Abstract
Chronic abuse of cocaine or alcohol is associated with structural, neuropathological and cognitive impairments that have been documented extensively. Little is known, however, about neurobiochemical changes in chronic substance abusers. We performed MRI and multi-slice brain proton magnetic resonance spectroscopic imaging (MRSI) to assess neuronal viability (via N-acetylaspartate (NAA)) and white matter metabolite status in 22 4-months-abstinent individuals dependent on crack cocaine only and on both crack cocaine and alcohol. Compared to 11 non-dependent controls we found (1) significantly lower NAA measures in the dorsolateral prefrontal cortex of the combined cocaine-dependent groups; (2) comparable spatial distribution and magnitude of these NAA effects for both cocaine-dependent groups; (3) higher choline-containing metabolites in frontal white matter of individuals dependent on both cocaine and alcohol; (4) absence of brain atrophy in both abstinent cocaine-dependent samples; and (5) partial recovery from prefrontal cortical NAA loss, primarily with abstinence from alcohol. The MRSI findings suggest preferential neuronal damage to the frontal cortex of both cocaine-dependent samples and gliosis in frontal white matter of individuals dependent on both alcohol and cocaine, conditions that persist for more than 4 months of abstinence.
Introduction
Alcohol use is common among cocaine-dependent subjects.1-3 Consumed in large quantities alcohol is neurotoxic in animals, leading to dendritic pruning and ultimately to nerve cell death.4 Chronic alcohol abuse in humans is associated with structural, neuropathological and functional impairments.5 Most positron emission tomography studies show lower frontal cerebral blood flow and glucose metabolism,6-8 while single photon emission computed tomography demonstrates a higher than normal incidence of frontal perfusion deficits.6,9 Computed tomography and magnetic resonance imaging (MRI) demonstrate sulcal widening, ventricular dilation, cerebellar degeneration and gray and white matter volume losses.10-12 Although most of these changes are reversible with abstinence within weeks to months,13,14 especially in younger alcoholics, atrophy is most persistent in the dorsolateral prefrontal cortex. Neuropathological studies in chronic drinkers15 show neuron loss primarily in the superior frontal cortex and in the cerebellum. Gross cortical volume loss is not consistently observed in all chronic alcoholics.15
In contrast to alcohol, there is little evidence for direct toxic effects of cocaine on brain neurons. Cocaine did not cause cytotoxic damage to neurons in animals.16,17 In humans, chronic cocaine use is associated with cerebrovascular events such as hemorrhages,18,19 cerebral vasculitis,20 infarctions21 and stroke, associated with MRI evidence of cerebral infarction22 and increased prevalence of white matter signal hyperintensities. Computed tomography studies demonstrated cerebral atrophy, accompanied by primarily sulcal enlargements.23 Cerebral blood flow defects were demonstrated in about 70% of cocaine abusers during and shortly after withdrawal compared to only 29% in controls.24,25 Cerebral blood flow,26 glucose metabolic rates27 and perfusion28-31 were reduced primarily in frontal, lateral frontal and cingulate cortices in the absence of structural imaging evidence of infarction and were even present in individuals abstinent for more than 3 months.26,27,29 More recently, perfusion defects in frontal and parietal cortices of 3-day-abstinent cocaine abusers were found to be associated with concurrent alcohol abuse,32 suggesting that concurrent alcohol abuse is primarily responsible for perfusion defects in cocaine-dependent subjects.30,32 Blood flow or perfusion studies are not particularly sensitive to white matter abnormalities, and these studies did not correct for possible brain tissue loss.
This proton MR spectroscopic imaging (1H MRSI) study was performed to determine effects of chronic crack cocaine dependence and crack cocaine plus alcohol dependence on MR-detectable brain metabolites. In vivo 1H MRSI assesses regional neuronal/axonal viability via the amino acid N-acetylaspartate (NAA) and gliosis or inflammation via measurement of choline-containing metabolites (Cho).33 Previous 1H MRS findings of metabolite abnormalities in active34 or abstinent cocaine-dependent individuals35 have been inconsistent. Moreover, most MRS studies have had limited ability to measure metabolites in the lateral frontal cortex, precisely the brain region of most interest in chronic substance abusers. Recent improvements in 1H MRSI methodology36 now allow metabolite measurements in cortical brain regions. The specific aims of this study were to measure regional cerebral (including cortical) proton metabolite levels in crack cocaine-dependent and crack cocaine/alcohol-dependent individuals abstinent from drugs for several months. Our a priori hypothesis was that NAA levels are diminished in the prefrontal cortex of cocaine-dependent subjects, reflecting compromised neuronal viability. We also sought to determine whether chronic alcohol dependence potentiates brain damage in chronic cocaine-dependent individuals, and whether potential metabolite alterations are associated with measures of the severity of drug use or with the presence and severity of cognitive impairments.
Methods
Subjects
With approval from the local committee on human research and informed consent, we studied 22 crack cocaine-dependent individuals (mean age 41.4 ± 6.0 years) and 11 controls (37.3 ± 8.5 years). Of the crack cocaine-dependent sample, seven were only dependent on crack cocaine, while 15 were dependent on both crack cocaine and alcohol. Subject characteristics and substance use measures are presented in Table 1. All cocaine-dependent subjects were recruited from local substance abuse treatment programs; controls were recruited from the research laboratory and from the community via fliers and word-of-mouth. Prior to the MRI study, participants were interviewed to assess medical and drug history. Exclusion criteria for all subjects were pre-existing cardiovascular or neurological diseases (e.g. seizures, head injury, stroke), a history of head trauma with loss of consciousness and medical disorders with known neurological effects (e.g. hypertension, diabetes, HIV infection). All cocaine-dependent participants met DSM -IV criteria for life-time dependence on cocaine and/or alcohol and no cocaine-dependent participant met DSM-IV criteria for life-time dependence on any substance other than alcohol or cocaine. All cocaine-dependent participants were heavy cocaine users for more than 5 years, while alcohol/cocaine-dependent participants also drank more than 70 alcohol-containing drinks per week on average for at least 12 years prior to the study. On average, subjects were studied 16 weeks into abstinence from both cocaine and alcohol, after withdrawal symptoms had subsided. Urine screens were obtained from all former cocaine-dependent subjects weekly throughout abstinence and on the day of study to ensure the absence of drugs.
Table 1.
Subject demographics
| Subjects (n and gender) |
Age (years) |
Ethnicity | Cocaine use ($/week) |
Cocaine use duration (years) |
Duration of abstinence from cocaine (weeks) |
Alcohol use (drinks/week) |
Alcohol use duration (years) |
Duration of abstinence from alcohol (weeks) |
|---|---|---|---|---|---|---|---|---|
| Controls (9 m, 2 f) |
37.3 ± 8.5 (26–50) |
2 Afri.-Ame. 8 Caucasian 1 Native Am. |
0.0 | NA | NA | 6.5 ± 5.7 (1.0–17.5) |
Not determined | NAa |
| Cocaine (6 m, 1 f) |
41.0 ± 6.3 (32–50) |
6 Afri.-Ame. 1 Caucasian |
270 ± 260 (60–760) |
9.5 ± 3.5 (6.6–14.8) |
18.8 ± 11.8 (5–34) |
4.2 ± 6.2 (0.0–12.0) |
Not determined | NAb |
| Cocaine/alcohol (13 m, 2 f) |
41.6 ± 6.1 (27–52) |
6 Afri.-Ame. 4 Caucasian 5 Hispanic |
160 ± 170 (50–630) |
15.6 ± 5.7c (5.0–23.2) |
15.7 ± 11.1 (5–39) |
70.7 ± 57.1d (21–218) |
24.7 ± 7.0 (12–34) |
16.4 ± 10.8 e (5–31) |
Data show mean ± standard deviation, range given in parentheses.
Not abstinent from alcohol
three subjects abstinent from alcohol
p < 0.02 relative to cocaine-only-dependent group
peak use: 130 ± 75 drinks/week (range: 25–250)
n = 14, one subject abstinent for 10.6 years
Neuropsychological testing
Participants were administered the computerized MicroCog Assessment of Cognitive Functioning (standard version),37 which includes 18 subtests used to assess performance in the domains of attention, abstraction, spatial processing, immediate and delayed memory, learning and reaction time. The MicroCog assessment took approximately 45–60 minutes to complete. Age- and education-adjusted Z-scores were calculated for all MicroCog subtests. Each domain’s average Z-score was referred to the cumulative normal distribution as described in the original references,37 then converted to a percentile score and finally to a clinical impairment score. A clinical impairment score of 0 was assigned to domain Z-scores falling above the 15th percentile, a clinical impairment score of 1 was assigned to domain Z-scores falling at or below the 15th and above the 5th percentile, and a clinical impairment score of 2 was assigned to domain Z-scores falling at or below the 5th percentile. The domain clinical impairment scores were summed across domains to yield a Global Clinical Impairment Score (GCIS). Subjects with a GCIS of 0 or 1 were classified as cognitively normal. Subjects with a GCIS of 2 or greater showed evidence of clinical neuropsychological impairment in at least one cognitive domain, and were classified as having some clinical cognitive impairment; subjects with a GCIS of 2–5 were considered mild-to-moderately cognitively impaired, while those with GCIS of 6 or more were considered severely cognitively impaired.
MRI
Studies were performed on a 1.5 Tesla Magnetom VISION™ system (Siemens Inc., Iselin, NJ, USA) operating at 63.64 MHz with a standard circularly polarized head coil. The MRI protocol consisted of sagittal T1-weighted localizer scans, of oblique-axial double spin-echo imaging (TR (repetition time)/TE (echo time)1/TE2 = 2575/20/80 ms) using contiguous 3-mm thick slices angulated -10° from a line parallel to the planum sphenoidale as seen on a mid-sagittal localizer MRI, and of a three-dimensional magnetization prepared rapid gradient echo acquisition (TR/TI/TE = 10/250/4 ms, flip angle = 15°, in-plane resolution 1.0 × 1.0 mm2) with 1.5-mm thick partitions perpendicular to the optic nerve as seen on midsagittal localizer MRIs. All MRIs were interpreted by a board-certified radiologist who was blinded to subject status.
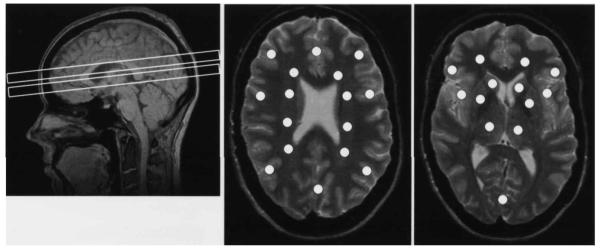
After MRI acquisition, the magnet was shimmed automatically to an average water-line width of 6±10 Hz in the spectroscopy region. After shimming, two-dimensional 1H MR spectroscopic imaging was perform ed simultaneously in two laterally unrestricted 15-mm-thick slices within 34 minutes. The home-written multi-slice inversion—recovery spin-echo sequence (TR/TE = 1800/135 ms) used a slice-selective 180° inversion pulse followed by a delay of 170 ms to achieve nulling of the lipid signal originating mainly from lipids of the scalp. Water suppression used three Gaussian pulses. The MRSI field of view (280 × 280 mm2) was sampled using a circular k-space scheme of 36 × 36 phaseencoding steps, resulting in a nominal in-plane resolution of 8.0 × 8.0 mm2 and 0.9 ml voxel size. Using the sagittal localizer and the oblique-axial spin-echo MRIs, two spectroscopic imaging slices were placed to encompass subcortical nuclei, thalami and cortices in one slice and central white matter and cortices in a periventricular slice, which was centered 21 mm in cranial direction from the subcortical slice (Fig. 1).
Figure 1.
Brain regions selected for proton MR spectroscopic imaging. The sagittal MR image (TR/TE = 500/6 ms) shows the typical placement of the two slices which yielded metabolite data (left). The slices are angulated at -10 degrees from the planum sphenoidale at the base of the frontal brain. Each slice is 15 mm thick and separated by a 6-mm thick gap to avoid cross-talk. The lower slice contains subcortical brain structures, the upper slice periventricular brain. The oblique-axial MR images (TR/TE = 2575/80 ms) are from the center of the periventricular MRSI slice (middle) and from the center of the subcortical MRSI slice (right). The overlaid white points correspond in size and position to the regions from where MRSI spectra were extracted for quantitative analysis. Sixteen spectra were extracted from the periventricular and 13 from the subcortical slice. The most anterior lateral cortical positions in the periventricular slice correspond to the dorsolateral prefrontal cortex. See text for exact voxel placements.
The 1H MRSI data were zero-filled to 64 × 64 × 1024 points, and the residual water signal was rem oved using a convolution filter. A 1-Hz Gaussian spectral filter was applied whereas no apodization was used in the spatial domains. These operations as well as Fourier transform, phase correction, data display and voxel extraction used standard Siemens software. Voxel placement and spectral processing was performed by the same rater who was blinded to the subject’s diagnosis. A summed image consisting of axial T2-weighted images from the top, middle and bottom of the MRSI slices was used as a guide for voxel placement, while the operator was blinded to the quality of the spectra at time of selection. At the levels of the lateral ventricles (periventricular slice), 16 single-voxel spectra were selected from cortical gray matter and from white matter (Fig. 1) as follows: bilateral gray matter spectra from anterior frontal cortex (‘dorsolateral prefrontal’), posterior frontal cortex and parietal cortex; additional gray matter spectra from frontal medial gray matter (‘anterior cingulate’) and posterior-parietal medial gray matter; bilateral white matter spectra were extracted from frontal and posterior white matter, and from white matter superior to the head of the caudate and thalami (‘central white matter’) in anterior and posterior centrum semiovale, respectively. At the level of the third ventricles (subcortical slice), 13 single-voxel spectra were selected to evaluate bilateral lenticular nuclei (voxel centered on the midline between globus pallidus and putamen), caudate (including at least 50% of caudate tissue as seen on the summed MRI) and central thalami, as well as bilateral orbitofrontal and insular cortices and occipital cortex. All extracted spectra were transferred to a SUN workstation (SUN Microsystems, Mountain View, CA, USA) and analyzed using NMR1 software (New Methods Research Inc, Syracuse, NY, USA). Spectral processing included inverse Fourier transform, convolution of time domain data by 1 – 0.5 × exp (-t × 40 Hz) to remove broad signal components from macromolecules and lipids, Fourier transform, linear baseline correction and phasing. Figure 2 shows typical spectra obtained from different brain regions of a cocaine/alcohol-dependent subject after the described processing procedures. Extracted spectra were excluded from data analysis when the signal-to-noise ratio of the NAA signal was below 4, when spectral line widths exceeded 13 Hz (in which case resonances from Cho and creatine-containing metabolites (Cr) could not be distinguished reliably) and when lipid resonances overlapped with NAA resonances. Peak fitting used Gaussian line shapes for NAA, Cho and Cr. The peak integrals were corrected for receiver gain and coil loading (based on the transmitter amplitude necessary to achieve a 180° flip angle pulse), allowing comparison of integrals between subjects. Because no relaxation time measurements were performed in this study, the peak integrals were not corrected for relaxation effects and, therefore, were not transformed into units of concentration. Nevertheless, the reported peak integrals are proportional to metabolite concentrations. Ratios of metabolite peak integrals were also computed, in order to eliminate dependence of absolute measures on partial volume (internal standardization) and because they are commonly used in the literature.
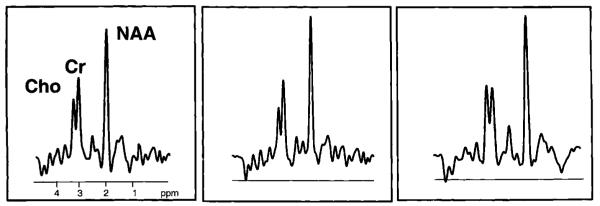
Figure 2.
1H MRSI spectra (TR/TE = 1800/135 ms) obtained from dorsolateral prefrontal cortex (left), posterior-parietal cortex (middle) and frontal white matter (right) indicated on the oblique-axial MRI in Fig. 1 of a 27-year-old alcohol-dependent cocaine addict abstinent for 4 months. The three major peaks are from n-acetyl-containing metabolites (primarily N-acetylaspartate or NAA) at 2.02 p.p.m., creatine-containing metabolites (Cr) (primarily creatine and phosphocreatine) at 3.03 p.p.m. and from choline-containing metabolites (Cho) (primarily glycerophosphocholine and phosphoch oline) at 3.23 p.p.m.
MRI tissue type segmentation
The MR images were segmented by software developed in-house and described previously.38 They were used to estimate the tissue composition of the MRSI voxels by examining the tissuesegmented images with the MRSI voxels superimposed and by taking into consideration the MRSI spatial response function and metabolitespecific caudo-cranial chemical shift displacements (i.e. contributions of tissue and cerebral spinal fluid were determined for all three voxels of the chemically shifted resonances, NAA, Cr and Cho). With the premise that NAA is not detected in cerebral spinal fluid, tissue-corrected NAA intensities were then computed by dividing the NAA integrals by the amount of tissue enclosed in a voxel. In addition, to verify that metabolite effects were not simply an artifact of differences between groups in the tissue composition of the spectroscopy voxels, the gray matter, white matter and white matter signal hyperintensity content in each of the extracted MRSI voxels was used as a covariate in statistical analyses. This MRI/MRSI co-analysis could only be performed in 25/33 subjects (eight controls, six crack cocaine-dependent and 11 crack cocaine/alcohol-dependent subjects). Six subjects had incomplete MRI examinations due to time constraints, while technical problems prevented the application of the co-analysis program in two other cases.
Statistical analysis
Statistical analyses were performed using SAS™ software (SAS Institute Inc., Cary, NC, USA). Groups were controls, crack cocaine-dependent and crack cocaine/alcohol-dependent. Student’s t-test was used to test for differences between groups of demographic measures. Metabolite differences between groups by region were tested using repeated-measures analysis of variance with dorsolateral prefrontal gray matter being the a priori region of interest. Metabolite measures from left and right voxels in the same brain region were averaged to give regional gray matter and white matter measures. Overall gray matter and overall white matter measures were obtained by averaging all measures obtained in gray matter or white matter of the same slice. Adjustments for multiple comparisons (Bonferroni corrections) were applied when analyzing metabolite differences in white matter regions. Measurement variables were peak integrals for NAA, Cho and Cr, and peak integral ratios. Metabolite ratios were the primary dependent variables in this analysis because of presumed better statistical variance by virtue of internal standardization. Peak integrals were examined to prevent misinterpretation of the ratio data but are prone to random instrument instabilities over the course of the project that could not be accounted for by periodic stability checks. Years of education and cocaine use duration were different between groups and were used as covariates to determine the degree to which these variables could account for group differences in the metabolite measures. Although groups were age-matched, age was used as covariate because of its possible effect on metabolite measures. MRI segmentation yielded tissue types (gray matter, white matter, cerebral spinal fluid and white matter signal hyperintensities) for all voxels. Repeated-measures analysis of covariance was used to determine the extent to which potential differences of MRSI voxel tissue composition contributed to any group differences in metabolite measures. Associations of metabolite measures with demographic and drug use data were assessed using Spearman correlations. In all tests, the statistical significance criteria p was set at 0.05. Results are expressed as mean ± one standard deviation.
Results
Study population
Subject demographics are summarized in Table 1. Mean age and age range were similar for all groups (p < 0.2). The controls as a group had more years of education (16.6 ± 2.3 years) than cocaine-only (13.4 ± 2.4, p < 0.02) or coabusing subjects (12.7 ± 2.4, p < 0.001). Average GCIS was 1.3 ± 1.6 for the cocaine-dependent sample (i.e. individuals were mildly impaired) and 2.8 ± 3.1 for the cocaine/alcohol-dependent sample (i.e. individuals evidenced moderate impairments); the difference was not statistically significant. Control subjects showed no impairments. The amount of cocaine used did not differ between groups. Within the cocaine/alcohol-dependent sample there was no correlation between the amount of alcohol and the amount of cocaine consumed daily. The sample dependent on alcohol and cocaine used cocaine for a significantly longer time (15.6 ± 5.7 years) than the sample dependent on cocaine (9.5 ± 3.5 years, p < 0.02). By design, alcohol use was higher in the alcohol/dependent group relative to both the group dependent only on cocaine (p < 0.02) and the control group (p < 0.01).
MRI
According to the neuroradiologist’s reading, all controls had normal MRI readings and none of the abstinent cocaine-dependent individuals had atrophy on MRI. Three of the 22 substancedependent subjects (14%) had abnormal MRI readings: one cocaine-dependent individual had ethmoidal paranasal sinus disease, one cocaine/alcohol-dependent individual had punctate white matter signal hyperintensities, while another presented with large confluent areas of white matter signal hyperintensities that were presumed ischemic. 1H MR spectra were not obtained from regions of white matter signal hyperintensities.
We observed no differences between groups in the amount of white matter, gray matter, white matter signal hyperintensities or cerebral spinal fluid contributing to the MRSI voxels. For example, in central white matter, the white matter fraction was 95% in all three groups and the gray matter fraction varied between 3 and 5% with the rem ainder taken up by white matter signal hyperintensities. Tissue from voxels selected in dorsolateral prefrontal gray matter regions consisted of 68–76% gray matter, 24–32% white matter and no white matter signal hyperintensities, while CSF contributed between 13% and 15% to the volume of dorsolateral prefrontal gray matter voxels (percentages are group averages). In summary, we did not detect atrophy in the bilateral frontal MRSI voxels of abstinent substance abusers.
1H MR spectroscopic imaging
Spectral quality
In the periventricular slice, the quality of spectra (as judged by signal-to-noise, spectral resolution and spectral contamination by lipid resonances as described in Methods) from all lateral cortical and occipital gray matter regions and from all white matter regions, except frontal, was excellent. Dorsolateral prefrontal gray matter spectra from only one subject and white matter spectra from another individual did not meet inclusion criteria. Qualitatively acceptable spectra from the anterior cingulate gyrus were obtained in only 55% of the subjects. In the subcortical slice, the quality of frontal spectra was generally worse than that of periventricular spectra, with the worst spectra obtained from frontal white matter and caudate. Spectral quality in these regions is degraded due to the proximity of the frontal sinus, eye sockets and major blood vessels, causing susceptibility differences that cannot be completely eliminated by magnet shimming. In these regions, broadening of metabolite resonances and shifting of the water resonance compromised the quality of water suppression as demonstrated previously.39 In addition, the quality of subcortical spectra was further degraded by fat resonances from eye and scalp muscles and from adipose tissue originating in badly shimmed neighboring brain regions. Given the spectral quality assurance criteria described in Methods, approximately onequarter of all subcortical spectra had to be excluded from data analysis, while at the same time the overall quality of most of the remaining subcortical spectra was of somewhat lower quality than spectra from periventricular regions. This lowered the power of the study to detect potential metabolite group differences in subcortical brain.
Periventricular metabolite findings
Table 2 lists metabolite ratios from the periventricular slice in substance abusers and controls, Figure 3 shows corresponding absolute metabolite integrals. The major findings compared to the control group were as follows.
Table 2.
Selected periventricular metabolite ratios in abstinent substance abusers and controls
| Group | NAA/Cr overall white matter |
NAA/Cho overall white matter |
NAA/Cr central white matter |
NAA/Cho central white matter |
NAA/Cr overall gray matter |
NAA/Cho overall gray matter |
NAA/Cr dorsolat. prefrontal gray matter |
NAA/Cho dorsolat. prefrontal gray matter |
|---|---|---|---|---|---|---|---|---|
| Controls (n = 11) |
2.04 ± 0.21 | 1.75 ± 0.18 | 2.34 ± 0.42a | 2.09 ± 0.33a | 1.90 ± 0.18 | 2.42 ± 0.45 | 1.98 ± 0.44 | 2.57 ± 0.62 |
| Cocaine (n = 7) |
1.96 ± 0.31 | 1.59 ± 0.24 | 2.00 ± 0.34 | 1.79 ± 0.25 p < 0.1 |
1.71 ± 0.07 p < 0.02 |
2.33 ± 0.39 | 1.59 ± 0.16 p < 0.03 |
2.22 ± 0.43 |
| Cocaine/alcohol (n = 15) |
2.01 ± 0.29 | 1.74 ± 0.43 | 2.05 ± 0.36b p < 0.1 |
1.81 ± 0.42b p < 0.1 |
1.73 ± 0.26 p < 0.1 |
2.30 ± 0.32 | 1.63 ± 0.27b p < 0.2 |
2.08 ± 0.44b p < 0.5 |
| Substance abusers pooled (n = 22) |
1.99 ± 0.30 | 1.69 ± 0.38 | 2.04 ± 0.35c p < 0.05 |
1.80 ± 0.36c p < 0.05 |
1.72 ± 0.22 p < 0.03 |
2.31 ± 0.33 | 1.61 ± 0.24c p < 0.00 |
2.13 ± 0.43c p < 0.05 |
Data show mean ± standard deviation; significance level p relative to controls.
n = 10
n = 14
n = 21
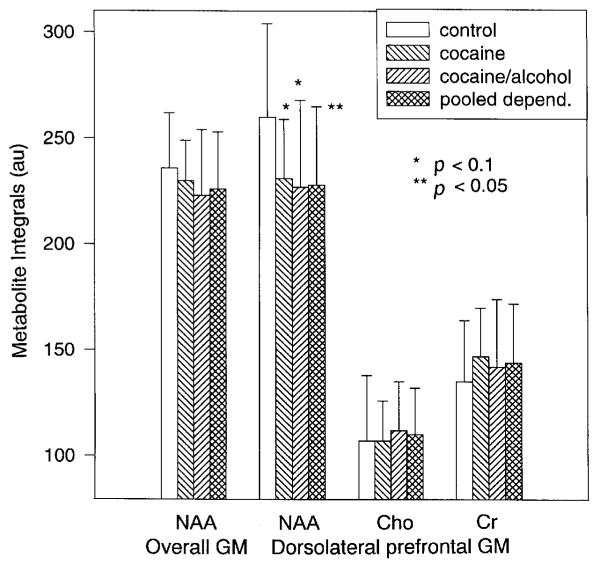
Figure 3.
Bar graph of periventricular metabolite integrals (mean ± standard deviation) by group: NAA in overall gray matter (GM) and NAA, Cho and Cr in dorsolateral prefrontal gray matter. Overall GM is the mean of all gray matter measures.
Sample dependent on cocaine only
In this sample, average NAA/Cr from all periventricular gray matter locations was 10% lower than in controls (p = 0.02), with the strongest NAA/Cr loss in dorsolateral prefrontal gray matter (-20%, p = 0.04). These gray matter NAA/Cr losses were independent of age as assessed by covariate analysis. NAA/Cho averaged over all gray matter voxels and in dorsolateral prefrontal gray matter were unchanged between groups (p > 0.2) and independent of age in covariate analyses. While NAA averaged over all gray matter regions was similar between groups, NAA in dorsolateral prefrontal gray matter tended to be 11% lower in the cocaine-dependent sample compared to controls (p = 0.1) (see Fig. 3). White matter NAA ratios of the cocaine-dependent sample were not different from controls, except for lower NAA/Cho (p = 0.02) and a trend to higher Cho (+18%, p = 0.06) in white matter superior to the caudate. Correction for multiple comparisons further reduced the significance of these white matter findings. Thus, these data show trends to lower NAA in dorsolateral prefrontal gray matter and trends to higher Cho in white matter superior to the caudate of cocaine-dependent subjects compared to controls.
Sample dependent on both cocaine and alcohol
In this sample, both dorsolateral prefrontal gray matter NAA ratios were significantly lower compared to controls (NAA/Cho, -19%, p = 0.03; NAA/Cr, -18%, p = 0.02), while NAA ratios averaged over all gray matter voxels were similar in these groups. In the dorsolateral prefrontal gray matter, lower NAA ratios were due to a trend to lower NAA (-13%, p = 0.07), while Cho and Cr were unchanged between groups (see Fig. 3). The observed NAA/Cr difference was independent of age or years of education. Frontal gray matter NAA/Cho decreased with fewer years of education (p = 0.02), but rem ained significant for group when co-varying for years of education (p = 0.03). NAA, Cho and Cr measures averaged over all white matter voxels were unchanged in cocaine/alcohol-dependent subjects compared to controls. Both NAA ratios in central white matter adjacent to the thalami, however, were more than 14% lower in the cocaine/alcohol-dependent sample (NAA/Cr, p = 0.02; NAA/Cho, p = 0.1) and NAA in this region tended to be lower (p = 0.1). In bilateral frontal white matter, Cho was 22% higher (p = 0.02), NAA tended to be lower (p = 0.1), and NAA/Cho was 27% lower (1.10 ± 0.21) than in controls (1.55 ± 0.26, p = 0.005) while frontal white matter NAA/Cr was unchanged. Corrected for multiple comparisons, only bilateral frontal white matter NAA/Cho remained significantly lower in cocaine/alcohol-dependent individuals than in controls (note, however, that qualitatively acceptable frontal white matter measures were only obtained in 7/15 cocaine/alcohol-dependent subjects and in 9/11 controls). Lower frontal white matter NAA/Cho in the cocaine/alcohol-dependent sample was independent of age, education or duration of cocaine use. In summary, metabolite measures reflect lower NAA in frontal gray matter and possibly higher Cho in frontal white matter in cocaine/alcohol-dependent subjects compared to controls.
Pooled cocaine-dependent samples
The pooled cocaine-dependent samples compared to controls exhibited (a) 19% lower NAA/Cr (p = 0.005) and 17% lower NAA/Cho (p = 0.02) in dorsolateral prefrontal gray matter and (b) 13% lower NAA/Cr (p = 0.04) and 14% lower NAA/Cho (p = 0.04) in central white matter adjacent to the thalami. These metabolite ratios were lower due to 12% lower NAA in dorsolateral prefrontal gray matter (p = 0.04) and due to a trend to lower NAA in central white matter adjacent to the thalami (-8%, p = 0.07). The central white matter NAA differences lost significance when adjusted for multiple comparisons. No significant group differences were observed for Cho, Cr or Cho/Cr in any of the regions analyzed (p > 0.2) so that the observed metabolite abnormalities in the pooled cocaine-dependent samples were solely due to NAA losses. Metabolite measures in posterior frontal, parietal or medial cortices were not different from controls.
Since age has been shown to influence brain metabolite measures, we analyzed the effects of age on outcome measures. Within the pooled cocaine-dependent samples, age varied from 27 to 50 years and was negatively correlated with NAA/Cho in dorsolateral prefrontal gray matter (Spearman’s r = -0.60, p < 0.01). This correlation became somewhat stronger when controls (age range 25–50 years) were included in the analysis (r = -0.67, p < 0.0001). No such correlations were observed for the corresponding NAA/Cr measure, suggesting that age-dependent Cho elevation is at least partly responsible for the observed correlation and for NAA/Cho group differences. Absolute Cho integrals, however, did not vary with age. Nevertheless, when age was used as a covariate, dorsolateral prefrontal gray matter NAA/Cho remained significantly lower in the pooled cocaine dependent sample vs. controls (p < 0.02). This, together with the corresponding NAA/Cr findings, suggests that frontal cortical NAA loss was independent of age. In contrast, in white matter, covariate analysis showed that age, not group, was the major determining factor for the NAA/Cho group difference (p < 0.01). Similarly, NAA/Cr in central white matter covaried with age (p < 0.03), but remained almost significant for group (p < 0.06). This suggests that NAA loss in central white matter was due primarily to age but possibly also due to cocaine- and/or alcohol-dependence.
MRI/MRSI co-analysis
The most important findings of this study were lower NAA measures in dorsolateral prefrontal gray matter of cocaine-dependent individuals in the absence of atrophy in the MRSI voxels. When the coil loading-corrected NAA peak areas in dorsolateral prefrontal gray matter voxels were normalized to the fraction of tissue contributing to the voxel volume, the resulting coefficient of variation was increased by almost 20%, resulting in reduced effect sizes and reduced power to detect group differences. Furthermore, in an analysis where tissue fraction or cerebral spinal fluid fraction was used as covariate because of their effects on metabolite measures, dorsolateral prefrontal gray matter NAA measures were found to be independent of the tissue fraction of gray matter, white matter or cerebral spinal fluid, presumably because of little variation in the tissue composition of MRSI voxels. As described above, NAA peak integrals in central white matter adjacent to the thalami tended to be lower in the pooled cocaine-dependent subjects vs. controls. This trend became statistically significant after correction for tissue fraction (-11%, p = 0.04), strengthening the suggestion above of axonal damage in central white matter of the pooled cocaine-dependent subjects.
Associations of drug use and demographic data with metabolite measures
Within the pooled cocaine-dependent samples, regionally specific metabolite ratios were associated with measures of drug use. While present, these correlations must be interpreted with caution because of the large number of correlations computed. Using Spearman ranked tests, we found that cocaine/alcohol-dependent subjects with the longest time of abstinence from alcohol had the highest NAA/Cr ratios in overall gray matter and in dorsolateral prefrontal cortex (both r = 0.57, p = 0.03). Trends to such correlations were observed with length of abstinence from cocaine (r > 0.44, p < 0.1). These limited cross-sectional data suggest that these NAA/Cr measures are even lower in active drug users or in former drug users abstinent for fewer than 16 weeks, and that some metabolic recovery does occur with abstinence from alcohol, but not as much with abstinence from cocaine. None of the NAA measures in gray matter or white matter of the pooled cocaine-dependent samples were correlated with duration of drug use, except that lower NAA/Cho in central white matter was associated with the duration of cocaine use (r = -0.62, p = 0.02). This correlation, however, was probably due to higher Cho, since duration of cocaine use was also positively correlated with Cho/Cr averaged over all periventricular white matter voxels (r = 0.54, p = 0.04), and tended to be correlated with Cho/Cr in central white matter (r = 0.45, p < 0.10). Duration of cocaine use and age were not correlated (p = 0.2) so that white matter Cho measures in the pooled substance abusers, although not significantly higher than in controls, were associated with prolonged cocaine use, but not with age. Similarly, subjects with the longest history of alcohol use had the highest white matter Cho ratios (Cho/Cr averaged over all periventricular white matter voxels: r = 0.53, p < 0.04; Cho/Cr in central white matter: r = 0.55, p < 0.04). Since the duration of alcohol use and age of the pooled cocaine-dependent subjects was positively correlated (r = 0.66, p < 0.01), we perform ed an ANCOVA with both duration of alcohol use and age as covariates to determine which was responsible for higher Cho ratios. Only the correlation of overall white matter Cho/Cr with alcohol use duration remained statistically significant (p < 0.02), suggesting white matter Cho/Cr increases with prolonged alcohol use in cocaine-dependent subjects. Furthermore, drinking severity (assessed by the number of daily alcoholic drinks averaged over life-time) showed no correlation with any of the metabolite measures. Taken together these correlations suggest (1) higher white matter Cho measures, but not lower NAA measures, associated with longer duration of both cocaine and cocaine/alcohol abuse and (2) recovery from low dorsolateral prefrontal gray matter NAA ratios over time with abstinence from alcohol, but not with abstinence from cocaine. Global clinical impairment (assessed by GCIS) was not associated with any of the metabolite measures; this was not surprising considering the relatively narrow range of cognitive impairment in the subject cohort.
Subcortical metabolite findings
Table 3 lists subcortical metabolite ratios in substance abusers and controls. In this brain region there were no significant group differences for any of the metabolite measures. There was a trend to lower NAA/Cr in bilateral orbitofrontal gray matter of crack cocaine-dependent subjects (-20%, p = 0.08); however, the same measure was unchanged in cocaine/alcohol-dependent subjects. Orbitofrontal gray matter NAA/Cho was unchanged between either of the groups. Similarly, metabolite levels in insular gray matter did not differ among groups. It is possible that the smaller number of qualitatively acceptable spectra obtained from the subcortical SI slice (see above) reduced the power of this study to detect significant subcortical metabolite group differences. Alternatively, subcortical brain, including the orbitofrontal and insular gray matter regions investigated, may be less vulnerable to the damaging effects of chronic drug abuse than dorsolateral prefrontal gray matter (see Introduction).
Table 3.
Selected subcortical metabolite ratios in abstinent substance abusers and controls
| Group | NAA/Cr lenticular nucleus |
NAA/Cho lenticular nucleus |
NAA/Cr thalamus |
NAA/Cho thalamus |
NAA/Cr overall gray matter |
NAA/Cho overall gray matter |
NAA/Cr orbitofrontal gray matter |
NAA/Cho orbitofrontal gray matter |
|---|---|---|---|---|---|---|---|---|
| Controls (n = 9) |
1.47 ± 0.39 | 1.87 ± 0.64 | 1.93 ± 0.48 | 1.82 ± 0.38 | 1.72 ± 0.33 | 2.30 ± 0.51 | 1.69 ± 0.42 | 2.43 ± 0.60 |
| Cocaine (n = 6) |
1.46 ± 0.24a | 1.67 ± 0.55a | 1.69 ± 0.20 | 1.79 ± 0.40 | 1.58 ± 0.27 | 2.17 ± 0.41 | 1.35 ± 0.12 p = 0.08 |
2.29 ± 0.68a |
| Cocaine/alcohol (n = 14) |
1.60 ± 0.54b | 1.61 ± 0.43b | 1.85 ± 0.27 | 1.82 ± 0.51 | 1.60 ± 0.23 | 2.02 ± 0.41 | 1.71 ± 0.34c | 2.23 ± 0.35c |
Data show mean ± standard deviation; significance level p relative to controls.
n = 5
n = 11
n = 12.
Discussion
The major findings of this cross-sectional 1H MRS study of recently abstinent cocaine and cocaine/alcohol-dependent individuals were: (1) absolute NAA, NAA/Cr and NAA/Cho from dorsolateral prefrontal cortex of the combined cocaine dependent groups were significantly lower than in controls. These NAA reductions were less prominent in central white matter regions. (2) The regional distribution and magnitude of these NAA effects were comparable in the samples dependent only on cocaine and dependent on both cocaine and alcohol. (3) There was evidence suggestive of higher than normal frontal white matter Cho in cocaine/alcohol-dependent subjects. (4) The MRI-derived tissue composition of the MRSI voxels did not differ between groups, indicating that the metabolite findings are not an artifact of structural differences between groups in the regions studied.
Lower than normal NAA in the dorsolateral prefrontal cortex of cocaine-dependent subjects (as assessed by both absolute and relative NAA measures) suggests lower neuronal viability, independent of the presence or absence of concomitant alcohol dependence. Low white matter NAA and high white matter Cho were found primarily in the frontal brain of individuals dependent on both alcohol and cocaine. Diminished NAA suggests axonal damage and increased Cho reactive gliosis in frontal white matter. These metabolite abnormalities were present after an average of 4 months of abstinence from both cocaine and alcohol, indicating lack of comprehensive metabolic recovery during this time period. Our correlation analyses, however, suggest at least partial recovery from prefrontal cortical NAA loss, primarily with abstinence from alcohol. The finding in these cocaine-dependent subjects of long-lasting diminished NAA levels in the frontal but not in the parietal cortex, together with lack of atrophy on MRI, is similar to and consistent with our earlier 1H MRSI findings in 9-months-abstinent elderly alcoholics.40
Recently, we reported marginally smaller ( < 10%) dorsolateral prefrontal cortical volume in a larger cocaine-dependent sample abstinent for about 8 weeks.41 In polysubstance abusers who were abstinent from drugs for at least 15 days, a similar 10% volume loss was observed by MRI in prefrontal cortical gray matter.42 These are relatively small regional changes, which are not necessarily reflected in the cortical spectroscopy voxels of the smaller cohort studied here, or which may have resolved 16 weeks into abstinence.
Our prefrontal cortical NAA findings were similar in magnitude in the cocaine-dependent and cocaine/alcohol-dependent samples. Thus, the results do not support the hypothesis of alcohol potentiating prefrontal neuronal damage in crack cocaine abusers. The possibly higher frontal white matter Cho measures in the coabusing group appear to be associated with longer cocaine abuse duration or with concurrent alcohol abuse. Since our finding of reduced NAA suggests impairment of neuronal processes, one would expect that cerebral blood flow and glucose metabolism is reduced to the extent that neurons are compromised. Therefore, diminished NAA is consistent with neuroimaging findings of reduced cerebral blood flow and glucose metabolism in chronic substance abuse and with neuropathological and neurocognitive findings, all of which implicate involvement of frontal cortex. In contrast to recent perfusion studies, which suggest that concurrent alcohol abuse is primarily responsible for perfusion defects in cocaine dependent subjects,30,32 our metabolic imaging results suggest neuronal damage also in samples dependent on cocaine only. The observation that the metabolite alterations were independent of the tissue composition of the spectroscopy voxels indicates that 1H MRSI allows detection of metabolite changes separate from morphological alterations. Our findings suggest sensitivity of cortical 1H MRSI measures to chronic drug effects, providing insight into the neuropathology of drug dependence that is consistent with long-lasting effects on prefrontal cortex.
In a single-volume 1H MRS study of male cocaine users abstinent for about 12 months no NAA loss was found in midoccipital gray matter, but NAA was lower in midfrontal gray and frontal white matter and Cr and myo-inositol were higher in frontal and temporoparietal white matter35 (and Linda Chang, personal communication). In a preliminary single-volume 1H MRS study of chronic active cocaine users, NAA/Cr was lower in white and medial gray matter of the frontal lobe by approximately 15% and in the basal ganglia by approximately 10%.34 These earlier studies suggest frontal cortical neuron loss and white matter damage in cocaine abusers, consistent with our spectroscopic imaging data of specific regional metabolite alterations. Damage to white matter of cocaine-dependent polysubstance abusers has also been observed via measuring phospholipid metabolites by phosphorous MR spectroscopy.43
Although this study failed to show increased neurotoxicity in abstinent alcohol-abusing crack cocaine users, animal studies suggest an increased risk of toxicity with concurrent cocaine and alcohol use. This increased risk of toxicity might be linked to the formation of cocaethylene, a more potent compound with lower stimulant properties than cocaine. One could argue that the lower potency of cocaethylene might lead to higher usage of cocaine in alcohol-dependent cocaine abusers. However, in this study the amount of alcohol used was not associated with the amount of cocaine used. This may be associated with the fact that higher plasma concentrations of cocaine are achieved when alcohol is used simultaneously,44-46 possibly off-setting the lower stimulant properties of cocaethylene.
Previously, we demonstrated NAA loss in dorsolateral prefrontal gray matter relative to bilateral parietal gray matter in elderly, 9-months-abstinent alcoholics without morphological changes. This suggests that frontal relative to parietal neuron loss and gliosis may underlie the persistent neuropsychological deficits evident in this elderly population.40 That study’s MRS methodology was different from the current study and the subjects were older, precluding direct comparisons. Both studies, however, are consistent with long-term chronic abuse of alcohol, cocaine or both, leading to 1H MRS detectable damage to neurons in the dorsolateral prefrontal cortex that is not completely reversible after several months of abstinence. Also, in both of these studies frontal cortical NAA measures were positively correlated with the duration of abstinence from alcohol. This suggests that these metabolite changes are, at least in part, reversible with abstinence from alcohol and that group differences are possibly more pronounced in active substance abusers or earlier in abstinence.
Acknowledgements
This study was supported by DA08365 (G. F.) and AA10788 (D. J. M.). We are thankful for Mr Gilbert Salas’ relentless efforts and expertise in recruiting subjects for this study.
References
- 1.Higgins ST, DeLaney DD, Budney AJ, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psych. 1991;148:1218–24. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 2.Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54:199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- 3.Grant BF, Hartford TC. Concurrent and simultaneous use of alcohol with cocaine: results of a national survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- 4.Harper C, Kril J. An introduction to alcoholinduced brain damage and its causes. Alcohol Alcohol. 1994;2(suppl.):237–43. [PubMed] [Google Scholar]
- 5.Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolas JM, Catafau AM, Estruch R, et al. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med. 1993;34:1452. [PubMed] [Google Scholar]
- 7.Volkow ND, Hitzemann R, Wolf AP, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res Neuroimaging. 1990;35:39. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 8.Matthew R, Wilson W. Substance abuse and cerebral blood flow (review) Am J Psychiatry. 1991;148:292. doi: 10.1176/ajp.148.3.292. [DOI] [PubMed] [Google Scholar]
- 9.Melgaard B, Henriksen L, Ahlgren P, Danielsen UT, Sorensen H, Paulson OB. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurol Scand. 1990;82:87. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 10.Jernigan T, Butters N, DiTraglia G, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alc Clin Exp Res. 1991;15:418. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 11.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alc Clin Exp Res. 1997;21:521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbloom M, Pfefferbaum A, Sullivan E. Structural brain alterations associated with alcoholism. Alcohol Health Res World. 1995;19:266–72. [PMC free article] [PubMed] [Google Scholar]
- 13.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alc Clin Exp Res. 1995;19:1177–91. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 14.Trabert W, Niewald M, Huber G. Significant reversibility of alcoholic brain shrinkage within 3 weeks of abstinence. Acta Psychiatr Scand. 1995;92:87–90. doi: 10.1111/j.1600-0447.1995.tb09548.x. [DOI] [PubMed] [Google Scholar]
- 15.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 16.Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther. 1988;245:485–92. [PubMed] [Google Scholar]
- 17.Kleven MS, Woolverton WL, Seiden LS. Lack of long-term following continuous or repeated exposure to cocaine. Brain Res Bull. 1988;21:233–337. doi: 10.1016/0361-9230(88)90236-5. [DOI] [PubMed] [Google Scholar]
- 18.Capella JA, Atetrero JMC, Rei JF. Complications of cocaine receptors. Ann Intern Med. 1987;107:940. doi: 10.7326/0003-4819-107-6-940. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein SR, Colins SD, Massa SM, McKinney HE, Benowitz N, Simon RP. The neurologic complications of cocaine abuse. Neurology. 1987;37(suppl. 1):195. [Google Scholar]
- 20.Krendel DA, Ditter SM, Frankel MR, Ross WK. Biopsy: proven cerebral vasculitis associated with cocaine abuse. Neurology. 1990;40:1092–4. doi: 10.1212/wnl.40.7.1092. [DOI] [PubMed] [Google Scholar]
- 21.Seaman ME. Acute cocaine abuse associated with cerebral infarction. Ann Emerg Med. 1990;19:34–7. doi: 10.1016/s0196-0644(05)82137-7. [DOI] [PubMed] [Google Scholar]
- 22.Strickland TL, Mena I, Villanueva-Meyer J, et al. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5:419–27. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- 23.Pascual-Leone A, Dhuna A, Anderson DC. Cerebral atrophy in habitual cocaine abusers: a planimetric CT study. Neurology. 1991;41:34–8. doi: 10.1212/wnl.41.1.34. [DOI] [PubMed] [Google Scholar]
- 24.Volkow N, Mullani N, Gould K, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psych. 1988;152:641. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Fowler JS, Wolf AP, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psych. 1991;148:621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Hitzemann K, Wang G-J, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 27.Porrino LJ, Flowers DL, Fahey FH, Wood FB. Patterns of cerebral metabolism of cocaine abusers vary with length of abstinence. Coll Problems Drug Depend. 1998;1:115. [Google Scholar]
- 28.Levin J, Holman B, Mendelson J, et al. Gender differences in cerebral perfusion in cocaine abuse: technetium-99m-HM PAO SPECT study of drugabusing women. J Nucl Med. 1994;35:1902–9. [PubMed] [Google Scholar]
- 29.Strickland TL, Mena I, Villanueva-Meyer J, et al. Cerebral perfusion and neuropsychological consequence of chronic cocaine use. J Neuropsych Clin Neurosci. 1993;5:419–27. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- 30.Holman B, Garada B, Johnson K, et al. A comparison of brain perfusion SPECT in cocaine abuse and AIDS dementia complex. J Nucl Med. 1992;33:1312. [PubMed] [Google Scholar]
- 31.Gatley SJ, Volkow ND. Addiction and imaging of the living human brain. Drug Alcohol Depend. 1998;51:97–108. doi: 10.1016/s0376-8716(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 32.Kosten TR, Cheeves C, Palumbo J, et al. Regional cerebral blood flow during acute and chronic abstinence from combined cocaine-alcohol abuse. Drug Alcohol Depend. 1998;50:187–95. doi: 10.1016/s0376-8716(98)00038-6. [DOI] [PubMed] [Google Scholar]
- 33.Vion-Dury J, Meyerhoff DJ, Cozzone PJ, Weiner MW. What might be the impact on neurology of the analysis of brain metabolism by in vivo magnetic resonance spectroscopy? J Neurol. 1994;241:354–71. doi: 10.1007/BF02033352. [DOI] [PubMed] [Google Scholar]
- 34.Li SJ, Wang Y, Prost RW, et al. Reversible neurochemical alterations in cocaine abusers assessed by 1H magnetic resonance spectroscopy. In: Hoffman EA, editor. Medical imaging. Vol. 3337. International Society for Optical Engineering; 1998. pp. 203–9. [Google Scholar]
- 35.Chang L, Mehringer M, Ernst T, et al. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997;42:1105–14. doi: 10.1016/s0006-3223(97)00135-2. [DOI] [PubMed] [Google Scholar]
- 36.Weiner MW, Maudsley AA, Schuff N, et al. Multislice 1H magnetic resonance spectroscopic imaging: assessment of epilepsy, Alzheimer’s disease, and amyotrophic lateral sclerosis. Proc Int Soc Optical Eng. 1999 in press. [Google Scholar]
- 37.Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog assessment of cognitive functioning. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 38.Cardenas VA, Ezekiel F, Disclafani V, Gomberg B, Fein G. Reliability of tissue volumes and their spatial distribution for segmental magnetic resonance images. submitted. [DOI] [PubMed]
- 39.Meyerhoff DJ, Weiner MW, Fein G. Deep gray matter structures in HIV infection: a 1H MR spectroscopic imaging study. Am J Neuroradiol. 1996;17:973–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Fein G, Meyerhoff DJ, DiSclafani V, et al. 1H magnetic resonance spectroscopic imaging separates neuronal from glial changes in alcohol-related brain atrophy. NIAAA Res Monogr. 1994;27:227–41. [Google Scholar]
- 41.Di Sclafani V, Bloomer C, Tolou-Shams M, Clark W, Norman D, Fein G. Alcohol and cocaine-codependent subjects abstinent 7.6 weeks do not evidence global atrophy on MRI. Alc Clin Exp Res. 1997;21:19A. [Google Scholar]
- 42.Liu X, Matochik JA, Cadet J-L, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–52. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- 43.MacKay S, Meyerhoff DJ, Dillon WP, Weiner MW, Fein G. Alteration of brain phospholipid metabolites in cocaine-dependent polysubstance abusers. Biol Psych. 1993;34:261–4. doi: 10.1016/0006-3223(93)90080-w. [DOI] [PubMed] [Google Scholar]
- 44.McNeil JF, Kouri EM, Lundahl LH, Lukas SE. Plasma cocaine and metabolite levels in subjects with and without a family history of alcoholism. Coll Problems Drug Depend. 1998;1:92. [Google Scholar]
- 45.McCance-Katz E. Cocaine and alcohol effects in humans and the role of cocaethylene. Presented at the Research Society on Alcoholism Annual Meeting.1998. [Google Scholar]
- 46.Hedaya MA, Pan WJ. Effect of alcohol coadministration on the plasma and brain concentrations of cocaine in rats. Drug Metab Dis. 1997;25:647–9. [PubMed] [Google Scholar]