Abstract
Mechanical loads placed on connective tissues alter gene expression in fibroblasts through mechanotransduction mechanisms by which cells convert mechanical signals into cellular biological events, such as gene expression of extracellular matrix components (e.g., collagen). This mechanical regulation of ECM gene expression affords maintenance of connective tissue homeostasis. However, mechanical loads can also interfere with homeostatic cellular gene expression and consequently cause the pathogenesis of connective tissue diseases such as tendinopathy and osteoarthritis. Therefore, the regulation of gene expression by mechanical loads is closely related to connective tissue physiology and pathology. This article reviews the effects of various mechanical loading conditions on gene regulation in fibroblasts and discusses several mechanotransduction mechanisms. Future research directions in mechanoregulation of gene expression are also suggested.
Keywords: Mechanical loads, Fibroblasts, Extracellular matrix, Gene expression, Mechanotransduction
1. Introduction
Mechanical loads play a key role in the maintenance of tissue homeostasis. Connective tissues in the body deserve special attention because they are constantly subjected to mechanical loads and, as a result, respond by changing their structure and function. This change is brought about to a large extent by connective tissue fibroblasts. These cells are most abundant and mechanoresponsive through mechanotransduction mechanisms by which fibroblasts convert mechanical signals into a series of biological events such as expression of numerous genes, including those responsible for extracellular matrix (ECM). As a result of such mechanoregulation of gene expression in fibroblasts, mechanical loads influence connective tissue physiology and pathology (MacKenna et al., 2000; Riley, 2005).
Although many studies have investigated mechanotransduction mechanisms, the dynamic and complex cell ECM environments and their inter- and intra-connective networking molecular systems make such investigations challenging. Numerous studies have, however, pointed out that various types of cells, including endothelial cells, smooth muscle cells, osteoblasts, chondrocytes, and fibroblasts, appear to share common signaling pathways; nevertheless, the eventual response of a given cell type to mechanical load depends on mechanical loading conditions and the types of signaling molecules and transcription factors that are expressed. There is a vast difference in cellular mechanobiological response depending on the type of mechanical loading and the context in which it is applied. The mechanotransduction mechanisms can be through direct transduction to the cytoskeleton via integrins which directly or indirectly alter cellular gene expression, through triggering of soluble biochemical signals derived from a cluster of signaling molecules, or through ion fluxes via mechanosensitive ion channels. Although the models proposed for mechanotransduction have been regarded as distinct, an integration of the cytoskeleton and biochemical signaling molecules is ultimately required to yield an appropriate cellular response to mechanical load. In this article, we provide an overview of the mechanoregulation of gene expression of fibroblasts, with a focus on major load-responsive connective tissue fibroblasts belonging to tendon, ligament, and skin. We also review several mechanotransduction mechanisms proposed for fibroblasts in literature. Finally, we comment on research directions in mechanoregulation of gene expression.
2. Fibroblasts and mechanical loads
Fibroblasts are major type of mechanoresponsive cells and are highly heterogeneous. At present, a specific marker for identification of fibroblasts is still lacking. The relatively generic term fibroblast applies to connective tissue cells of diverse origins. These cells are found throughout connective tissues such as tendon, ligament, and skin. Fibroblasts are traditionally defined as the cells that produce collagens and are considered to be the primary source of most ECM components (Camelliti et al., 2005). Besides collagens, fibroblasts are also responsible for maintenance of other ECM components such as proteoglycans and production of various growth factors (e.g., TGF-β) and cytokines (e.g., TNF-α) (Camelliti et al., 2005). In addition, these cells secrete matrix degradative enzymes, MMPs. As such, fibroblasts play a central role in tissue remodeling and wound healing processes and are involved in the pathogenesis of connective tissue diseases. In vivo, fibroblasts are embedded in ECM and subjected to tension, compression, and shear stress, all of which differentially affect cellular gene expression of ECM components.
Tension, compression, and shear stress are the three basic types of mechanical loading, which, in the context of cell mechanobiology, is defined as the imposition of stresses or strains through the application of physical forces. A physical force may be applied in a variety of ways such as through substrate stretching or through movement of fluid or air. Three types of mechanical loading on cells can be categorized (Bao and Suresh, 2003), and each serves a different purpose. Atomic force microscopy (AFM) and magnetic twisting cytometry (MTC) are local probes that induce deformation in a portion of a cell and may be used in quantifying local cellular mechanical properties (Tao et al., 1992; Wang and Ingber, 1995). Micropipette aspiration and optical trapping exert mechanical loading on a whole cell and are useful for determining the mechanical properties of the entire cell (Hochmuth, 2000; Takahashi et al., 2003). To study the mechanobiological response of a population of fibroblasts, however, mechanical loadings have been applied mainly by various substrate stretching methods (Almekinders et al., 1993; Banes et al., 1999; Wang and Thampatty, 2006).
Although connective tissue fibroblasts in vivo are subjected to tension, compression, and shear stress, tensile load is most common, especially for fibroblasts in tendons and ligaments. To mimic in vivo environment, tensile mechanical loading on fibroblasts in vitro is often introduced using a substrate stretching method. This method is versatile; the loading parameters, including magnitude, frequency, and duration, are easy to control; and the mechanical properties of substrates as well as surface chemistry for cell attachment can be readily altered. The substrate deformation induced by substrate stretching is characterized by substrate surface strains.
To determine the effects of mechanical loading on fibroblasts, static or cyclic stretching is applied to a group of cells cultured on smooth, flexible elastic membranes (e.g., silicone elastomers). Surface strains induced by substrate stretching can be estimated based on both experimental and computational methods. For the experimental methods, substrate deformations are obtained by tracking spherical fluorescent beads attached to the substrate, and the strains are then calculated from the displacement of the fluorescent microspheres (Barbee et al., 1994; Lee et al., 1996). Images of bead movement are captured in video images which give the marker coordinates before and after substrate stretching. The same principle applies to individual cells for determining cell deformations (Lee et al., 1996). Because of variation in the adhesion strength of individual cells to underlying substrate and incomplete strain transmission from substrate to cells, the strains experienced by individual cells vary and are generally smaller than substrate surface strains (Wang et al., 2001).
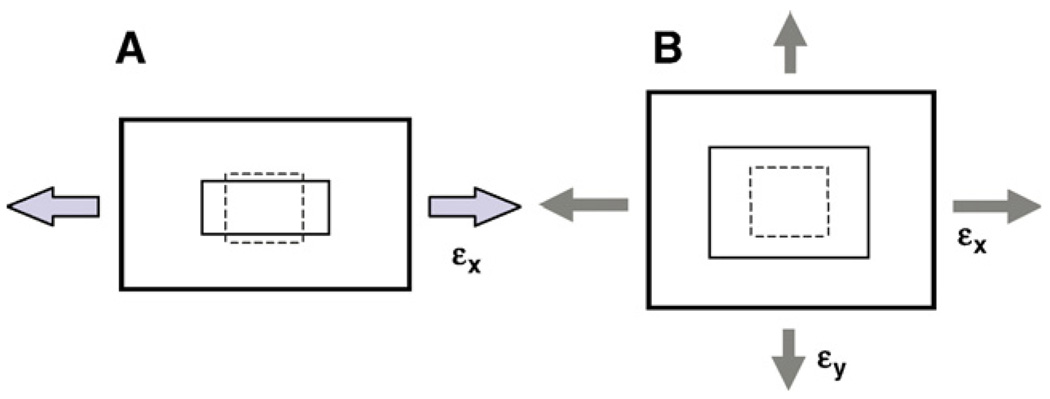
A substrate can be stretched either uniaxially or biaxially (Fig. 1). For uniaxial stretching, a rectangular substrate is lengthened in its stretching direction but shortened in its orthogonal direction (Wang and Thampatty, 2006). Such a uniaxial cell stretching system using compliant microgrooved silicone dishes, in which cells align mimicking in vivo cell alignment and orientation, has been successfully used in studies concerning mechanical loading effects on tendon fibroblasts (Yang et al., 2004, 2005). On the other hand, biaxial stretching can be either equibiaxial stretch, where substrate strains are the same in all directions, or non-equibiaxial stretch, where substrate surface strains change with respect to stretching direction (Banes et al., 1985; Lee et al., 1996; Wang et al., 2001). In both uniaxial and biaxial stretching systems, stretches can be applied either statically or dynamically.
Fig. 1.
An illustration of 2-D in vitro models for studying mechanobiological responses of fibroblasts. A. Uniaxial stretching. B. Biaxial stretching. When two stretches (εx and εy) are equal, it is referred to as equibiaxial stretching.
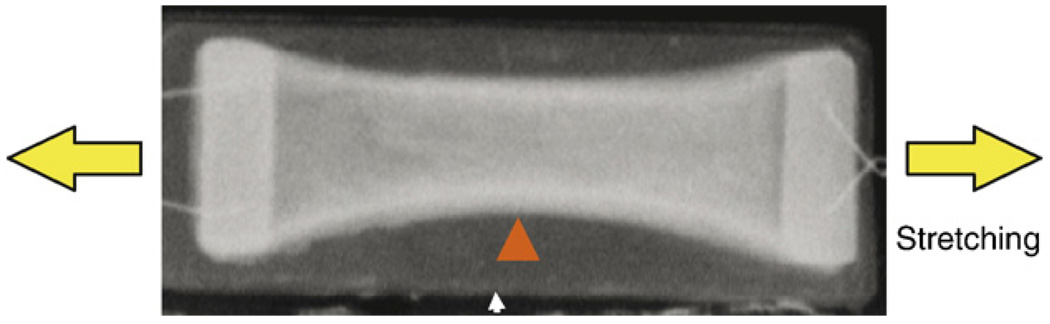
Although two-dimensional stretching studies have provided much information about the effects of mechanical loads on cells, they do not take into account the fact that cells in vivo are surrounded by ECM. Therefore, three-dimensional (3-D) matrices such as collagen gels have been used to provide cells with a more in vivo-like environment to aid in maintaining cell morphology and phenotype (Eastwood et al., 1998a,b). In fibroblast-populated collagen gels (FPCGs) (Fig. 2), which are the most commonly used 3-D matrices, fibroblasts are embedded in a 3-D network of fibrillar collagen type I, which is either attached to a culture dish or floats in medium (Grinnell, 2000, 2003). In addition to internally generated tensile stresses, external mechanical loading can also be applied to the FPCGs. A good example is the development of bioartificial tissues of tendon constructs cultured in 3-D collagen gels that can be mechanically loaded by a computer-driven, pressure-controlled system for fabrication of tissue engineered constructs (Garvin et al., 2003).
Fig. 2.
Shown is the fibroblast-populated collagen gel (FPCG). This type of 3-D in vitro model has been used to study the effects of internal mechanical gel tension on fibroblasts. Also, an external mechanical stretching can be applied to the gel in this model. Note that the fibroblasts in the gel generate tension, and as a result, the gel changes from rectangular (arrow) to parabolic shape (triangle).
3. Regulation of gene expression in fibroblasts by mechanical loads
Dense connective tissues such as tendons and ligaments are mainly subjected to tensile loads. In vivo, tendons vary in their ability to stretch depending on the species and also on the type and location of the tendon (Kjaer, 2004). Maximal strain of human wrist tendon is up to 5–6% when passively stretched, whereas avian flexor tendons and rabbit Achilles tendons can be elastically stretched up to 14% and 16%, respectively (Lieber et al., 1991; Loren and Lieber, 1995; Devkota and Weinhold, 2003), and superficial flexor tendons in equine models can withstand strains in excess of 12% (Abrahams, 1967; Herrick et al., 1978). Therefore, a 10% mechanical stretching of tendon fibroblasts is well within the physiologically relevant levels of force normally experienced by these tendon fibroblasts in vivo. The response of tendon fibroblasts to mechanical loading depends on various factors such as type of loading (e.g. static vs. cyclic, uniaxial vs. biaxial), stretching magnitude, frequency, and duration (Wang et al., 2004; Wang, 2006a).
The mechanoresponsive genes can be largely categorized into two types: ECM-related and inflammation-related genes. Numerous in vitro studies have shown that mechanical loading alters gene expression in fibroblasts from tendons, ligaments, and skin tissue (Table 1). The former includes collagen (e.g., I, III, and XII), MMPs (e.g.,−1, −3, and −13), TIMPs (e.g., TIMP-2), TGF-β1, tenascin-C, fibronectin, connectin, cystatin, and calmodulin; and the latter includes COX-2 and mPGES-1. For example, in tendon cells subjected to cyclic stretching of 5% strain at 1 Hz for 6 h in vitro, expression of collagen type I was increased at the transcriptional level, as was the expression of several other genes including connectin, cystatin, and calmodulin (Banes et al., 1999). Connectin is an elastic protein whose role may be as a shock cord to keep sarcomeres from pulling apart (Maruyama, 1997). Its presence in tendon could serve to prevent cell syncytia from pulling apart under dynamic loads. Cystatins are cystein protease inhibitors (Henskens et al., 1996), which could reduce or inhibit mechanical loading-induced matrix degradation by cystein proteases. Calmodulin is an important calcium-binding protein that binds to other proteins such as myosin light chain kinase (MLC), consequently affecting their activities.
Table 1.
Mechanoregulation of gene expression in fibroblasts
| Cells | Response | Type of load | Substrate | Significance | Reference |
|---|---|---|---|---|---|
| Chicken tendon fibroblasts |
Induction of several genes including collagen type I |
Cyclic biaxial stretch, 5%, 6 h |
Collagen type I-coated |
Induction of novel genes such as connectin and cyclophilins |
Banes et al. (1999) |
| Rabbit tendon fibroblasts |
Increase in MMP-3 mRNA in the presence of IL-1β |
Cyclic biaxial stretch, 5%, 6 h |
Collagen type I-coated |
Matrix degradation initiation by synergistic effect of cytokine such as IL-1β and mechanical load |
Archambault et al. (2002a,b) |
| Human tendon fibroblasts |
Increase in MMP-3, IL-1β and COX-2 gene expression |
Cyclic equibiaxial stain, 3.5%, 2 h |
Collagen type I-coated |
Matrix degradation initiation by synergistic effect of cytokine (IL-1β) and mechanical load |
Tsuzaki et al. (2003a,b) |
| Human tendon fibroblasts |
Stimulation of collagen type I and TGF-β1 gene expression |
Cyclic uniaxial stretch, 4%, 8%, 4 h |
ProNectin-F-coated and microgrooved |
Growth factor and stretching magnitude-dependent response |
Yang et al. (2004) |
| Human tendon fibroblasts |
Decrease in COX-2 and MMP-1 gene expression in the presence of IL-1β Increase in COX-2 and MMP-1 gene expression in the presence of IL-1β |
Cyclic uniaxial stretch, 4%, 4 h |
ProNectin-F-coated | Stretching magnitude- dependent differential matrix degradation and inflammatory gene regulation which is IL-1β mediated |
Yang et al. (2005) |
| Cyclic uniaxial stretch, 8%, 4 h | |||||
| Rat tail tendons | Up- or downregulation of collagen type I and MMP-1 gene expression |
3-D Collagen gel contraction |
N/A | Gene expression control (collagen type I and MMP-1) by alterations in cytoskeletal tension |
Lavagnino and Arnoczky (2005) |
| Human ligament (ACL) fibroblasts |
Increase in collagen types I and III mRNA, and TGF-β1 protein |
Cyclic uniaxial stretch, 10%, 24 h |
Collagen type I-coated |
Growth factor (TGF-β1)-mediated response |
Kim et al. (2002) |
| Human ligament (ACL and MCL) fibroblasts |
Increase in collagen type I mRNA with slight increase in collagen type III mRNA (ACL), increase in collagen type III mRNA with decrease in collagen type I mRNA (MCL) |
Cyclic equibiaxial stretch, 5%, 24 h |
Collagen type I-coated |
Differential response to different cell source (ACL vs. MCL) and strain magnitudes (5% vs. 7.5%) |
Hsieh et al. (2000) |
| Time-dependent increase in collagen type III mRNA (MCL) and collagen type I mRNA (ACL), no change in collagen type I mRNA (MCL), decrease in collagen (ACL) |
Cyclic equibiaxial stretch, 7.5%, 0.5–24 h |
||||
| Human ligament (PDL) fibroblasts |
Increase in COLIA1, MMP-2, and TIMP-2 mRNA Decrease in COL1A1 mRNA, increase in MMP-2 mRNA without change in TIMP-2 mRNA |
Static equibiaxial stretch, 10%, 24 h Static compression, 10%, 24 h |
Collagen type I-coated |
Differential ECM and MMP gene expression in response to different types of load (tension vs. compression) |
He et al. (2004) |
| Human ligament (PDL) fibroblasts |
Increase in collagen type I and fibronectin synthesis Increase in fibronectin synthesis, no change in collagen type I |
Cyclic biaxial stretch, 5%, 24 h |
Collagen type I-coated |
Differential ECM synthesis in response to different magnitudes of stretch |
Howard et al. (1998) |
| Cyclic biaxial stretch,10%, 24 h | |||||
| Human ligament (PDL) fibroblasts |
Induction of several genes including MSGen-15 |
Cyclic equibiaxial stretch, 18%, 16 h |
Collagen type I-coated |
Induction of novel transcription factor-like genes such as MSGen-15 |
Myokai et al. (2003) |
| Human ligament (PDL) fibroblasts |
Increase in c-fos mRNA | Cyclic equibiaxial stretch, 15%, 30 min |
Collagen type I-coated |
Rapid induction of transcription factor, c-fos |
Yamaguchi et al. (2002) |
| Human ligament cells |
Increase in gene expression of collagen types I, III, and V |
Cyclic biaxial stretch, 10%, 48 h |
Collagen type I-coated |
TGF-β-mediated gene expression |
Nakatani et al. (2002) |
| Human dermal fibroblasts |
Increase in procollagen mRNA levels and procollagen synthesis |
Cyclic biaxial stretch, 20%, 30 min |
Collagen type I-coated |
TGF-β-mediated gene expression |
Parsons et al. (1999) |
| Human dermal fibroblasts |
Induction of several ECM genes including α1(I), α2(I), and α1(III) collagen, tenascin-C, structural components of the cytoskeleton and focal adhesion sites |
3-D collagen gel contraction |
N/A | Induction of several genes coding for ECM proteins, focal adhesions, and cytoskeletal genes such as α-smooth muscle actin by contraction |
Kessler et al. (2001) |
| Further increase in α1(I) collagen gene expression in collagen coated silicone surfaces compared to 3-D collagen gels, plastic, and uncoated silicone, more induction in cyclically stretched cells compared to statically stretched and increase in MMP-1 mRNA in collagen coated silicone surface compared to plastic and 3-D collagen gels |
Cyclic and static equibiaxial stretch, 20%, 0.1 Hz, 24 h |
Collagen type I-coated |
Influence of substrate property (stiffness and coating) and type of strain (cyclic vs. static) on gene expression |
||
| Chicken skin fibroblasts |
Increase in tenascin-C and collagen type XII mRNA levels |
3-D collagen gels and cyclic equibiaxial strain, 10%, 1–6 h |
Collagen type I- or fibronectin coated |
Rho/ROCK-dependent actin cytoskeletal contractility in gene induction |
Trachslin et al., 1999; Chiquet-Ehrismann et al., 1994; Sarasa-Renedo and Chiquet, 2005 |
Collagen type I gene induction was also observed in cyclically stretched human tendon fibroblasts under serumfree conditions (Yang et al., 2004). Specifically, cyclic uniaxial stretching with 4% and 8% stretching magnitudes at 0.5 Hz for 4 h increased collagen type I gene expression in a stretching magnitude-dependent manner. Concomitant with collagen type I induction, TGF-β1 mRNA level was upregulated in cyclically stretched human tendon fibroblasts. Furthermore, inhibition of TGF-β1 with antibodies abolished stretching-induced collagen secretion, suggesting that TGF-β1 mediates stretching-induced collagen production in tendon fibroblasts (Yang et al., 2004).
The in vitro observations correlate well with the in vivo findings on the role of mechanical loading in collagen type I induction and the involvement of TGF-β1 in the process. For example, procollagen type I C-terminal peptide (PICP), a marker for type I collagen synthesis, rose 3- to 7-fold from basal level 72 h after acute exercise in human peritendinous tissue (Langberg et al., 1999, 2001; Kjaer et al., 2000). Also, the role of TGF-β1 in relation to exercise-induced type I collagen synthesis in human tendons has been reported. For instance, mechanical loading of human tendon during exercise elevates type I collagen production with parallel increase in TGF-β1 (Heinemeier et al., 2003). Thus, changes in the circulating and local TGF-β1 in response to exercise suggest a role of TGF-β1 in type I collagen synthesis in tendons in vivo.
Although TGF-β1 could link mechanical loading and collagen synthesis in tendons in vitro and in vivo, the precise molecular mechanisms are not clear. However, several putative regulatory elements that may determine the transcriptional efficiency of procollagen genes have been identified in their corresponding promoters (Schmidt et al., 1984; Bornstein et al., 1987; Jimenez et al., 1994). These include the consensus TATA and CCAAT motifs, which are the potential targets for the action of promoter-specific transcription factors (Chu et al., 1985; De Wet et al., 1987). Transcriptional activation of rat COL1A1 gene in cardiac fibroblasts subjected to mechanical stretching with a 10% strain at 1 Hz, similar to the physiological tension experienced by the cells in the heart, has been reported to be mediated by autocrine stimulation by TGF-β (Lindahl et al., 2002). This activation involves TGF-β response elements in the promoter region of the collagen gene and increased binding of CCAAT-binding factor (CBF/NF-Y) at the proximal promoter. Additionally, increased binding of transcription factor CBF to human COL1A1 proximal promoter has been demonstrated in human dermal fibroblasts (Saitta et al., 2000). Similar mechanisms can be suggested for TGF-β1-mediated collagen type I induction by mechanical load in tendon fibroblasts.
In contrast to the anabolic effects of TGF-β1 on mechanical load-induced collagen production, inflammatory cytokines such as IL-1β can significantly upregulate matrix degrading enzymes, MMPs, in the presence of mechanical loading (Archambault et al., 2002a; Tsuzaki et al., 2003a). IL-1β alone has been shown to induce mRNA expression of MMP-1, -3, and -13 in tendon cells (Archambault et al., 2002a,b; Tsuzaki et al., 2003b). However, in the presence of IL-1β (1000 pM), rabbit tendon cells subjected to a cyclic strain of 5% biaxial stretching at 0.33 Hz for 6 h expressed significantly higher MMP-3 gene and protein levels than did cells treated with IL-1β but without stretching (Archambault et al., 2002a). Cells subjected to stretching alone did not produce more MMP-3 than control cells. The results suggest that mechanical load and inflammatory cytokine together can initiate matrix destruction. Similar experiments in human tendon cells with a different loading protocol (3.5% biaxial stretching at 1 Hz for 2 h) showed induction of IL-1β, COX-2, and MMP-3 genes (Tsuzaki et al., 2003a). This result suggests that load-induced IL-1β may trigger matrix remodeling/destruction via COX-2 and MMP-3 expression. However, the load-induced cellular gene expression may be dependent on the loading type used in this study, biaxial stretching, instead of more physiological uniaxial stretching (see below).
Using uniaxial stretching protocol, MMP-1 gene expression of human tendon fibroblasts in the presence of IL-1β has been investigated (Yang et al., 2005). For example, tendon fibroblasts subjected to cyclic 4% uniaxial stretching at 0.5 Hz for 4 h in the presence of 10 pM IL-1β decreased MMP-1 gene expression, while similar conditions at 8% stretching increased it. Furthermore, 4% uniaxial stretching decreased COX-2 gene expression and PGE2 production, while 8% uniaxial stretching increased both, indicating that small-magnitude stretching is anti-inflammatory, whereas large-magnitude stretching is pro-inflammatory (Yang et al., 2005). These in vitro experimental findings correlate with the observation that acute exercise results in elevated interstitial amount of MMPs in human peritendinous tissue (Koskinen et al., 2004).
Besides IL-1β, PGE2 is another major catabolic factor that is considered to be involved in the development of tendinopathy and joint diseases (Almekinders et al., 1993; Laufer, 2003; Wang et al., 2003, 2004). Previously, it was shown that tendon fibroblasts under repetitive uniaxial mechanical stretching at 8% and 12% strains increased COX-2 expression and release of PGE2 (Wang et al., 2003). Microsomal PGE synthase type 1 (mPGES-1), which is involved in the conversion of PGH2 to PGE2, is an inducible enzyme functionally linked to COX-2. Recently, it has been reported that in cartilage explants under compressive forces, mPGES-1 is a mechanoresponsive gene (Gosset et al., 2006). Hence, similar induction of mPGES-1 gene may be involved in PGE2 release in tendon fibroblasts under mechanical loading conditions.
Similar to tendons, ligaments are under mechanical loading in vivo. For example, it has been shown that the anterior cruciate ligaments (ACL) and medial collateral ligaments (MCL) can be stretched to 4–5% strains during normal activities and to 7.7% during external application of loads to the knee joint (Beynnon et al., 1995, 1997; Hull et al., 1996).
Depending on the loading type and magnitude, mechanical loading in vitro differentially affects the gene expression of collagens and MMPs in ligament fibroblasts. Cyclic equibiaxial stretching of 5% magnitude induced differential expression of collagen types I and III mRNA in ACL and MCL fibroblasts (Hsieh et al., 2000). ACL fibroblasts responded to cyclic equibiaxial stretching by expression of higher levels of collagen type I mRNA with a slight increase in type III mRNA levels, whereas MCL fibroblasts exhibited a significant increase in type III collagen mRNA with a decrease in type I mRNA levels after 16–24 h. In addition, differences in mechanobiological responses by the fibroblasts from the two types of ligaments were observed between two applied stretching magnitudes. Specifically, 7.5% stretching induced a time-dependent (time period ranging from 0.5 to 24 h) increase in type III collagen mRNA levels in MCL fibroblasts, whereas 5% stretching did not. In contrast, type I collagen mRNA levels in ACL fibroblasts continued to increase at all time points at 7.5% stretching, although the increases were smaller than those found for 5% stretching. However, at lower than 7.5% stretching, collagen type III mRNA levels were lower than in the controls in ACL fibroblasts.
It should be noted that while equibiaxial stretching in the above study can apply uniform substrate strains to cells in vitro, ACL and MCL fibroblasts are not likely subjected to equibiaxial stretching in vivo; hence, this loading condition is considered to be less physiological compared to uniaxial stretching. Also, although both magnitudes (5% and 7.5%) are within physiological strain limits, the 7.5% strains are likely to be on the high end of the strain spectrum, which may explain the altered expressions of collagen types I and III between the two applied strains.
The differential gene expression between the two types of ligament fibroblasts in response to mechanical stress may explain their differential healing potentials. It was suggested that the mode of healing in MCL occurs through the bridging of the wound by scar tissue that is predominantly composed of type III collagen (Frank et al., 1983a,b). The initial expression of type III collagen, therefore, may be crucial for the bridging of scar tissue for adequate healing, and the overexpression of type I collagen may have limited functional significance during the early stages of ligament healing. All ligaments require the bridging of scar tissue for proper healing, and scar tissue is associated with increased levels of minor collagens such as collagen type III (Hildebrand and Frank, 1998). Collagen type III is also believed to provide a great advantage during the healing process because of its ability to form rapid cross links to stabilize the repair site (Liu et al., 1995). Therefore, the decrease in collagen type III mRNA due to 7.5% strains in the above described study of ACL fibroblasts may explain the poor healing capacity of the ACL.
In contrast to the biaxial stretching effects as described above, 10% cyclic uniaxial stretching of ACL fibroblasts at 0.33 Hz for 24 h increased the expression of both collagen types I and III mRNA levels; the ratios of stretch to control values of type I and type III collagen were 1.6 and 2.7, respectively (Kim et al., 2002). In parallel, TGF-β1 protein in the medium was increased in response to stretching. Additionally, in the presence of exogenous TGF-β1, expression of types I and III collagen mRNA levels increased in a dose-dependent manner. Also, anti-TGF-β1 antibody inhibited the stretching-induced mRNA expression of types I and III collagen. This observation supports the view that stretching-induced expression of collagen types I and III genes is mediated via an autocrine mechanism of TGF-β1 released from ligament cells. However, note that considering ACL is normally stretched up to 7.7% (Beynnon et al., 1995, 1997), the 10% stretching applied in the above study may be considered to be “injurious mechanical loading.”
In human ligamentum flavum cells subjected to cyclic mechanical stretching, gene expression of collagen types I, III, and V was increased (Nakatani et al., 2002). The increase in collagen mRNA expression due to stretching was markedly reduced by neutralizing endogenous TGF-β1 with anti-TGF-β1 antibody. These results suggested that TGF-β1 mediated these collagen gene expressions, which may result in enhanced collagen deposition and hence promote hypertrophy of the ligament.
Increase in collagen synthesis by TGF-β1 has been shown in ligament fibroblasts previously (DesRosiers et al., 1996; Marui et al., 1997). The TGF-β1 intracellular signaling occurs via serine/threonine kinases that phosphorylate downstream-mediator, a family of SMAD proteins. In turn, the SMADs transduce the signal through their oligomerization and transport to the nucleus where they act as transcription factors to stimulate collagen transcription (Ghosh et al., 2001; Runyan et al., 2006). It has been shown that TGF-β positively regulates α2(I) procollagen gene (COL1A2) promoter activity through the cellular SMAD signal transduction pathway (Chen et al., 1999; Ghosh et al., 2001). Mechanical load-induced TGF-β expression in ligament fibroblasts could initiate similar signaling pathways toward increased collagen gene expression.
In addition to the investigations of mechanical loading effects on gene expression in ACL and MCL fibroblasts, mechanoregulation of gene expression has been investigated in periodontal ligament (PDL) fibroblasts (Howard et al., 1998). PDL fibroblasts exist in an active mechanical environment by virtue of their distinct function to mechanically link a tooth to its bony socket. PDL fibroblasts may initiate the remodeling process in response to the load applied to them during tooth movement (Davidovitch, 1991). These fibroblasts have been shown to respond differentially to tensile and compressive strains in terms of ECM synthesis and degradation (He et al., 2004). Application of 10% compressive strain with a frequency of 0.5 Hz decreased COL1A1 mRNA levels and type I collagen and fibronectin protein but increased both MMP-2 mRNA and protein levels (both latent and active) without any change in TIMP-2 mRNA levels. On the other hand, 10% tensile strain at the same frequency increased COL1A1 mRNA, MMP-2 mRNA, and TIMP-2 mRNA. Under tensional loads, an increase in MMP-2 mRNA was paralleled by a similar increase in its inhibitor TIMP-2 mRNA, thereby promoting synthetic events, whereas under compressive loads, both MMP-2 mRNA and protein levels were increased without any change in TIMP-2 mRNA promoting matrix degradation. Moreover, these fibroblasts responded differently in terms of ECM protein synthesis to different stretching magnitudes: 5% biaxial stretching increased collagen type I and fibronectin synthesis, whereas 10% biaxial stretching had similar response for fibronectin but no change in collagen type I synthesis (Howard et al., 1998).
When orthodontic loads are applied to a tooth, the periodontal ligament is compressed on one side while it is stretched on the other. The compression side is characterized by bone resorption while the tension side is characterized by the bone synthesis (Reitan, 1967, 1969, 1970). This suggests that the type of load applied to tooth support structures plays a role in modulating the phenotype of the constituent cells.
In addition to the regulation of the known genes (i.e., collagen I, MMPs, TIMP-2, and fibronectin), mechanical loads also affect certain genes putatively related to transcription factors which have been identified in PDL cells. When PDL cells were subjected to 18% biaxial stretching at 0.1 Hz for 16 h, several mechanosensitive genes, which have sequence homology to ribosomal protein S27, androgen-binding protein, cathepsin H, and cytochrome c, were induced. Analysis of these mechanical stress-induced genes (MSGens) showed marked induction of MSGen-15 mRNA expression in the cells subjected to mechanical loading (Myokai et al., 2003). Interestingly, MSGen-15 showed homology with MRG-15 (mortality factor on a chromosome-4-related gene), which belongs to a novel family of transcription factor-like genes whose RNA level changes during cell growth and at times of senescence (Bertram et al., 1999). In human PDL cells, mRNA for transcription factors such as c-fos is also shown to be induced after mechanical stretching without any significant change in the matrix proteins (Yamaguchi et al., 2002). It should be noted that to assess physiological relevance of these findings in the above studies, the loading type (e.g., biaxial stretching) and magnitude (up to 18% strain) have to be verified on periodontal ligaments.
Skin is another example of connective tissue that is subjected to mechanical loads, which are known to regulate procollagen synthesis and processing in dermal fibroblasts. Application of cyclic mechanical load at 20% strain and frequency of 1 Hz up to 48 h enhanced procollagen mRNA levels and procollagen synthesis in the presence of TGF-β1 in human dermal fibroblasts (Parsons et al., 1999). Procollagen mRNA levels were increased by 2-fold in stretched cells compared to unstretched cells with enhancement of procollagen C-proteinase (PCP) levels. PCP is matrix metalloproteinase which cleaves the C-terminal propeptide from procollagen chains to form insoluble collagen that is required for fiber formation (Li et al., 1996). Based on these in vitro data, it is hypothesized that mechanical load, which acts in synergy with growth factors present at the wound site, increases procollagen synthesis and also modulates enzyme activity, thus increasing collagen deposition, which is implicated in dermal scar formation.
There is a growing body of evidence indicating that specific ECM substrates and culture conditions may determine the level of collagen and proteoglycan synthesis by fibroblasts subjected to mechanical load (Reynolds and Bishop, 1998; Atance et al., 2004). For example, collagen I mRNA synthesis was greater in human lung fibroblasts stretched on laminin and elastin than in fibroblasts stretched on fibronectin (Breen, 2000). Cell–ECM interaction may directly regulate cell functions through cell surface receptor-mediated signaling mainly via integrins and non-integrin receptors, syndecan, and CD44 (Hynes, 1992; Couchman et al., 2001; Cichy and Pure, 2003). Through αβ pairing, specific integrin ligands can interact with ECM proteins and different ECM macromolecules may selectively stimulate specific types of signal transduction pathways for the modulation of gene expression (Jalali et al., 2001; Rosso et al., 2004). Increased expression of cell surface proteoglycan genes CD44 and syndecan has been observed in tendon fibroblasts in collagen type I 3-D gels compared to that in monolayer cultures (Sawaguchi et al., 2006).
Mechanoregulation of gene expression has been widely studied in chicken skin fibroblasts, and various mechanisms have been proposed for the mechanoregulation of ECM genes (Chiquet-Ehrismann et al., 1994; Chiquet et al., 1996, 2003, 2004; Sarasa-Renedo and Chiquet, 2005; Sarasa-Renedo et al., in press). The genes of focus in these studies are tenascin-C and collagen fibril-associated type XII collagen. Tenascin-C, which is not normally expressed in adults, changes its expression patterns during embryogenesis and regenerative processes and appears under pathological conditions and adjacent to contracting wounds (Mackie et al., 1988; Silver et al., 2003). Tensile loading has been shown to control tenascin-C mRNA expression in chicken skin fibroblasts. When cyclic equibiaxial tensile stretch was applied (15%, 0.3 Hz) to these fibroblasts, tenascin-C mRNA was induced 2- to 4-fold compared to non-stretched cells within 3–6 h (Chiquet et al., 2003). When cells were pretreated with cytochalasin-B, an actin depolymerizing drug, and latrunculin A, an actin cytoskeleton disrupting drug, the stretching-induced tenascin-C mRNA expression was abolished, indicating that an intact cytoskeleton is required for stretching-induced tenascin-C mRNA expression (Chiquet et al., 2003; Sarasa-Renedo and Chiquet, 2005). Collagen XII has an expression pattern similar to that of tenascin-C and has been shown to co-assemble with collagen I during fibril formation (Koch et al., 1995). A mechanoresponsive control element was also localized in the collagen XII gene (Chiquet et al., 1998).
A major limitation with these cell stretching studies above is that the level of mechanical load applied is measured on the substrate, not actually on cells that attach to the substrate. Also, because some cells in a population may adhere to the underlying substrate more strongly than others, individual cells may experience different levels of mechanical loading, regardless of the mode of loading. Consequently, substantial differences in cellular response to mechanical loading exist. This situation makes it difficult to interpret the results of many studies despite the fact that the same cells were used but were cultured on substrates made of different materials or coated with different ECM proteins.
Another limitation of the above studies is that cells reside on 2-D substrate without the surrounding matrix which is typically present in vivo. To more closely mimic in vivo conditions, 3-D collagen gel matrix also has been used to investigate the effect of cytoskeletal tension on gene expression in rat tail tendon cells (Lavagnino and Arnoczky, 2005). In this study, cells seeded into collagen gels were able to establish a cytoskeletal tensional homeostasis through an isometric contraction against collagen gel matrices attached to the culture dish. This was characterized by the presence of organized stress fibers within the cytoskeleton and an upregulation of anabolic gene, α1(I) collagen. However, after a loss of cytoskeletal organization through chemical disruption or through detachment of the gel, which caused cell-mediated gel contraction, an upregulation of catabolic gene (MMP-1) expression and inhibition of anabolic gene (α1(I) collagen) expression were observed. The ability of the fibroblasts to remodel their ECM by virtue of an internal contractile mechanism has important implications in wound contracture and connective tissue morphogenesis (Harris et al., 1981; Grinnell, 1994).
Furthermore, using 3-D collagen gel model, mechanical tension on human dermal fibroblasts has been shown to induce 57 genes coding for ECM proteins, fibrogenic growth factors, protease inhibitors, components of focal adhesions, and the cytoskeleton (Kessler et al., 2001). Specifically, in comparison with mechanically relaxed collagen gels, the genes for α1(I) and α2(I) collagen, α1(III) collagen, all three α-chains of type VI collagen, fibronectin, and β-actin were found to be induced in stressed fibroblasts. Elastin gene was minimally induced, whereas MMP-1 gene was significantly repressed. In parallel, transcription of protease inhibitors (PAI1 and PAI2) and TIMP-1 and TIMP-3 was significantly induced by mechanical loading. All these genes may share mechanoresponsive regulatory elements, or they may be regulated by local growth factor release (Chiquet, 1999).
The steady-state levels for collagen type XII and tenascin-C mRNA were high under stretched conditions and low under relaxed conditions in skin fibroblasts cultured in collagen gels (Trachslin et al., 1999). The relative mRNA levels of fibronectin and MMP-2, however, were barely affected under these conditions. Expression levels of tenascin-C were found to be increased in chick embryo fibroblasts in restrained collagen gels (Chiquet-Ehrismann et al., 1994). In contrast, a group of MMPs (MMP-1, -3, -9, and -13) was upregulated in human dermal fibroblasts by dissipation of the tension upon retraction of collagen gel (Lambert et al., 2001).
4. Cellular mechanotransduction
Gene expression profiles induced by mechanical loading can be divided into immediate early response genes (e.g., c-fos, c-myc) and late response genes (e.g., collagens) (Ruwhof and van der Laarse, 2000). Activation and transportation of immediate early response genes such as c-fos and transcription factor NF-κB, which can bind to mechanoresponsive ECM genes, are suggested as two of the primary responses to mechanical loads (Khachigian et al., 1995; Sadoshima and Izumo, 1997; Mercurio and Manning, 1999a,b; Chen et al., 2003; Xiao, 2004). Transcription and synthesis of nuclear factors such as early growth response-1 (EGR-1) that can transactivate a specific ECM gene are considered secondary responses to mechanical loads (Schwachtgen et al., 1998; Liu et al., 2000). Finally, mechanisms that include triggering autocrine or paracrine release of growth factors such as TGF-β by mechanical loads to regulate the transcription of “mechanoresponsive genes” are also presented (Davies et al., 1997; Sadoshima and Izumo, 1997). Mechanical stress stimulates the release of TGF-β (Lindahl et al., 2002; Nakatani et al., 2002; Yang et al., 2004) as described in the previous section, as well as enhances its gene transcription through activation of EGR-1 (Liu et al., 2000). These indirect mechanotransduction mechanisms have been explained in detail previously (Chiquet, 1999; Sarasa-Renedo and Chiquet, 2005).
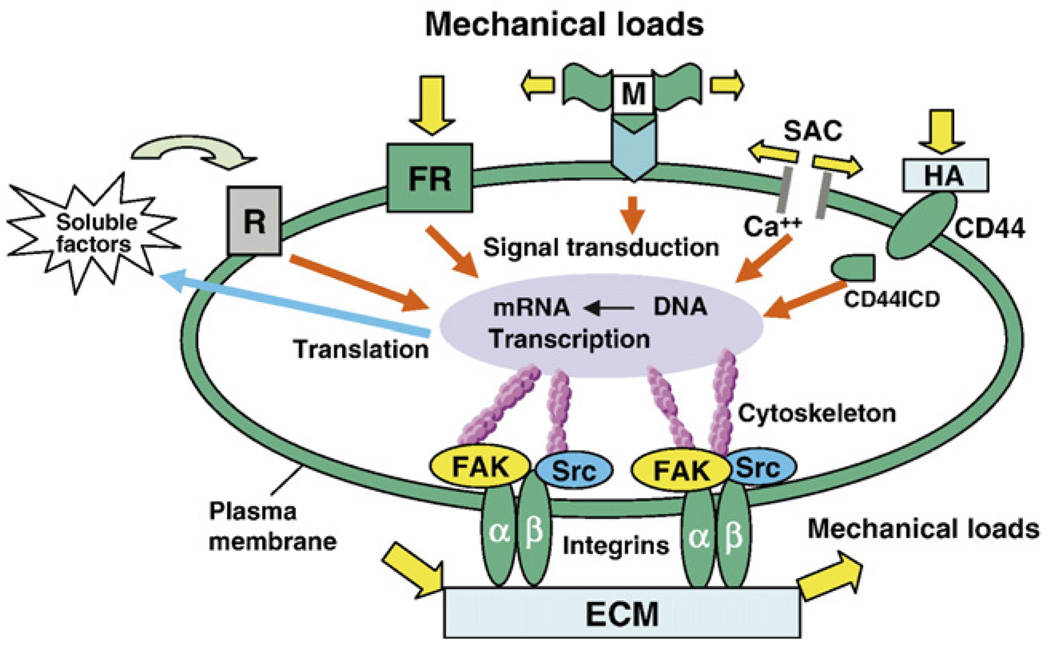
However, connective tissue fibroblasts likely use cell–matrix adhesions as the functional units to sense changes in the mechanical properties of the ECM (Chiquet, 1999). Therefore, mechanotransduction via adhesion components and cytoskeleton appears to be an attractive mechanism that transduces mechanical signals in such fibroblasts. Fibroblasts anchor to ECM by focal adhesions that physically link with actin cytoskeleton via integrin family of ECM receptors. Integrins are regarded as receptors capable of inducing biochemical signals that regulate gene expression (Ingber, 1991; Schwartz et al., 1995; Ross, 2004). The extracellular domain of integrins binds to ECM proteins such as fibronectin and collagen and functions as signaling receptors to them. Besides playing the role of indirect mechanosensors, integrins can act as direct mechanosensors, physically connecting the cytoskeleton to ECM, thus transmitting mechanical loads acting on the ECM (Hynes, 1992). The cytoplasmic domain of integrins interacts with cytoskeletal proteins (e.g., paxillin, talin and vinculin) and signaling molecules in the focal adhesion sites, e.g., FAK and c-Src (Hynes, 1992; Sastry and Horwitz, 1993; Schwartz and Shattil, 2000) (Fig. 3).
Fig. 3.
A conceptual illustration of several cellular mechanotransduction mechanisms. Mechanical loads can induce signal transduction by directly transmitting forces from the extracellular matrix (ECM) to integrins, the cytoskeleton, and the nucleus, eventually resulting in changes in gene transcription and protein translation. Also, mechanical stretching of cells opens stretching-activated channels (SACs), causing influx of ions (e.g., Ca+) and thus a series of downstream signaling events. Still, mechanical loads acting on a cell may unfold a domain of the extracellular protein (M) and expose a cryptic site that may serve as an activating ligand for a cell surface receptor, which results in a series of signaling events. Additionally, mechanical load applied to a “force receptor” (FR) may initiate signal transduction, which results in transcription, followed by translation. As a result, soluble factors are secreted into the ECM which act on the receptor (R) and then initiate a cascade of signaling events. Other signaling molecules that are involved in mechanotransduction can include CD44 transmembrane protein and its intracellular domain (CD44ICD), which is translocated into the nucleus causing gene transcription.
Mechanical loads applied to integrin ligands trigger the assembly and growth of focal contacts (Geiger and Bershadsky, 2001) and MAPK signaling (MacKenna et al., 2000). Stretching-induced conformational changes in the ECM may alter integrin structure, which leads to activation of several secondary messenger pathways in the cell. For example, mechanical stretching of NIH3T3 cells stimulates the conformational activation of integrin αvβ3, which is followed by an increase in integrin binding to ECM proteins (Katsumi et al., 2005). The stimulation of MAPK pathway (e.g., JNK) is dependent on this new integrin binding. Stimulation of integrin signals by mechanical force may transduce signals across cell membrane, leading to activation of key transcription factors (e.g., AP-1 and NF-κB) and gene expression, thus modifying cell behavior and differentiation (Shyy and Chien, 1997).
Focal adhesions (FAs) together with actin cytoskeleton are suggested to serve as mechanosensors in fibroblasts (Ingber, 1997). FAs provide attachment for stress fibers which are bundles of actin filaments, and they also serve as a site for mechanotransduction (Burridge and Chrzanowska-Wodnicka, 1996). FAs evolve from small focal complexes (Geiger and Bershadsky, 2001). The maturation of focal complexes requires externally applied mechanical force or internal actin cytoskeletal contractile force (Balaban et al., 2001; Hinz and Gabbiani, 2003). For instance, cyclic equibiaxial stretching of fibroblasts induce the formation of large matrix adhesions containing vinculin along their margins (Sarasa-Renedo and Chiquet, 2005). Moreover, after stretching, the actin stress fibers assume a crescent morphology and are aligned parallel to the long axis of the cell body; while in cells at rest, the stress fibers are straight and randomly oriented. These findings support the hypothesis that focal adhesions together with actin cytoskeleton act as mechanosensors. The actin cytoskeleton and myosin II are the key components of focal adhesion formation, and an intact actin cytoskeleton is required for effective signal transduction (Bershadsky et al., 2003).
Rho family GTPases are involved in integrin-mediated signaling primarily because of their capacity to induce cytoskeleton organization (Hall, 1998; Hall and Nobes, 2000). The most extensively studied members of the GTPase family are Rho, Rac, and Cdc42, each of which acts as a molecular switch, cycling between an active GTP-bound and an inactive GDP-bound state (Jaffe and Hall, 2005). Rho and Rac are involved in actin assembly and generation of cellular contractile force acting on the ECM, whereas Cdc42 triggers the formation of filopodia in fibroblasts.
Rho also induces assembly of FAs and stress fiber formation by stimulating myosin contractility (Chrzanowska-Wodnicka and Burridge, 1996). Rapid assembly of FAs and stress fibers by direct microinjection of activated Rho into quiescent fibroblasts has been demonstrated (Ridley and Hall, 1992). Rho-dependent kinase (ROCK) and mammalian homolog of Drosophila diaphanous (mDia1) are the two downstream effectors of Rho and are necessary to induce and maintain FAs (Nakano et al., 1999; Watanabe et al., 1999; Bishop and Hall, 2000). ROCK is a serine/threonine kinase, with two isoforms ROCKα and ROCKβ, necessary for stress fiber assembly (Bishop and Hall, 2000). ROCK is responsible for Rho-mediated enhancement of myosin-based contractility and also induces focal adhesions and stress fibers by activation of integrin and by integrin clustering via myosin-based contractility (Ishizaki et al., 1997; Narumiya et al., 1997). Two substrates of ROCK, myosin light chain (MLC) and myosin-binding subunit (MBS) of MLC phosphatase are thought to be important regulators of actin–myosin filament assembly (Bishop and Hall, 2000). ROCK stimulates actomyosin contractility either by direct phosphorylation of MLC or indirectly by inactivating MLC phosphatase (Amano et al., 1996, 1997; Kimura et al., 1996). The resulting increase in force at focal complexes triggers the transition of these structures into focal contacts. It has been shown that ROCK inhibitor attenuates cyclic stretching-mediated tenascin-C mRNA induction, and ROCK stimulators act synergistically with cyclic stretching to induce tenascin-C, suggesting a direct correlation between the tenascin-C gene induction and ROCK-mediated cytoskeletal tension (Chiquet et al., 2004; Sarasa-Renedo et al., in press).
Rho simulates F-actin assembly and enhances cellular stiffness via mDia1, and this cytoskeletal stiffening also might play a role in mechanotransduction (Riveline et al., 2001). mDia1 induces actin polymerization by recruiting actin-binding protein profilin to the site of Rho action (Narumiya et al., 1997; Watanabe et al., 1997; Bishop and Hall, 2000). It has been previously shown that fibroblasts generate an internal tension within the actin cytoskeleton by an actomyosin filament sliding mechanism, which allows them to maintain a constant cytoskeletal tension in response to changes in external loading (Takakuda and Miyairi, 1996; Brown et al., 1998). Alterations in this cytoskeletal tensional homeostasis have been reported to control gene expression in tendon cells (Lavagnino and Arnoczky, 2005). Gene expression in contracted collagen gel matrices seeded with tendon cells could be reversed through chemical disruption of cell cytoskeleton. According to the tensegrity model (Ingber, 1997), a stiffer cytoskeleton is at a better advantage in sensing external mechanical loading in contrast to a completely relaxed cytoskeleton. The integrity of actin cytoskeleton is shown to be essential for force generation, as disruption of actin microfilaments results in the disappearance of force (Kolodney and Wysolmerski, 1992). Therefore, it seems that both the integrity and contractility of the actin cytoskeleton are required for efficient mechanical signal transduction. In the light of such experimental evidence relating cytoskeletal integrity and contractility to gene induction, mechanotransduction in fibroblasts can be considered as a process in which cells sense mechanical forces and counteract these forces through intact actin cytoskeleton and actomyosin contraction.
Besides integrins, other various potential biochemical mediators of mechanotransduction such as mechanosensitive cell surface receptors including receptor tyrosine kinases (RTKs) and heterotrimeric guanine nucleotide-binding proteins (G proteins) also have been suggested in fibroblasts (Ruwhof and van der Laarse, 2000; Wang, 2006b; Wang and Thampatty, 2006). Non-integrin cell surface receptors such as CD44 and syndecan, which serve as receptors for cell adhesion molecules, growth factors, and cytokines, are also suggested as candidates for mechanotransduction (Boraldi et al., 2003; Yoneda and Couchman, 2003; Gigant-Huselstein et al., 2004). The downstream signals from these mediators in force transduction can modify each other to produce various cell responses.
G proteins are important cell surface receptors in mechanical signaling, the subunits of which are localized at the site of focal adhesions (Hansen et al., 1994). Co-localization of G proteins and integrins allows a single signal to activate two transmembrane receptors, indicating the indirect involvement of G proteins in integrin-mediated signaling (Lehoux and Tedgui, 2003). Stimulation of early activation of G proteins by cyclic stretch has been reported in cardiac fibroblasts (Gudi et al., 1998). Activation of G proteins can further activate PLC that subsequently can activate PKC (Jalili et al., 1999). Growth factor receptors that are tyrosine kinases (RTKs) also take part in mechanotransduction. Growth factors such as EGF and PDGF bind to RTKs inducing secondary signaling events via MAPK pathways (Ullrich and Schlessinger, 1990). RTK pathway may not function in isolation; direct associations of RTKs and integrins have been identified. Integrin receptor occupancy and clustering may be required for RTK phosphorylation and activation (Miyamoto et al., 1996).
CD44 is transmembrane protein that serves as an adhesion molecule for hyaluronan (HA), an ECM component (Aruffo et al., 1990; Nagano and Saya, 2004). CD44-dependent cell– matrix interaction and signaling pathway are regulated by its proteolytic cleavage (Lesley and Hyman, 1998; Cichy and Pure, 2003). The cleavage generates CD44ICD (CD44 intracellular domain), which acts as a signal transduction molecule (Fig. 3). CD44ICD is transported to the nucleus and activates transcription. Cleavage of CD44 is triggered by extracellular Ca2+ influx, and the activation of PKC and Rho GTPases (Okamoto et al., 1999; Shi et al., 2001). These factors are indeed triggered by the mechanical stretch (Nagano and Saya, 2004). CD44 is an important mediator in the regulation of interaction between ECM and cytoskeleton and is expressed in the ECM of all connective tissues (Gunthert, 1993). Intra-cytoplasmic domain of CD44 has binding sites for actin filaments. Mechanical load alters ECM synthesis, which may affect the expression of CD44 receptors and modulate the interactions with actin (Fig. 3).
Syndecans are a family of similar proteoglycans involved in cell signaling (Yoneda and Couchman, 2003). They are present in FAs and interact with numerous extracellular ligands and are considered to be co-receptors for matrix molecules (e.g. fibronectin) and growth factors (Bernfield et al., 1999). Syndecan-4 is involved in cytoskeletal reorganization, assembly of stress fibers, and FA formation. In fact, it has been shown to be required for FA formation in addition to integrins (Saoncella et al., 1999; Woods et al., 2000). Recruitment of syndecan-4 to FAs is dependent on PKC activation, and multimerization of the cytoplasmic domain of syndecan-4 is required for kinase binding and activation (Baciu and Goetinck, 1995). Regulation through phosphorylation of syndecan-4 cytoplasmic tail may also exist in syndecan-4 signaling (Horowitz et al., 1999). The involvement of Rho activity in the formation FAs and stress fibers through syndecan-4 signaling has also been suggested (Saoncella et al., 1999; Couchman et al., 2001).
Besides all signaling molecules described above, stretch-activated ion channels are considered to be candidates for mechanotransduction mechanisms (Hu and Sachs, 1997; Sadoshima and Izumo, 1997). One of the earliest signals that are generated after application of mechanical stretching is the influx of calcium ions through stretch-activated ion channels in fibroblasts (Wu et al., 1999). Cytoskeletal and membrane stress open cation channels allowing passage of ions such Ca2+, Na+, and K+ (Sackin, 1995a,b). A large increase in intracellular Ca2+ ion concentration has been reported in fibroblasts responding to a wide variety of stretching protocols (Arora et al., 1994; Bibby and McCulloch, 1994; Glogauer et al., 1997). In addition, stretch-induced increases in intracellular Ca2+ can be blocked by pre-incubation with blockers (gadolinium and streptomycin) of stretching-activated channels (SACs). Both gadolinium and streptomycin inhibit immediate early genes and protein synthesis, suggesting their direct involvement in mechanotransduction (Sadoshima and Izumo, 1993; Gannier et al., 1994; Lacampagne et al., 1994). Elevations in Ca2+ activate membrane kinases to specifically phosphorylate other signaling molecules. For example, Ca2+ influx induces tyrosine phosphorylation of the epidermal growth factor receptor (EGFR) to levels that can activate MAPK signaling pathways (Rosen and Greenberg, 1996). Elevated Ca2+ may enhance calcium/ calmodulin-dependent protein kinase (CAM kinase), which can phosphorylate and activate transcription factors such as c-AMP response element-binding protein (CREB) (Sadoshima and Izumo, 1997; Iqbal and Zaidi, 2005).
Tendon and ligament fibroblasts respond to mechanical loads by increasing their intracellular Ca2+ levels. Tendon cells form gap junctions comprised of connexin 32 and 43, through which Ca2+ waves are propagated via the passage of inositol triphosphate (IP3) (Wall and Banes, 2005). MCL fibroblasts subjected to mechanical stretch propagated Ca2+ wave better than cells that were not subjected to mechanical stretch (Jones et al., 2005). The entry of extracellular Ca2+ through SACs is also involved in PKC activation and cell proliferation in lung fibroblasts (Liu and Post, 2000). PKC may activate transcriptional factors that bind to stress response element (SRE) of the promoter region of the target gene. Upregulation of COX-2 mRNA expression by uniaxial cyclic stretch has been shown to be mediated via SACs in lung fibroblasts (Kato et al., 1998). Human COX-2 promoter region contains transcription factors such as AP-1 and NF-κB, which may be induced by cyclic stretching (Yang et al., 1997). In fact, it is shown that uniaxial cyclic stretch activates and translocates NF-κB via Ca2+ influx through SACs in human lung fibroblasts (Inoh et al., 2002). When the cells were stretched in the absence of extracellular Ca2+ or in the presence of gadolinium, translocation of NF-κB was significantly inhibited. The activation of NF-κB induces expression of COX-2 (Chen, 2006), the first rate limiting enzyme in the synthesis of PGE2 in cases of tissue inflammation (Wang et al., 2004).
SACs do not work in isolation, but are modulated by contractile actin cytoskeleton. It is suggested that there is a direct link between the regulation of Ca2+ and cytoskeletal network (Kalapesi et al., 2005). Ca2+ ions are known to regulate the physical linkage of integrins and the cytoskeleton (Nebe et al., 1996). Regulation of stretch-activated intracellular Ca2+ transients by actin filaments has been demonstrated using collagen-magnetic bead model, in which application of well-defined forces induced immediate Ca2+ influx (Wu et al., 1999). Cytochalasin D treatment greatly increased the amplitude of stretch-activated Ca2+ transients, indicating that actin filaments modulate stretch-activated Ca2+ transients.
Stretching forces applied through flexible substrates have been shown to induce increases in intracellular Ca2+ concentration, traction forces, and cell migration in NIH3T3 fibroblasts (Munevar et al., 2004). Stretch-activated increase in Ca2+ was inhibited by treatment with gadolinium, suggesting that response must rely on the entry of extracellular Ca2+ through stretch-sensitive channels. Gadolinium treatment also decreased cellular traction force and expression of vinculin and phosphotyrosine at FAs (Munevar et al., 2004). Previous studies have suggested that mechanical force application to integrins can activate mechanosensitive ion channels and trigger Ca2+ entry into cells. Ca2+ entry may influence cell mechanics by modulating cytoskeletal structure or contractility (Glogauer et al., 1997, 1998). Ca2+-activated proteases, such as calpain are associated with FAs (Glading et al., 2002). Their putative targets include structural and regulatory components such as paxillin and Rho (Geiger and Bershadsky, 2001).
Besides the explorations of mechanotransduction mechanisms at the cellular levels, recent investigations are opening up new frontiers at the molecular level. Single molecule studies using AFM and single molecule force spectroscopy (SMFS) have revealed structural motifs of proteins that can be mechanically switched between conformations potentially translating force into functional changes in proteins (Vogel, 2006; Vogel and Sheetz, 2006). Of special interest with respect to mechanosensing is the cell adhesion protein fibronectin, which consists of tandem repeat sequences. Many cryptic binding sites have been identified that are buried within individual fibronectin modules in the folded state. Applied mechanical force can turn on a biochemical switch, exposing these cryptic sites by three proposed mechanisms. First, the force applied on the protein module can stretch it into a conformation in which the binding site is exposed, enabling it to bind to the receptor. Second, the applied force can change the relative distance of two binding sites that bind the same receptor molecule. Third, the force can change the geometry of the binding site. Force deforms a binding pocket, making its position favorable for ligand binding. In addition, the disulfide bonds, which are present in many ECM proteins, have been recently proposed to act as redox switches, controlling the extent of a protein domain that is accessible to mechanical unfolding (Sandal and Samori, 2006) (Fig. 3).
5. Concluding remarks
In this paper, we have reviewed the mechanoregulation of gene expression in fibroblasts, an abundant cell type in connective tissues responsible for maintaining not only tissue homeostasis but also pathology. Evidence from previous studies indicates that gene regulation in fibroblasts depends on mechanical loading conditions: type (e.g., tension vs. compression), magnitude, frequency, and duration. The mechanoregulation of gene expression in fibroblasts also depends on the tissue location from which fibroblasts are derived, as well as ECM protein with which cells interact. To better understand how tissue homeostasis is maintained and how pathological conditions initiate and develop, it is necessary to study the effects of various mechanical loading conditions on fibroblasts. Also, the interaction between the mechanical loading conditions and soluble factors, including growth factors (e.g., TGF-β and PDGF) and cytokines (e.g., IL-1β and TNF-α), should be investigated in order to discern how changes in mechanical loads are associated with remodeling of connective tissues as well as onset and development of connective tissue diseases. Furthermore, future research will require novel theoretical and experimental methodologies to determine the precise details of mechanotransduction mechanisms, such as which mechanical factors (e.g., stress, strain and strain-rate) cells respond to and how they transduce them into a cascade of precise biological responses including the expression of ECM genes. One challenging task in future research is to understand how a cell “decides” its response from “crosstalks” of many mechanotransduction signals, since mechanotransduction mechanisms do not function in isolation, but rather by an integrated network of various signaling pathways. Another particularly challenging task is to identify specific “force receptors,” for which specific proteins at the membrane–cytoskeletal interface (e.g., integrins and G proteins) are good candidates. Ultimately, additional research in mechanoregulation of gene expression in fibroblasts will aid in developing new therapeutic strategies and new approaches to engineering tissue constructs for improved repair and regeneration of connective tissues.
Acknowledgements
We thank Mr. Michael Lin for his assistance in preparing this review. We also gratefully acknowledge the funding support of the Arthritis Investigator Award and NIH grant AR049921 (JHW).
Abbreviations
- ACL
Anterior cruciate ligament
- AFM
Atomic force microscopy
- CAM kinase
Calcium/calmodulin-dependent protein kinase
- CBF/ NF-Y
CCAAT binding factor
- CD44ICD
CD44 intracellular domain
- COX-2
Cyclooxygenase-2
- CREB
c-AMP response element-binding protein
- ECM
Extracellular matrix
- EGR-1
Early growth response-1
- FA
Focal adhesion
- FAK
Focal adhesion kinase
- FPCGs
Fibroblast-populated collagen gels
- HA
Hyaluronan
- IL-1β
Interleukin 1-beta
- IP3
Inositol triphosphate
- MAPK
Mitogen-activated protein kinase
- MCL
Medial collateral ligament
- mDial
Mammalian homolog of Drosophila diaphanous
- MLCK
Myosin light chain kinase
- MMP
Matrix metalloproteinase
- mPGES-1
Microsomal PGE synthase
- MSGens
Mechanical stress-induced genes
- MTC
Magnetic twisting cytometry
- PCP
Procollagen C-proteinase
- PDGF
Platelet-derived growth factor
- PDL
Periodonatal ligament
- PGE2
Prostaglandin E2
- PICP
Procollagen type I C-terminal peptide
- PKC
Protein kinase C
- PLC
phospholipase C
- ROCK
Rho-dependent kinase
- RTK
Receptor tyrosine kinase
- SAC
stretching-activated channel
- SMAD
Regulators of transcription that transduce signals from TGF-β receptors
- SMFS
Single molecule force spectroscopy
- TGF-β
Transforming growth factor beta
- TIMP
Tissue inhibitor of metalloproteinase
- TNF-α
Tumor necrosis factor alpha
References
- Abrahams M. Mechanical behaviour of tendon in vitro. A preliminary report. Med. Biol. Eng. 1967;5:433–443. doi: 10.1007/BF02479137. [DOI] [PubMed] [Google Scholar]
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med. Sci. Sports Exerc. 1993;25:603–607. [PubMed] [Google Scholar]
- Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J. Orthop. Res. 2002a;20:36–39. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J. Biomech. 2002b;35:303–309. doi: 10.1016/s0021-9290(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Arora PD, Bibby KJ, McCulloch CA. Slow oscillations of free intracellular calcium ion concentration in human fibroblasts responding to mechanical stretch. J. Cell. Physiol. 1994;161:187–200. doi: 10.1002/jcp.1041610202. [DOI] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Atance J, Yost MJ, Carver W. Influence of the extracellular matrix on the regulation of cardiac fibroblast behavior by mechanical stretch. J. Cell. Physiol. 2004;200:377–386. doi: 10.1002/jcp.20034. [DOI] [PubMed] [Google Scholar]
- Baciu PC, Goetinck PF. Protein kinase C regulates the recruitment of syndecan-4 into focal contacts. Mol. Biol. Cell. 1995;6:1503–1513. doi: 10.1091/mbc.6.11.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J. Cell Sci. 1985;75:35–42. doi: 10.1242/jcs.75.1.35. [DOI] [PubMed] [Google Scholar]
- Banes AJ, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthr. Cartil. 1999;7:141–153. doi: 10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- Bao G, Suresh S. Cell and molecular mechanics of biological materials. Nat. Matters. 2003;2:715–725. doi: 10.1038/nmat1001. [DOI] [PubMed] [Google Scholar]
- Barbee KA, Macarak EJ, Thibault LE. Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation. Ann. Biomed. Eng. 1994;22:14–22. doi: 10.1007/BF02368218. [DOI] [PubMed] [Google Scholar]
- Bernfield M, et al. Functions of cell surface heparan sulfate proteoglycans. Ann. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Bertram MJ, et al. Identification of a gene that reverses the immortal phenotype of a subset of cells and is a member of a novel family of transcription factor-like genes. Mol. Cell. Biol. 1999;19:1479–1485. doi: 10.1128/mcb.19.2.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renstrom PA, Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am. J. Sports Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Johnson RJ, Fleming BC, Stankewich CJ, Renstrom PA, Nichols CE. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension. A comparison of an open and a closed kinetic chain exercise. Am. J. Sports Med. 1997;25:823–829. doi: 10.1177/036354659702500616. [DOI] [PubMed] [Google Scholar]
- Bibby KJ, McCulloch CA. Regulation of cell volume and [Ca2+]i in attached human fibroblasts responding to anisosmotic buffers. Am. J. Physiol. 1994;266:C1639–C1649. doi: 10.1152/ajpcell.1994.266.6.C1639. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Boraldi F, et al. Cell-matrix interactions of in vitro human skin fibroblasts upon addition of hyaluronan. Tissue Cell. 2003;35:37–45. doi: 10.1016/s0040-8166(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Bornstein P, McKay J, Morishima JK, Devarayalu S, Gelinas RE. Regulatory elements in the first intron contribute to transcriptional control of the human alpha 1(I) collagen gene. Proc. Natl. Acad. Sci. U. S. A. 1987;84:8869–8873. doi: 10.1073/pnas.84.24.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EC. Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J. Appl. Physiol. 2000;88:203–209. doi: 10.1152/jappl.2000.88.1.203. [DOI] [PubMed] [Google Scholar]
- Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 1998;175:323–332. doi: 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Chen CC. Signal transduction pathways of inflammatory gene expressions and therapeutic implications. Curr. Pharm. Des. 2006;12:3497–3508. doi: 10.2174/138161206778343028. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-beta: involvement of Smad 3. J. Invest. Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Chen NX, Geist DJ, Genetos DC, Pavalko FM, Duncan RL. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33:399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Matthisson M, Koch M, Tannheimer M, Chiquet-Ehrismann R. Regulation of extracellular matrix synthesis by mechanical stress. Biochem. Cell. Biol. 1996;74:737–744. doi: 10.1139/o96-080. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Mumenthaler U, Wittwer M, Jin W, Koch M. The chick and human collagen alpha1(XII) gene promoter-activity of highly conserved regions around the first exon and in the first intron. Eur. J. Biochem. 1998;257:362–371. doi: 10.1046/j.1432-1327.1998.2570362.x. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Sarasa-Renedo A, Tunc-Civelek V. Induction of tenascin-C by cyclic tensile strain versus growth factors: distinct contributions by Rho/ROCK and MAPK signaling pathways. Biochim. Biophys. Acta. 2004;1693:193–204. doi: 10.1016/j.bbamcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, et al. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J. Cell Biol. 1994;127:2093–2101. doi: 10.1083/jcb.127.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ML, de Wet W, Bernard M, Ramirez F. Fine structural analysis of the human pro-alpha 1 (I) collagen gene. Promoter structure, AluI repeats, and polymorphic transcripts. J. Biol. Chem. 1985;260:2315–2320. [PubMed] [Google Scholar]
- Cichy J, Pure E. The liberation of CD44. J. Cell Biol. 2003;161:839–843. doi: 10.1083/jcb.200302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR, Chen L, Woods A. Syndecans and cell adhesion. Int. Rev. Cyt. 2001;207:113–150. doi: 10.1016/s0074-7696(01)07004-8. [DOI] [PubMed] [Google Scholar]
- Davidovitch Z. Tooth movement. Crit. Rev. Oral Biol. Med. 1991;2:411–450. doi: 10.1177/10454411910020040101. [DOI] [PubMed] [Google Scholar]
- Davies PF, et al. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu. Rev. Physiol. 1997;59:527–549. doi: 10.1146/annurev.physiol.59.1.527. [DOI] [PubMed] [Google Scholar]
- de Wet W, et al. Organization of the human pro-alpha 2(I) collagen gene. J. Biol. Chem. 1987;262:16032–16036. [PubMed] [Google Scholar]
- DesRosiers EA, Yahia L, Rivard CH. Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J. Orthop. Res. 1996;14:200–208. doi: 10.1002/jor.1100140206. [DOI] [PubMed] [Google Scholar]
- Devkota AC, Weinhold PS. Mechanical response of tendon subsequent to ramp loading to varying strain limits. Clin. Biomech. (Bristol, Avon) 2003;18:969–974. doi: 10.1016/s0268-0033(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Eastwood M, McGrouther DA, Brown RA. Fibroblast responses to mechanical forces. Proc. Inst. Mech. Eng. H J. Eng. Med. 1998a;212:85–92. doi: 10.1243/0954411981533854. [DOI] [PubMed] [Google Scholar]
- Eastwood M, Mudera VC, McGrouther DA, Brown RA. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil. Cytoskelet. 1998b;40:13–21. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Frank C, Schachar N, Dittrich D. Natural history of healing in the repaired medial collateral ligament. J. Orthop. Res. 1983a;1:179–188. doi: 10.1002/jor.1100010209. [DOI] [PubMed] [Google Scholar]
- Frank C, Woo SL, Amiel D, Harwood F, Gomez M, Akeson W. Medial collateral ligament healing. A multidisciplinary assessment in rabbits. Am. J. Sports Med. 1983b;11:379–389. doi: 10.1177/036354658301100602. [DOI] [PubMed] [Google Scholar]
- Gannier F, White E, Lacampagne A, Garnier D, Le Guennec JY. Streptomycin reverses a large stretch induced increases in [Ca2+]i in isolated guinea pig ventricular myocytes. Cardiovasc. Res. 1994;28:1193–1198. doi: 10.1093/cvr/28.8.1193. [DOI] [PubMed] [Google Scholar]
- Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–979. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Yuan W, Mori Y, Chen S, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J. Biol. Chem. 2001;276:11041–11048. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- Gigant-Huselstein C, et al. Expression of adhesion molecules and collagen on rat chondrocyte seeded into alginate and hyaluronate based 3D biosystems. Influence of mechanical stresses. Biorheology. 2004;41:423–431. [PubMed] [Google Scholar]
- Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- Glogauer M, Arora P, Yao G, Sokholov I, Ferrier J, McCulloch CA. Calcium ions and tyrosine phosphorylation interact coordinately with actin to regulate cytoprotective responses to stretching. J. Cell Sci. 1997;110(Pt 1):11–21. doi: 10.1242/jcs.110.1.11. [DOI] [PubMed] [Google Scholar]
- Glogauer M, Arora P, Chou D, Janmey PA, Downey GP, McCulloch CA. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J. Biol. Chem. 1998;273:1689–1698. doi: 10.1074/jbc.273.3.1689. [DOI] [PubMed] [Google Scholar]
- Gosset M, et al. Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene. Arthritis Res. Ther. 2006;8:R135. doi: 10.1186/ar2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Gudi SR, Lee AA, Clark CB, Frangos JA. Equibiaxial strain and strain rate stimulate early activation of G proteins in cardiac fibroblasts. Am. J. Physiol. 1998;274:C1424–C1428. doi: 10.1152/ajpcell.1998.274.5.C1424. [DOI] [PubMed] [Google Scholar]
- Gunthert U. CD44: a multitude of isoforms with diverse functions. Curr. Top. Microbiol. Immunol. 1993;184:47–63. doi: 10.1007/978-3-642-78253-4_4. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CA, Schroering AG, Carey DJ, Robishaw JD. Localization of a heterotrimeric G protein gamma subunit to focal adhesions and associated stress fibers. J. Cell Biol. 1994;126:811–819. doi: 10.1083/jcb.126.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- He Y, Macarak EJ, Korostoff JM, Howard PS. Compression and tension: differential effects on matrix accumulation by periodontal ligament fibroblasts in vitro. Connect. Tissue Res. 2004;45:28–39. doi: 10.1080/03008200490278124. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J. Appl. Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- Henskens YM, Veerman EC, Nieuw Amerongen AV. Cystatins in health and disease. Biol. Chem. Hoppe-Seyler. 1996;377:71–86. doi: 10.1515/bchm3.1996.377.2.71. [DOI] [PubMed] [Google Scholar]
- Herrick WC, Kingsbury HB, Lou DY. A study of the normal range of strain, strain rate, and stiffness of tendon. J. Biomed. Mater. Res. 1978;12:877–894. doi: 10.1002/jbm.820120610. [DOI] [PubMed] [Google Scholar]
- Hildebrand KA, Frank CB. Scar formation and ligament healing. Can. J. Surg. 1998;41:425–429. [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003;14:538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. J. Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Murakami M, Gao Y, Simons M. Phosphatidylinositol-4,5-bisphosphate mediates the interaction of syndecan-4 with protein kinase C. Biochemistry. 1999;38:15871–15877. doi: 10.1021/bi991363i. [DOI] [PubMed] [Google Scholar]
- Howard PS, Kucich U, Taliwal R, Korostoff JM. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J. Periodontal Res. 1998;33:500–508. doi: 10.1111/j.1600-0765.1998.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Hsieh AH, et al. Time-dependent increases in type-III collagen gene expression in medical collateral ligament fibroblasts under cyclic strains. J. Orthop. Res. 2000;18:220–227. doi: 10.1002/jor.1100180209. [DOI] [PubMed] [Google Scholar]
- Hu H, Sachs F. Stretch-activated ion channels in the heart. J. Mol. Cell. Cardiol. 1997;29:1511–1523. doi: 10.1006/jmcc.1997.0392. [DOI] [PubMed] [Google Scholar]
- Hull ML, Berns GS, Varma H, Patterson HA. Strain in the medial collateral ligament of the human knee under single and combined loads. J. Biomech. 1996;29:199–206. doi: 10.1016/0021-9290(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber D. Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Inoh H, et al. Uni-axial cyclic stretch induces the activation of transcription factor nuclear factor kappaB in human fibroblast cells. FASEB J. 2002;16:405–407. doi: 10.1096/fj.01-0354fje. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem. Biophys. Res. Commun. 2005;328:751–755. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, et al. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jalali S, et al. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili T, Takeishi Y, Walsh RA. Signal transduction during cardiac hypertrophy: the role of G alpha q, PLC beta I, and PKC. Cardiovasc. Res. 1999;44:5–9. doi: 10.1016/s0008-6363(99)00211-4. [DOI] [PubMed] [Google Scholar]
- Jimenez SA, et al. Functional analysis of human alpha 1(I) procollagen gene promoter. Differential activity in collagen-producing and -nonproducing cells and response to transforming growth factor beta 1. J. Biol. Chem. 1994;269:12684–12691. [PubMed] [Google Scholar]
- Jones BF, Wall ME, Carroll RL, Washburn S, Banes AJ. Ligament cells stretch-adapted on a microgrooved substrate increase intercellular communication in response to a mechanical stimulus. J. Biomech. 2005;38:1653–1664. doi: 10.1016/j.jbiomech.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Kalapesi FB, Tan JC, Coroneo MT. Stretch-activated channels: a mini-review. Are stretch-activated channels an ocular barometer? Clin. Exp. Ophthalmol. 2005;33:210–217. doi: 10.1111/j.1442-9071.2005.00981.x. [DOI] [PubMed] [Google Scholar]
- Kato T, Ishiguro N, Iwata H, Kojima T, Ito T, Naruse K. Up-regulation of COX2 expression by uni-axial cyclic stretch in human lung fibroblast cells. Biochem. Biophys. Res. Commun. 1998;244:615–619. doi: 10.1006/bbrc.1998.8335. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J. Biol. Chem. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- Kessler D, et al. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J. Biol. Chem. 2001;276:36575–36585. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J. Clin. Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Akaike T, Sasagaw T, Atomi Y, Kurosawa H. Gene expression of type I and type III collagen by mechanical stretch in anterior cruciate ligament cells. Cell Struct. Funct. 2002;27:139–144. doi: 10.1247/csf.27.139. [DOI] [PubMed] [Google Scholar]
- Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kjaer M, et al. In vivo studies of peritendinous tissue in exercise. Scand. J. Med. Sci. Sports. 2000;10:326–331. doi: 10.1034/j.1600-0838.2000.010006326.x. [DOI] [PubMed] [Google Scholar]
- Koch M, Bohrmann B, Matthison M, Hagios C, Trueb B, Chiquet M. Large and small splice variants of collagen XII: differential expression and ligand binding. J. Cell Biol. 1995;130:1005–1014. doi: 10.1083/jcb.130.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen SO, Heinemeier KM, Olesen JL, Langberg H, Kjaer M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J. Appl. Physiol. 2004;96:861–864. doi: 10.1152/japplphysiol.00489.2003. [DOI] [PubMed] [Google Scholar]
- Lacampagne A, Gannier F, Argibay J, Garnier D, Le Guennec JY. The stretch-activated ion channel blocker gadolinium also blocks L-type calcium channels in isolated ventricular myocytes of the guinea-pig. Biochim. Biophys. Acta. 1994;1191:205–208. doi: 10.1016/0005-2736(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Lambert CA, Colige AC, Munaut C, Lapiere CM, Nusgens BV. Distinct pathways in the over-expression of matrix metalloproteinases in human fibroblasts by relaxation of mechanical tension. Matrix Biol. 2001;20:397–408. doi: 10.1016/s0945-053x(01)00156-1. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J. Physiol. 1999;521(Pt 1):299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J. Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer S. Role of eicosanoids in structural degradation in osteoarthritis. Curr. Opin. Rheumatol. 2003;15:623–627. doi: 10.1097/00002281-200309000-00017. [DOI] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J. Orthop. Res. 2005;23:1211–1218. doi: 10.1016/j.orthres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lee AA, Delhaas T, Waldman LK, MacKenna DA, Villarreal FJ, McCulloch AD. An equibiaxial strain system for cultured cells. Am. J. Physiol. 1996;271:C1400–C1408. doi: 10.1152/ajpcell.1996.271.4.C1400. [DOI] [PubMed] [Google Scholar]
- Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J. Biomech. 2003;36:631–643. doi: 10.1016/s0021-9290(02)00441-4. [DOI] [PubMed] [Google Scholar]
- Lesley J, Hyman R. CD44 structure and function. Front. Biosci. 1998;3:d616–d630. doi: 10.2741/a306. [DOI] [PubMed] [Google Scholar]
- Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Leonard ME, Brown CG, Trestik CL. Frog semitendinosis tendon load-strain and stress-strain properties during passive loading. Am. J. Physiol. 1991;261:C86–C92. doi: 10.1152/ajpcell.1991.261.1.C86. [DOI] [PubMed] [Google Scholar]
- Lindahl GE, et al. Activation of fibroblast procollagen alpha 1(I) transcription by mechanical strain is transforming growth factor-beta-dependent and involves increased binding of CCAAT-binding factor (CBF/ NF-Y) at the proximal promoter. J. Biol. Chem. 2002;277:6153–6161. doi: 10.1074/jbc.M108966200. [DOI] [PubMed] [Google Scholar]
- Liu M, Post M. Invited review: mechanochemical signal transduction in the fetal lung. J. Appl. Physiol. 2000;89:2078–2084. doi: 10.1152/jappl.2000.89.5.2078. [DOI] [PubMed] [Google Scholar]
- Liu SH, Yang RS, Al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. Clin. Orthop. Relat. Res. 1995:265–278. [PubMed] [Google Scholar]
- Liu C, Yao J, Mercola D, Adamson E. The transcription factor EGR-1 directly transactivates the fibronectin gene and enhances attachment of human glioblastoma cell line U251. J. Biol. Chem. 2000;275:20315–20323. doi: 10.1074/jbc.M909046199. [DOI] [PubMed] [Google Scholar]
- Loren GJ, Lieber RL. Tendon biomechanical properties enhance human wrist muscle specialization. J. Biomech. 1995;28:791–799. doi: 10.1016/0021-9290(94)00137-s. [DOI] [PubMed] [Google Scholar]
- MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc. Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Halfter W, Liverani D. Induction of tenascin in healing wounds. J. Cell Biol. 1988;107:2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui T, et al. Effect of growth factors on matrix synthesis by ligament fibroblasts. J. Orthop. Res. 1997;15:18–23. doi: 10.1002/jor.1100150104. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Connectin/titin, giant elastic protein of muscle. FASEB J. 1997;11:341–345. doi: 10.1096/fasebj.11.5.9141500. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Manning AM. Multiple signals converging on NF-kappaB. Curr. Opin. Cell Biol. 1999a;11:226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Manning AM. NF-kappaB as a primary regulator of the stress response. Oncogene. 1999b;18:6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munevar S, Wang YL, Dembo M. Regulation of mechanical interactions between fibroblasts and the substratum by stretch-activated Ca2+ entry. J. Cell Sci. 2004;117:85–92. doi: 10.1242/jcs.00795. [DOI] [PubMed] [Google Scholar]
- Myokai F, et al. Unique genes induced by mechanical stress in periodontal ligament cells. J. Periodontal Res. 2003;38:255–261. doi: 10.1034/j.1600-0765.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–935. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, et al. Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol. Biol. Cell. 1999;10:2481–2491. doi: 10.1091/mbc.10.8.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T, Marui T, Hitora T, Doita M, Nishida K, Kurosaka M. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factor-beta1. J. Orthop. Res. 2002;20:1380–1386. doi: 10.1016/S0736-0266(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Nebe B, Bohn W, Sanftleben H, Rychly J. Induction of a physical linkage between integrins and the cytoskeleton depends on intracellular calcium in an epithelial cell line. Exp. Cell Res. 1996;229:100–110. doi: 10.1006/excr.1996.0348. [DOI] [PubMed] [Google Scholar]
- Okamoto I, et al. Regulated CD44 cleavage under the control of protein kinase C, calcium influx, and the Rho family of small G proteins. J. Biol. Chem. 1999;274:25525–25534. doi: 10.1074/jbc.274.36.25525. [DOI] [PubMed] [Google Scholar]
- Parsons M, Kessler E, Laurent GJ, Brown RA, Bishop JE. Mechanical load enhances procollagen processing in dermal fibroblasts by regulating levels of procollagen C-proteinase. Exp. Cell Res. 1999;252:319–331. doi: 10.1006/excr.1999.4618. [DOI] [PubMed] [Google Scholar]
- Reitan K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am. J. Orthod. 1967;53:721–745. doi: 10.1016/0002-9416(67)90118-2. [DOI] [PubMed] [Google Scholar]
- Reitan K. Principles of retention and avoidance of posttreatment relapse. Am. J. Orthod. 1969;55:776–790. doi: 10.1016/0002-9416(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Reitan K. Evaluation of orthodontic forces as related to histologic and mechanical factors. SSO Schweiz. Monatsschr. Zahnheilkd. 1970;80:579–596. [PubMed] [Google Scholar]
- Reynolds L, Bishop JE. Stimulation of cardiac fibroblast procollagen synthesis is enhanced by mechanicalload and fibronectin. J. Mol. Cell. Cardiol. 1998;30:A180. [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand. J. Med. Sci. Sports. 2005;15:241–251. doi: 10.1111/j.1600-0838.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Riveline D, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen LB, Greenberg ME. Stimulation of growth factor receptor signal transduction by activation of voltage-sensitive calcium channels. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1113–1118. doi: 10.1073/pnas.93.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc. Res. 2004;63:381–390. doi: 10.1016/j.cardiores.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J. Cell. Physiol. 2004;199:174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- Runyan CE, Poncelet AC, Schnaper HW. TGF-beta receptor-binding proteins: complex interactions. Cell. Signal. 2006;18:2077–2088. doi: 10.1016/j.cellsig.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc. Res. 2000;47:23–37. doi: 10.1016/s0008-6363(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Sackin H. Mechanosensitive channels. Annu. Rev. Physiol. 1995a;57:333–353. doi: 10.1146/annurev.ph.57.030195.002001. [DOI] [PubMed] [Google Scholar]
- Sackin H. Stretch-activated ion channels. Kidney Int. 1995b;48:1134–1147. doi: 10.1038/ki.1995.397. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Mechanotransduction in stretch-induced hypertrophy of cardiac myocytes. J. Recept. Res. 1993;13:777–794. doi: 10.3109/10799899309073692. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- Saitta B, Gaidarova S, Cicchillitti L, Jimenez SA. CCAAT binding transcription factor binds and regulates human COL1A1 promoter activity in human dermal fibroblasts: demonstration of increased binding in systemic sclerosis fibroblasts. Arthritis. Rheum. 2000;43:2219–2229. doi: 10.1002/1529-0131(200010)43:10<2219::AID-ANR9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sandal MGF, Samori B. Single molecule force specroscopy discovers mechanochemical switches in biology: the case of the disufide bond. Polymer. 2006;47:2571–2579. [Google Scholar]
- Saoncella S, et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports. 2005;15:223–230. doi: 10.1111/j.1600-0838.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Sarasa-Renedo A, Tunc-Civelek V, Chiquet M. Role of RhoA/ROCK-dependent actin contractility in the induction of tenascin-C by cyclic tensile strain. Exp. Cell Res. doi: 10.1016/j.yexcr.2005.12.025. in press. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr. Opin. Cell Biol. 1993;5:819–831. doi: 10.1016/0955-0674(93)90031-k. [DOI] [PubMed] [Google Scholar]
- Sawaguchi N, et al. Extracellular matrix modulates expression of cell-surface proteoglycan genes in fibroblasts. Connect Tissue Res. 2006;47:141–148. doi: 10.1080/03008200600685459. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Yamada Y, de Crombrugghe B. DNA sequence comparison of the regulatory signals at the 5′ end of the mouse and chick alpha 2 type I collagen genes. J. Biol. Chem. 1984;259:7411–7415. [PubMed] [Google Scholar]
- Schwachtgen JL, Houston P, Campbell C, Sukhatme V, Braddock M. Fluid shear stress activation of egr-1 transcription in cultured human endothelial and epithelial cells is mediated via the extracellular signal-related kinase 1/2 mitogen-activated protein kinase pathway. J. Clin. Invest. 1998;101:2540–2549. doi: 10.1172/JCI1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci. 2000;25:388–391. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shi M, Dennis K, Peschon JJ, Chandrasekaran R, Mikecz K. Antibody-induced shedding of CD44 from adherent cells is linked to the assembly of the cytoskeleton. J. Immunol. 2001;167:123–131. doi: 10.4049/jimmunol.167.1.123. [DOI] [PubMed] [Google Scholar]
- Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr. Opin. Cell Biol. 1997;9:707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res. Technol. 2003;9:3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- Takahashi I, et al. Effect of stretching on gene expression of beta1 integrin and focal adhesion kinase and on chondrogenesis through cell-extracellular matrix interactions. Eur. J. Cell Biol. 2003;82:182–192. doi: 10.1078/0171-9335-00307. [DOI] [PubMed] [Google Scholar]
- Takakuda K, Miyairi H. Tensile behaviour of fibroblasts cultured in collagen gel. Biomaterials. 1996;17:1393–1397. doi: 10.1016/0142-9612(96)87280-2. [DOI] [PubMed] [Google Scholar]
- Tao NJ, Lindsay SM, Lees S. Measuring the microelastic properties of biological material. Biophys J. 1992;63:1165–1169. doi: 10.1016/S0006-3495(92)81692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachslin J, Koch M, Chiquet M. Rapid and reversible regulation of collagen XII expression by changes in tensile stress. Exp. Cell Res. 1999;247:320–328. doi: 10.1006/excr.1998.4363. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J. Cell Biochem. 2003a;89:556–562. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J. Orthop. Res. 2003b;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell. Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J. Musculoskelet Neuronal Interact. 2005;5:70–84. [PubMed] [Google Scholar]
- Wang J-HCT. B.P: Mechanoregulation of Fibroblast Function. Taylor and Francis; 2006a. [Google Scholar]
- Wang JH. Mechanobiology of tendon. J. Biomech. 2006b;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE. Probing transmembrane mechanical coupling and cytomechanics using magnetic twisting cytometry. Biochem. Cell. Biol. 1995;73:327–335. doi: 10.1139/o95-041. [DOI] [PubMed] [Google Scholar]
- Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech. Model Mechanobiol. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001;34:1563–1572. doi: 10.1016/s0021-9290(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Wang JH, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect. Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- Wang JH, Yang G, Li Z, Shen W. Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J. Biomech. 2004;37:573–576. doi: 10.1016/j.jbiomech.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Watanabe N, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. Embo. J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- Woods A, Longley RL, Tumova S, Couchman JR. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wong K, Glogauer M, Ellen RP, McCulloch CA. Regulation of stretch-activated intracellular calcium transients by actin filaments. Biochem. Biophys. Res. Commun. 1999;261:419–425. doi: 10.1006/bbrc.1999.1057. [DOI] [PubMed] [Google Scholar]
- Xiao W. Advances in NF-kappaB signaling transduction and transcription. Cell Mol. Immunol. 2004;1:425–435. [PubMed] [Google Scholar]
- Yamaguchi N, Chiba M, Mitani H. The induction of c-fos mRNA expression by mechanical stress in human periodontal ligament cells. Arch. Oral Biol. 2002;47:465–471. doi: 10.1016/s0003-9969(02)00022-5. [DOI] [PubMed] [Google Scholar]
- Yang X, Hou F, Taylor L, Polgar P. Characterization of human cyclooxygenase 2 gene promoter localization of a TGF-beta response element. Biochim. Biophys. Acta. 1997;1350:287–292. doi: 10.1016/s0167-4781(96)00225-4. [DOI] [PubMed] [Google Scholar]
- Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J. Biomech. 2004;37:1543–1550. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Couchman JR. Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol. 2003;22:25–33. doi: 10.1016/s0945-053x(03)00010-6. [DOI] [PubMed] [Google Scholar]