI is a synthetic cationic polymer that comes with either linear or branched forms. Although PEI is toxic and non-biodegradable, it has long been used for gene delivery thanks to its ability to complex with DNA, facilitate endosomal release via the “proton sponge effect”, and guide intracellular trafficking of DNA into the nucleus.94 Numerous copolymers have been synthesized to synergize the advantages and overcome the disadvantages of each component. For example, Bhattarai et al synthesized PEG-grafted chitosan copolymer that has improved solubility in neutral pH, and it was utilized as injectable thermoreversible hydrogels.95 Veiseh et al demonstrated that PEG-grafted PEI copolymers can both complex DNA to facilitate cell transfection, and exhibit low-toxic and non-fouling profile necessary for molecular targeting.96
There are two major approaches to form the coatings of MNPs: in situ coating (during the “one-pot” synthesis) and post-synthetic coating. During the in situ coating process, precursors of magnetic cores and coating materials are dissolved in the same reaction solution, and the nucleation of magnetic cores occurs on the coating materials. Therefore, the magnetic cores and the coatings of MNPs form at the same time.33, 36, 69, 97–100 In the post-synthetic coating process, first, the magnetic cores are synthesized, and optionally capped by surfactants protecting against agglomeration. Then, the coating materials are introduced to the surface of magnetic cores by either direct grafting,12, 35, 90, 91 ligand exchange,11, 26, 57, 59, 92, 101–104 or hydrophobic interactions.32, 60, 105–108
Typically, two strategies have been used to link coating materials on the surface of magnetic cores: the end-grafting strategy and the surface-encapsulation strategy. Schematic representations of those coating strategies are listed in Figure 2. End-grafting strategy utilizes a single surface-capping group on one end of coating molecules to connect the magnetic core and coating molecules. The end-grafting process can be achieved either by in situ coating or post-synthetic coating. Various surface binding groups have been explored in the past in order to form stable surface coatings. Initially, carboxyl groups have been utilized to anchor coating molecules on transitional metal oxide surfaces,97, 100 while thiol groups have been utilized for capping noble metal surfaces.59, 104 Recently, other high affinity surface-capping groups have been explored to improve the stability of the coating. Hydroxamic acid,109 phosphine oxide,106 and catechol-based57, 101, 103, 104 ligands have been reported to form stable coatings on the surfaces of various MNPs. Some surface-capping molecules can form cross-linked “mesh” on the core surface, resulting in coatings with better chemical stability. For example, Jun et al reported coating of ferrites MNPs with dimercaptosuccinic acid (DMSA).26 Some of thiol groups on the DMSA coating formed disulfide bridges to enhance stability, while remaining free thiols were still available for further bioconjugation. Alkoxysilanes have also been studied extensively since they can form highly stable polysiloxane shells on the surface of metal oxide MNPs.12, 35, 89–91, 110, 111 However, due to limited amount of surface-capping groups per coating molecule, linkages between the end-grafted coatings and magnetic cores may easily breakdown, resulting in lost of coatings and agglomeration of MNPs. Furthermore, some of those high-affinity capping groups are bulky, causing low surface density of end-grafted polymers. As a result, the overall colloidal stability of MNPs, as well as the availability of functional groups for bioconjugation, could be affected.
Figure 2.
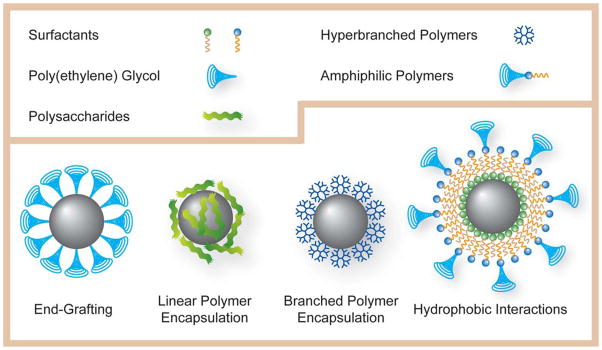
Graphic illustration of different types of coating strategies for hydrophilic MNPs: end-grafting, encapsulation by linear polymers, encapsulation by hyperbranched polymers, and coating via hydrophobic interactions.
To overcome the limitations of end-grafting strategy, nanoparticle cores can be fully encapsulated by surface-encapsulation strategy. Polymers with multiple surface-interacting groups (i.e. multidentate polymers) can be introduced as the coatings of MNPs by either in situ or post-synthetic coating methods. The collective effect of multidentate connections results in stronger interactions and greater stability of the coating compared to those coatings produced by end-grafting method. The first category of polymers is linear and partially branched polymers. The flexibility of these polymers enables them to be wrapped around the magnetic cores. Various polymers, including dextran,98 PAA,112 modified hyaluronic acid,113 and various copolymers,69 have been utilized as the coatings of MNPs. The second category of polymers is hyperbranched polymers, which can form close-packed layer of surface coatings. Examples of those polymers include branched PEI and other types of dendrimers. Scherer et al developed PEI-coated MNPs for gene delivery.15 Landmark et al have developed Fe3O4 nanoparticles coated with poly(amidoamine) (PAMAM) dendrimers.102
A special case of surface-encapsulation strategy is formation of coatings via hydrophobic interactions. As mentioned in the previous section, magnetic nanocrystals that are synthesized by thermal decomposition method are often capped by hydrophobic surfactants. Those hydrophobic magnetic cores can be coated with amphiphilic molecules, forming micelle-like structures. An amphiphilic molecule (e.g. phospholipids) consists of both hydrophilic and hydrophobic regions. The hydrophilic regions can interact with water by either ionic interactions or hydrogen bonding, stabilizing MNPs-containing micelles in aqueous solutions. The hydrophobic regions are typically long-chain hydrocarbons that can interact with hydrophobic surfactants on the core surface. The aqueous stability of those MNPs-containing micelles can be improved by using amphiphilic copolymers derived from PEG11, 60, 105, 107 or polysaccharides.114 Large amphiphilic copolymers with multiple hydrophilic and hydrophobic regions can be wrapped around the magnetic cores, resulting in great chemical and thermal stability of MNPs-containing micelles.108, 115 Since the coating process via micelle formation is independent of the chemical composition of magnetic cores, this strategy can apply to not only MNPs, but also other nanomaterials including quantum dots, carbon nanotubes, and noble metal nanocrystals.108, 114–116 Some of those micelle structures can be further modified to mimic high-density lipoprotein (HDL), a naturally occurred nanoparticle. Those HDL nanoparticles have demonstrated their ability to target the expression of macrophage.116
The quality of the coatings on MNPs can be monitored by measuring the hydrodynamic size, surface charge and other physicochemical parameters. The hydrodynamic size of MNPs can be obtained by dynamic light scattering (DLS), and is often studied under various solution conditions, such as pH, ionic strength, and temperature.103, 117 The surface charge of MNPs is examined by measuring the zeta potential of MNPs. Once MNPs are well characterized after the synthesis and coating stages, further derivation and evaluation toward biomedical applications will begin.
4. Achieving biological functionality
The primary goal of MNPs-based MRI contrast agents is to improve the detectability of medical imaging. This goal can be achieved by adding target-specificity and/or multimodality features of MNPs. In addition to detectability improvement, multifunctional MNPs can further expand their applications on other areas, such as therapeutics. Along with those goals, safety is the baseline of MNPs’ biomedical applications, and toxicity and biodistribution must be studied to assure their safety prospect.
The detectability of MRI can be significantly improved by the preferential accumulation of MNP contrast agents in the diseased tissue. This is known as active or specific targeting, achieved by immobilized molecules that have high affinity toward unique signatures of malignant cells. Those molecules are called targeting ligands, including small molecules, peptides, proteins, antibodies and aptamers.14 Those targeting ligands can be immobilized on the outer surface of MNPs via various established bioconjugate chemistries.118 One advantage of nanoparticle-based imaging agents over small molecule counterparts is that much higher amount of targeting ligands can be conjugated to nanoparticles, resulting in higher affinity towards the molecular targets, which is known as multivalency effect.119, 120 Targeted MRI have broad applications on diagnosis and staging of diseases, evaluation of therapeutic outcome, and other applications.
While MRI is capable of providing high-resolution for pre- and post-operative imaging of patients, it is not appropriate for intraoperative monitoring. To broaden the usage of the MNP systems, MNPs are often modified to enable the detection by other imaging modalities. Those imaging modalities include optical imaging, computed X-ray tomography (CT), and positron emission tomography (PET).121 Veiseh et al developed a target-specific dual-modality MNP system.12 By attaching Cy5.5, a near infrared (NIR) fluorophore onto the surface of PEG-coated iron oxide nanoparticles, this dual-modality MNP system is detectable by both MR and fluorescent imaging. Fluorescent MNP systems also include quantum dots-MNP heterostructures122, 123 and nanocomposites.124, 125 PET imaging has very high sensitivity, while image has poor resolution and provides no anatomical information. Combined with MRI, Choi et al reported a PET/MRI dual-modality MNP system by attaching 124I, a radionuclide to the coatings of MnFe2O4-based MNPs.126 Colocalized PET/MRI fusion images can be generated as a result of the highly complementary nature of those two modalities (Figure 3). Other examples of dual-modality MNP systems include optical reflection/MRI104 and X-ray/MRI127.
Figure 3.
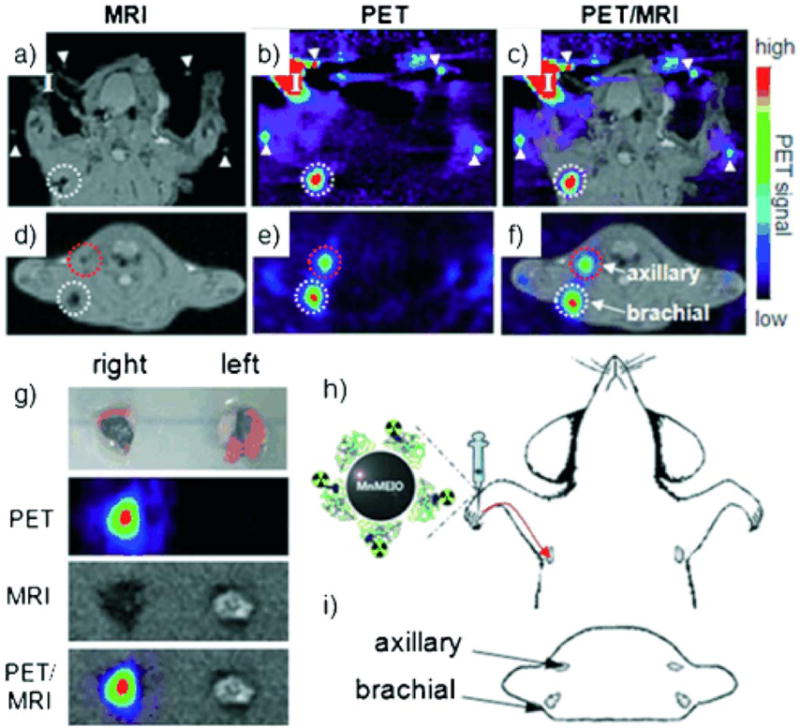
a–f) PET/MR images of SLNs in a rat at 1 h post injection of 124I-SA-MnMEIO into the right forepaw (I=nanoprobe injection site). Coronal a) MR and b) PET images in which a brachial LN (white circle) is detected. c) The position of the brachial LN is well matched in a PET/MR fusion image. Four small pipette tips containing Na124I solution are used as a fiducial marker (white arrowheads) for the concordant alignment in PET/MR images. In the transverse images, axillary (red circle) and brachial LNs (white circle) are detected in the d) MR and e) PET images, and images of each node are nicely overlapped in the corresponding PET/MR fusion image (f). g) The explanted brachial LN also shows consistent results with in vivo images by PET and MR. Only the LN from the right-hand side of the rat containing 124I-SA-MnMEIO shows strong PET and dark MR images. The schematics of the rat in the h) coronal and i) transverse directions show the locations of the LNs. Reproduced with permission from John Wiley & Sons Ltd.126
The application of MNP systems can be further expanded beyond medical imaging by incorporating multifunctionality. By exploiting the large surface-to-volume ratio of MNP, a large quantity of functional molecules can be immobilized into or around the coatings of MNPs. A common example is that MNPs can serve as vehicles for delivering therapeutic payloads, such as conventional chemotherapeutic drugs, therapeutic peptides, proteins and genes.10, 13, 15, 96 For example, by conjugating chlorotoxin (CTX), a peptide that bind specifically to brain cancer cells, and methotrexate (MTX), a chemotherapeutic drug to PEG-coated SPIONs, Sun et al developed an imaging/drug delivery multifunctional system that demonstrated promising results in vivo.10 Engineering consideration of such MNP-based nanocarriers include: adequate protection of therapeutic payloads during circulation, target-specific biodistribution, sufficient cellular internalization, controlled payload-releasing profile, and organelle-specific delivery.128 Successful MNP-based nanocarriers can provide imaging tools for real-time monitoring the delivery of therapeutic payload.
Finally, MNP systems must minimize toxicity to assure that they do not harm prospective patients. Attention should be paid on the toxicity of each individual components and MNPs as a whole system, as well as the toxicity of the byproducts during the degradation process.129, 130 The biodistribution of MNPs is another critical aspect on the safety of MNPs applications since unfavorable biodistribution may lead to strong side effects.130 Although there is no universal set of criteria, standardizing preclinical characterization of nanoparticles can help better elucidate their structure-activity relationships (SARs).131, 132
5. Summary and outlook
During the past decade, the breakthrough of nanotechnology, molecular biology and novel therapeutics greatly accelerated the development of MNP systems. Numerous milestones have been achieved during the progress of MNP development. Aiming at the solutions for future diagnostics and therapeutics, the development goal of MNP systems is to achieve higher imaging contrast, better stability, and superior biocompatibility. With better understanding of chemistry and physics, a diverse variety of nanoparticles, such as metal oxide/metal/alloy nanocrystals, heterostructures and noncrystalline nanoparticles have been developed. Improvements on the magnetic properties of MNPs increase the ability of contrast enhancement, resulting in higher sensitivity and less dosage for potential applications. Numerous biocompatible coating materials have been developed. The implementation of PEG and other non-fouling polymers as surface coatings of MNPs has dramatically increased the blood circulation time, offering more opportunities for MNPs to reach their destination. Different coating strategies, such as end-grafting and surface encapsulation, have been applied along with in situ and post-synthetic coating techniques, to maximize the stability of MNP systems and simplify the overall procedure. Target-specific, multimodality MNPs have helped medical imaging to be more sensitive and accurate. Multifunctional MNP systems can deliver therapeutics with minimized side effects, while allowing noninvasive monitoring of therapeutic outcome.
Although much progress has been made on the fabrication of MNPs with delicate structure and fascinating properties, it has been difficult to precisely probe the in vivo behavior of those MNPs, such as accumulation, degradation and clearance. Novel characterization tools need to be developed in order to trace MNPs inside living objects. Quantitative testing protocols are required to reveal the correlation between the basic physicochemical properties of MNPs and their in vivo behavior. Some fundamental issues, such as long-term toxicity, targeting efficiency, have to be addressed before the clinical entry of MNPs. Once those problems are solved, MNPs will finally prevail, bringing unprecedented solutions to the diagnosis, treatment and prevention of most devastating diseases, such as cancer, cardiovascular diseases, and neurological diseases.
Figure 4.
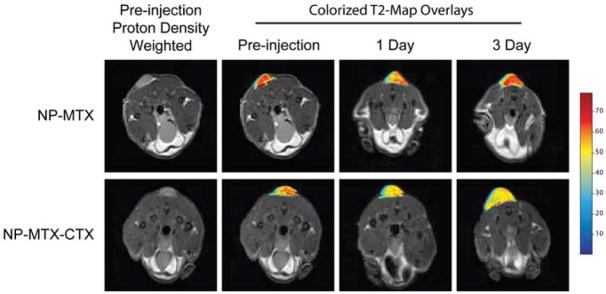
Axial cross sections displaying 9L tumors of mice before injection of nanoparticle conjugates and 1 and 3 days post-injection. T2 map overlays of the tumor region show decreased T2 for both NP-MTX and NP-MTX-CTX nanoprobe conjugates 1 day after administration. However, the reduction is more significant and uniform in tumor of mouse receiving NP-MTX-CTX. A total of 3 days post-injection, the tumor T2 values of the mouse receiving NP-MTX-CTX remained at the decreased level, while those of mouse receiving NP-MTX returned to the post-injection level suggesting clearance of NP-MTX from tumor tissue. Reproduced with permission from Future Medicine Ltd.10
Acknowledgments
The work was supported in part by grants (R01CA119408, R01EB006043, and R01CA134213) from the U.S. National Institute of Health.
Biographies

Chen Fang received his B.S. (2001) and M.S. (2004) in materials science and engineering from Shanghai Jiaotong University. He is currently pursuing his Ph.D. at University of Washington under the supervision of Prof. Miqin Zhang.

Miqin Zhang received her Ph.D. in Materials Science from University of California at Berkeley in 1999. She is currently a professor in Department of Materials Science and Engineering at University of Washington. Her research directions include nanotechnology for cancer diagnosis and therapy, tissue engineering for tissue regeneration and controlled drug delivery, and cell-based sensors for toxin detection and drug screening.
References
- 1.Nie SM, Xing Y, Kim GJ, Simons JW. Annu Rev Biomed Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 2.Pankhurst QA, Connolly J, Jones SK, Dobson J. J Phys D - Appl Phys. 2003;36:R167–R181. [Google Scholar]
- 3.Mornet S, Vasseur S, Grasset F, Duguet E. J Mater Chem. 2004;14:2161–2175. [Google Scholar]
- 4.Sun C, Lee JSH, Zhang MQ. Adv Drug Deliv Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MA, Semelka RC. MRI: Basic Principles and Applications. Wiley–Liss; New York: 2003. [Google Scholar]
- 6.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 7.Massoud TF, Gambhir SS. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 8.Moghimi SM, Hunter AC, Murray JC. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 9.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun C, Fang C, Stephen Z, Veiseh O, Hansen S, Lee D, Ellenbogen RG, Olson J, Zhang MQ. Nanomed. 2008;3:495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Na HB, Lee J, An K, Park YI, Park M, Lee IS, Nam D, Kim ST, Kim S, Kim S, Lim K, Kim K, Kim S, Hyeon T. Angew Chem Int Ed. 2007;46:5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 12.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang MQ. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 13.Pan D, Caruthers SD, Hu G, Senpan A, Scott MJ, Gaffney PJ, Wickline S, Lanza GM. J Am Chem Soc. 2008;130:9186–9187. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veiseh O, Gunn J, Zhang M. Adv Drug Deliv Rev. doi: 10.1016/j.addr.2009.11.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer F, Anton M, Schillinger U, Henkel J, Bergemann C, Kruger A, Gansbacher B, Plank C. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 16.Rudin M, Weissleder R. Nat Rev Drug Discov. 2003;2:123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- 17.McDonald DM, Choyke PL. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 18.Weissleder R, Pittet MJ. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 20.Dias MHM, Lauterbur PC. Magn Reson Med. 1986;3:328–330. doi: 10.1002/mrm.1910030218. [DOI] [PubMed] [Google Scholar]
- 21.Bonnemain B. J Drug Target. 1998;6:167–174. doi: 10.3109/10611869808997890. [DOI] [PubMed] [Google Scholar]
- 22.Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 23.Wang YXJ, Hussain SM, Krestin GP. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 24.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. N Engl J Med. 2003;348:2491–U2495. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 25.Koenig SH, Kellar KE. Magn Reson Med. 1995;34:227–233. doi: 10.1002/mrm.1910340214. [DOI] [PubMed] [Google Scholar]
- 26.Jun Yw, Huh YM, Choi Js, Lee JH, Song HT, Kim Sj, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. J Am Chem Soc. 2005;127:5732–5733. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 27.Duan HW, Kuang M, Wang XX, Wang YA, Mao H, Nie SM. J Phys Chem C. 2008;112:8127–8131. [Google Scholar]
- 28.Tromsdorf UI, Bigall NC, Kaul MG, Bruns OT, Nikolic MS, Mollwitz B, Sperling RA, Reimer R, Hohenberg H, Parak WJ, Forster S, Beisiegel U, Adam G, Weller H. Nano Lett. 2007;7:2422–2427. doi: 10.1021/nl071099b. [DOI] [PubMed] [Google Scholar]
- 29.Na HB, Lee J, An K, Park YI, Park M, Lee IS, Nam D, Kim ST, Kim S, Kim S, Lim K, Kim K, Kim S, Hyeon T. Angew Chem Int Ed Engl. 2007;46:5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 30.Bulte JWM, Kraitchman DL. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 31.Mahajan SV, Dickerson JH. Nanotechnology. 2007;18:325605. [Google Scholar]
- 32.Seo W, Lee J, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang P, Mcconnell M, Nishimura D, Dai H. Nat Mater. 2006;5:971–976. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 33.Evanics F, Diamente PR, van Veggel FCJM, Stanisz GJ, Prosser RS. Chem Mater. 2006;18:2499–2505. [Google Scholar]
- 34.An K, Kwon SG, Park M, Na HB, Baik SI, Yu JH, Kim D, Son JS, Kim YW, Song IC, Moon WK, Park HM, Hyeon T. Nano Lett. 2008 doi: 10.1021/nl8019467. [DOI] [PubMed] [Google Scholar]
- 35.Bridot JL, Faure AC, Laurent S, Rivière C, Billotey C, Hiba B, Janier M, Josserand V, Coll JL, Elst LV, Muller R, Roux S, Perriat P, Tillement O. J Am Chem Soc. 2007;129:5076–5084. doi: 10.1021/ja068356j. [DOI] [PubMed] [Google Scholar]
- 36.Hifumi H, Yamaoka S, Tanimoto A, Citterio D, Suzuki K. J Am Chem Soc. 2006;128:15090–15091. doi: 10.1021/ja066442d. [DOI] [PubMed] [Google Scholar]
- 37.Taylor KM, Rieter WJ, Lin W. J Am Chem Soc. 2008;130:14358–14359. doi: 10.1021/ja803777x. [DOI] [PubMed] [Google Scholar]
- 38.Taylor KML, Jin A, Lin WB. Angew Chem Int Ed. 2008;47:7722–7725. doi: 10.1002/anie.200802911. [DOI] [PubMed] [Google Scholar]
- 39.Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, Jacobs P, Lewis J. Am J Roentgenol. 1989;152:167–173. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 40.Schwertmann U, Cornell RM. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. Wiley-VCH; Weinheim: 2003. [Google Scholar]
- 41.Mornet S, Vasseur S, Grasset F, Duguet E. Journal of Materials Chemistry. 2004;14:2161–2175. [Google Scholar]
- 42.Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Chemical Reviews. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 43.Park J, An K, Hwang Y, Park J, Noh H, Kim J, Park J, Hwang N, Hyeon T. Nat Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 44.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. J Am Chem Soc. 2004;126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 45.Bárcena C, Sra A, Chaubey G, Khemtong C, Liu J, Gao J. Chem Commun. 2008:2224. doi: 10.1039/b801041b. [DOI] [PubMed] [Google Scholar]
- 46.Heer S, Kompe K, Gudel HU, Haase M. Advanced Materials. 2004;16:2102. [Google Scholar]
- 47.Cao YC. J Am Chem Soc. 2004;126:7456–7457. doi: 10.1021/ja0481676. [DOI] [PubMed] [Google Scholar]
- 48.Si R, Zhang Y, You L, Yan C. Angew Chem Int Ed Engl. 2005;44:3256–3260. doi: 10.1002/anie.200462573. [DOI] [PubMed] [Google Scholar]
- 49.Si R, Zhang Y, Zhou H, Sun L, Yan C. Chem Mater. 2007:18–27. [Google Scholar]
- 50.Evanics F, Diamente PR, van Veggel FCJM, Stanisz GJ, Prosser RS. Chem Mat. 2006:2499–2505. [Google Scholar]
- 51.Gilad A, Walczak P, Mcmahon M, Na HB, Lee J, An K, Hyeon T, Van Zijl P, Bulte J. Magn Reson Med. 2008;60:1–7. doi: 10.1002/mrm.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu AH, Salabas EL, Schuth F. Angewandte Chemie-International Edition. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 53.Park JI, Cheon J. J Am Chem Soc. 2001;123:5743–5746. doi: 10.1021/ja0156340. [DOI] [PubMed] [Google Scholar]
- 54.Peng S, Wang C, Xie J, Sun S. J Am Chem Soc. 2006;128:10676–10677. doi: 10.1021/ja063969h. [DOI] [PubMed] [Google Scholar]
- 55.Sun SH. Advanced Materials. 2006;18:393–403. [Google Scholar]
- 56.Hong R, Fischer NO, Emrick T, Rotello VM. Chem Mat. 2005;17:4617–4621. [Google Scholar]
- 57.Gao J, Liang G, Cheung JS, Pan Y, Kuang Y, Zhao F, Zhang B, Zhang X, Wu EX, Xu B. J Am Chem Soc. 2008;130:11828–11833. doi: 10.1021/ja803920b. [DOI] [PubMed] [Google Scholar]
- 58.Figuerola A, Fiore A, Di Corato R, Falqui A, Giannini C, Micotti E, Lascialfari A, Corti M, Cingolani R, Pellegrino T, Cozzoli PD, Manna L. J Am Chem Soc. 2008;130:1477–1487. doi: 10.1021/ja078034v. [DOI] [PubMed] [Google Scholar]
- 59.Choi J, Jun Y, Yeon SI, Kim HC, Shin JS, Cheon J. J Am Chem Soc. 2006;128:15982–15983. doi: 10.1021/ja066547g. [DOI] [PubMed] [Google Scholar]
- 60.Choi SH, Na HB, Park YI, An K, Kwon SG, Jang Y, Park MH, Moon J, Son JS, Song IC, Moon WK, Hyeon T. J Am Chem Soc. 2008;130:15573–15580. doi: 10.1021/ja805311x. [DOI] [PubMed] [Google Scholar]
- 61.Zeng H, Sun SH. Advanced Functional Materials. 2008;18:391–400. [Google Scholar]
- 62.Xu C, Xie J, Ho D, Wang C, Kohler N, Walsh EG, Morgan JR, Chin YE, Sun S. Angew Chem Int Ed Engl. 2008;47:173–176. doi: 10.1002/anie.200704392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao J, Zhang B, Gao Y, Pan Y, Zhang X, Xu B. J Am Chem Soc. 2007;129:11928–11935. doi: 10.1021/ja0731017. [DOI] [PubMed] [Google Scholar]
- 64.Shin JS, Anisur R, Ko M, Im G, Lee L, Lee D. Angewandte Chemie International Edition. 2009;48:321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Sun C, Kohler N, Zhang MQ. Biomedical Microdevices. 2004;6:33–40. doi: 10.1023/b:bmmd.0000013363.77466.63. [DOI] [PubMed] [Google Scholar]
- 66.Park J, Joo J, Kwon SG, Jang Y, Hyeon T. Angewandte Chemie-International Edition. 2007;46:4630–4660. doi: 10.1002/anie.200603148. [DOI] [PubMed] [Google Scholar]
- 67.Josephson L, Tung CH, Moore A, Weissleder R. Bioconjugate Chem. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 68.Shen T, Weissleder R, Papisov M, Bogdanov A, Brady TJ. Magnetic Resonance in Medicine. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 69.Lutz JF, Stiller S, Hoth A, Kaufner L, Pison U, Cartier R. Biomacromolecules. 2006;7:3132–3138. doi: 10.1021/bm0607527. [DOI] [PubMed] [Google Scholar]
- 70.Lee HS, Kim EH, Shao HP, Kwak BK. 2005:102–105. [Google Scholar]
- 71.Pileni MP. Nat Mater. 2003;2:145–150. doi: 10.1038/nmat817. [DOI] [PubMed] [Google Scholar]
- 72.Ingert D, Pileni MP. Advanced Functional Materials. 2001;11:136–139. [Google Scholar]
- 73.Park J, Lee E, Hwang NM, Kang M, Kim SC, Hwang Y, Park JG, Noh HJ, Kim J, Park JH, Hyeon T. Angew Chem Int Ed Engl. 2005;44:2872–2877. doi: 10.1002/anie.200461665. [DOI] [PubMed] [Google Scholar]
- 74.Smith AM, Nie S. Angew Chem Int Ed Engl. 2008;47:9916–9921. doi: 10.1002/anie.200804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun S, Zeng H. J Am Chem Soc. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 76.Jana NR, Chen YF, Peng XG. Chem Mater. 2004:3931–3935. [Google Scholar]
- 77.Li Z, Wei L, Gao MY, Lei H. Advanced Materials. 2005;17:1001. [Google Scholar]
- 78.Latham AH, Williams ME. Accounts of Chemical Research. 2008;41:411–420. doi: 10.1021/ar700183b. [DOI] [PubMed] [Google Scholar]
- 79.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chemical Reviews. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 80.Taylor KML, Jin A, Lin WB. Angewandte Chemie-International Edition. 2008;47:7722–7725. doi: 10.1002/anie.200802911. [DOI] [PubMed] [Google Scholar]
- 81.Aime S, Botta M, Fasano M, Terreno E. Chemical Society Reviews. 1998;27:19–29. [Google Scholar]
- 82.LaConte LEW, Nitin N, Zurkiya O, Caruntu D, O’Connor CJ, Hu XP, Bao G. Journal of Magnetic Resonance Imaging. 2007;26:1634–1641. doi: 10.1002/jmri.21194. [DOI] [PubMed] [Google Scholar]
- 83.Duan HW, Kuang M, Wang XX, Wang YA, Mao H, Nie SM. Journal of Physical Chemistry C. 2008;112:8127–8131. [Google Scholar]
- 84.Tromsdorf UI, Bigall NC, Kaul MG, Bruns OT, Nikolic MS, Mollwitz B, Sperling RA, Reimer R, Hohenberg H, Parak WJ, Forster S, Beisiegel U, Adam G, Weller H. Nano Letters. 2007;7:2422–2427. doi: 10.1021/nl071099b. [DOI] [PubMed] [Google Scholar]
- 85.Bonnemain B. Journal of Drug Targeting. 1998;6:167–174. doi: 10.3109/10611869808997890. [DOI] [PubMed] [Google Scholar]
- 86.Wang YXJ, Hussain SM, Krestin GP. European Radiology. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 87.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. New England Journal of Medicine. 2003;348:2491–U2495. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 88.Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang MQ. Small. 2006;2:785–792. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y, Kohler N, Zhang MQ. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Sun C, Kohler N, Zhang MQ. Biomed Microdevices. 2004;6:33–40. doi: 10.1023/b:bmmd.0000013363.77466.63. [DOI] [PubMed] [Google Scholar]
- 91.Kohler N, Fryxell GE, Zhang MQ. J Am Chem Soc. 2004;126:7206–7211. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- 92.Fang C, Bhattarai N, Sun C, Zhang M. Small. In press. [Google Scholar]
- 93.Kumar M, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chem Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 94.Kircheis R, Wightman L, Wagner E. Adv Drug Deliv Rev. 2001;53:341–358. doi: 10.1016/s0169-409x(01)00202-2. [DOI] [PubMed] [Google Scholar]
- 95.Bhattarai N, Matsen FA, Zhang M. Macromol Biosci. 2005;5:107–111. doi: 10.1002/mabi.200400140. [DOI] [PubMed] [Google Scholar]
- 96.Veiseh O, Kievit FM, Gunn JW, Ratner BD, Zhang MQ. Biomaterials. 2009;30:649–657. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Z, Wei L, Gao MY, Lei H. Adv Mater. 2005;17:1001–1005. [Google Scholar]
- 98.Shen T, Weissleder R, Papisov M, Bogdanov A, Brady TJ. Magn Reson Med. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 99.Smith AM, Nie S. Angew Chem Int Ed. 2008;47:9916–9921. doi: 10.1002/anie.200804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Adv Mater. 2006;18:2553. [Google Scholar]
- 101.Hong R, Fischer NO, Emrick T, Rotello VM. Chem Mater. 2005;17:4617–4621. [Google Scholar]
- 102.Landmark KJ, DiMaggio S, Ward J, Kelly C, Vogt S, Hong S, Kotlyar A, Myc A, Thomas TP, Penner-Hahn JE, Baker JR, Holl MMB, Orr BG. Acs Nano. 2008:773–783. doi: 10.1021/nn800034w. [DOI] [PubMed] [Google Scholar]
- 103.Xie J, Xu C, Kohler N, Hou Y, Sun S. Adv Mater. 2007;19:3163–3166. [Google Scholar]
- 104.Xu C, Xie J, Ho D, Wang C, Kohler N, Walsh EG, Morgan JR, Chin YE, Sun S. Angew Chem Int Ed. 2008;47:173–176. doi: 10.1002/anie.200704392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bárcena C, Sra A, Chaubey G, Khemtong C, Liu J, Gao J. Chem Comm. 2008:2224. doi: 10.1039/b801041b. [DOI] [PubMed] [Google Scholar]
- 106.Na NH, Lee IS, Seo H, Il Park Y, Lee JH, Kim SW, Hyeon T. Chem Comm. 2007:5167–5169. doi: 10.1039/b712721a. [DOI] [PubMed] [Google Scholar]
- 107.Shin JS, Anisur R, Ko M, Im G, Lee L, Lee D. Angew Chem Int Ed. 2009;48:321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 108.Yu WW, Chang E, Falkner JC, Zhang JY, Al-Somali AM, Sayes CM, Johns J, Drezek R, Colvin VL. J Am Chem Soc. 2007;129:2871–2879. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- 109.Kim M, Chen YF, Liu YC, Peng XG. Adv Mater. 2005;17:1429. doi: 10.1002/adma.200401991. [DOI] [PubMed] [Google Scholar]
- 110.Jana NR, Earhart C, Ying JY. Chem Mater. 2007;19:5074–5082. [Google Scholar]
- 111.De Palma R, Peeters S, Van Bael MJ, Van den Rul H, Bonroy K, Laureyn W, Mullens J, Borghs G, Maes G. Chem Mater. 2007;19:1821–1831. [Google Scholar]
- 112.Zhang TR, Ge JP, Hu YP, Yin YD. Nano Lett. 2007;7:3203–3207. doi: 10.1021/nl071928t. [DOI] [PubMed] [Google Scholar]
- 113.Lee YH, Lee H, Kim YB, Kim JY, Hyeon T, Park H, Messersmith PB, Park TG. Adv Mater. 2008;20:4154. doi: 10.1002/adma.200800756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goodwin AP, Tabakman SM, Welsher K, Sherlock SP, Prencipe G, Dai H. J Am Chem Soc. 2009;131:289–296. doi: 10.1021/ja807307e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin CAJ, Sperling RA, Li JK, Yang TY, Li PY, Zanella M, Chang WH, Parak WGJ. Small. 2008;4:334–341. doi: 10.1002/smll.200700654. [DOI] [PubMed] [Google Scholar]
- 116.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, Fisher EA, Fayad ZA, Mulder WJ. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu H, Zhu H, Zhuang J, Yang S, Liu C, Cao YC. Angew Chem Int Ed. 2008;47:3730–3734. doi: 10.1002/anie.200800434. [DOI] [PubMed] [Google Scholar]
- 118.Hermanson GT. Bioconjugate Techiques. Academic Press; Amsterdam, The Netherlands: 2008. [Google Scholar]
- 119.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 120.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. J Med Chem. 2006;49:6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 121.Cai WB, Chen XY. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 122.Gao J, Liang G, Cheung JS, Pan Y, Kuang Y, Zhao F, Zhang B, Zhang X, Wu EX, Xu B. J Am Chem Soc. 2008;130:11828–11833. doi: 10.1021/ja803920b. [DOI] [PubMed] [Google Scholar]
- 123.Gao J, Zhang B, Gao Y, Pan Y, Zhang X, Xu B. J Am Chem Soc. 2007;129:11928–11935. doi: 10.1021/ja0731017. [DOI] [PubMed] [Google Scholar]
- 124.Corr SA, Rakovich YP, Gun’ko YK. Nanoscale Res Lett. 2008;3:87–104. [Google Scholar]
- 125.Quarta A, Di Corato R, Manna L, Ragusa A, Pellegrino T. IEEE Trans Nanobiosci. 2007;6:298–308. doi: 10.1109/tnb.2007.908989. [DOI] [PubMed] [Google Scholar]
- 126.Choi JS, Park JC, Nah H, Woo S, Oh J, Kim KM, Cheon GJ, Chang Y, Yoo J, Cheon J. Angew Chem Int Ed. 2008;47:6259–6262. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]
- 127.Galperin A, Margel S. J Biomed Mater Res Part B. 2007;83B:490–498. doi: 10.1002/jbm.b.30821. [DOI] [PubMed] [Google Scholar]
- 128.Peer D, Karp JM, Hong S, FaroKhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 129.Lewinski N, Colvin V, Drezek R. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 130.Vega-Villa KR, Takemoto JK, Yanez JA, Remsberg CM, Forrest ML, Davies NM. Adv Drug Deliv Rev. 2008;60:929–938. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 131.Patri AK, Dobrovolskaia MA, Stern ST, McNeil SE. In: Nanotechnology for Cancer Therapy. Amiji MM, editor. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- 132.Garnett MC, Kallinteri P. Occup Med (Lond) 2006;56:307–311. doi: 10.1093/occmed/kql052. [DOI] [PubMed] [Google Scholar]