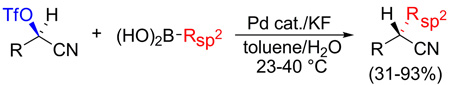
Abstract
Scalemic α-cyanohydrin triflates undergo Pd-catalyzed cross-coupling with aryl, heteroaryl, and vinyl boronic acids under mild conditions. Coupling proceeds with complete inversion of configuration at the stereogenic carbon. The resultant nitrile can be easily converted into a variety of alternative functional groups of value in organic synthesis and thus achieves a higher level of molecular complexity than traditional Suzuki reactions.
Suzuki and related transition metal catalyzed cross-couplings have had a profound influence on how carbon-carbon bonds are created and, by extension, on the craft of organic synthesis.1,2 While most often applied to sp- and sp2-hybridized halides and pseudohalides, significant progress has been achieved in recent years towards extending cross-coupling methodology to primary alkyl electrophiles.3 Secondary alkyl electrophiles, as might be anticipated, are more challenging due largely to their decreased rate of oxidative addition and the greater ease of β-hydride elimination. Nevertheless, pioneer work by (i) the Fu laboratory, beginning in 2004, with a series of Ni-catalyzed cross-couplings of activated and unactivated secondary alkyl electrophiles;4 (ii) Breit and Studte using zinc-catalyzed Grignard additions to chiral α-hydroxy ester triflates;5 and (iii) Asensio et al. with scalemic α-bromosulfoxides,6 cogently demonstrated the feasibility of transferring chiral sp3-carbons, often with pendant functionality that increases molecular complexity. In continuation of our own exploration of transition metal catalyzed cross-couplings of functionalized stereogenic carbon centers and their application to natural products total synthesis,7 we describe herein the Pd-catalyzed stereospecific cross-coupling of alkyl α-cyanohydrin triflates with aryl and alkenyl boronic acids (eq 1).
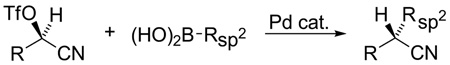 |
(1) |
α-Cyanohydrins attracted our attention because of their ready availability, often in either enantiomeric form, via addition of cyanide or its equivalents to aldehydes and ketones.8 Additionally, the cyano is a versatile functional group that can be easily transformed into a range of other moieties, e.g., aldehyde, ketone, carboxylate, amide, or primary amine, without compromising the chiral center. Since suitably derivatized α-cyanohydrins are known to undergo displacement by a variety of nucleophiles,8a we sought to exploit the corresponding triflates as Suzuki cross-coupling partners with arylboronic acids.
 |
(2) |
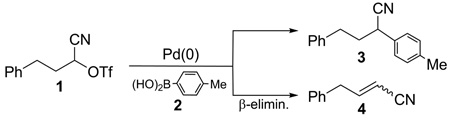
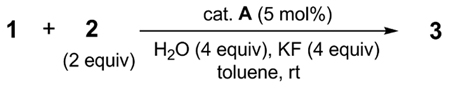
Preliminary screening of catalytic systems was performed with triflate 1 and 4-tolylboronic acid (2) (eq 2). As expected,1b the electron withdrawing cyano facilitated the rate of oxidative addition of 1 with palladium catalysts, even at room temperature. Unfortunately, for most catalytic systems, including Pd(OAc)2or Pd2(dba)3 in combination with various phosphine ligands, the reaction underwent β-hydride elimination to give 4 as the only product. The exception was Pd(PtBu3)2 which gave a very modest (20%), but reliable yield of 3 in the presence of K2CO3. Further screening of other palladium catalysts confirmed that the bulky, electron-rich PtBu3 ligand effectively minimized β-hydride elimination whereas other ligands gave essentially none of the desired product.
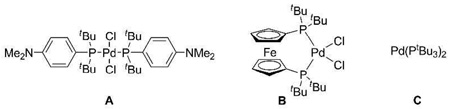
Extensive optimization of reaction parameters eventually led to a 93% yield of 3 using bis(di-tert-butyl(4-dimethylaminophenyl)phosphine)dichloropalladium(II) (catalyst A) and KF in moist toluene at room temperature (Table 1, entry 1). Reducing the amount of boronic acid 2 resulted in a more or less proportionate decrease in the yield of 3 (entry 2). A modest increase in the reaction temperature boosted the reaction rate, as expected, without affecting the yield of 3 (entry 3), but a simultaneous reduction in the amount of catalyst was detrimental (entry 4). Consistent with previous studies,9the presence of a small amount of water was helpful, but had to be carefully controlled; too much (entry 5) or none (entries 6 and 7) proved unfavorable. Other common solvents (entry 8–10), reduced KF (entry 11), or K2CO3 as base (entry 12) were less effective. Numerous other commercial catalysts and loadings were investigated, e.g., catalysts B and C were more reactive than A, but generally β-hydride elimination was simultaneously accelerated resulting in a lower yield of 3 (entry 13–15). Other typical organoborane sources were also tested, inter alia, boronate esters, potassium trifluoroborates and MIDA boronates, but showed no reactivity under the test conditions; the exception was triphenylborane which gave a moderate yield of cross-coupled adduct (67%), possibly the result of in situ hydrolysis to the corresponding boronic acid.
Table 1.
Suzuki Cross-coupling of α-Cyanohydrin Triflate 1 with Boronic Acid 2 Effect of Reaction Parameters.a
 | ||
|---|---|---|
| entry | variations from "standard" conditions | yield 3(%) |
| 1 | none | 93 |
| 2 | boronic acid 2 (1.5 equiv) | 71 |
| 3 | 40 °C | 91 |
| 4 | 40 °C, catalyst A (3 mol%) | 79 |
| 5 | H2O (40 equiv) | 74 |
| 6 | noH2O | 82 |
| 7 | no H2O, catalyst A (10 mol%) | 85 |
| 8 | THF as solvent | 60 |
| 9 | DME as solvent | 30 |
| 10 | benzene as solvent | 74 |
| 11 | KF (2 equiv) | 76 |
| 12 | K2CO3 (4 equiv), no KF, no H2O | 68 |
| 13 | catalyst B (5 mol%) | 77 |
| 14 | catalyst B (3 mol%), 40 °C | 68 |
| 15 | catalyst C (5 mol%), −4 °C | 45 |
See Supporting Information for experimental procedure.
Application of the optimized reaction conditions, with small variations as noted to maximize yields, to a panel of representative triflates and boronic acids is summarized in Table 2. For non-racemic α-cyanohydrin triflates (S)-1 and (R)-1, cross-coupling with 2 and 5 stereospecifically furnished (S)-3 and (R)-6, respectively, in excellent yields (entries 1 and 2). For more sterically hindered substrates like ortho-substituted boronic acids 7 (entry 3) and 15 (entry 7), catalyst B was more efficacious than catalyst A, even when employed at room temperature. The influence of steric crowding was more evident when present near the α-cyanohydrin center and if a β-hydride was present, e.g., 19 vs. 21 (entries 9 and 10). Otherwise, the couplings were comparatively tolerant of electron donating (9, entry 4) and withdrawing substituents (11 and 13, entries 5 and 6). Likewise, vinyl boronic acid 17 was quite successful, smoothly generating adduct 18 in high yield (entry 8). Extensions to heteroaromatic boronic acids were positionally dependent, reflecting the well-known proclivity of boronic acids adjacent to a heteroatom to undergo decomposition following oxidative addition to a transition metal (23 and 25, entries 11 and 12). In contrast with the preceding results, aliphatic α-cyanohydrin mesylates were generally refractory to cross-coupling and decomposed under forcing conditions. However, benzylic mesylate 27 was atypical and gave rise to adduct 28 in good yield, if catalyst C was used (entry 13). Some racemization was observed, but control experiments indicated this was due to the lability of the benzhydryl proton in 28 under the reaction conditions and not a consequence of the coupling process.
Table 2.
Stereospecific Suzuki Cross-coupling of Alkyl α-Cyanohydrin Triflates.a
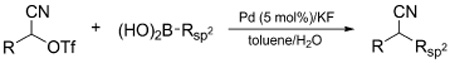 | ||||||
|---|---|---|---|---|---|---|
| entry | α-cyanohydrin | boronic acid | adduct | cat. (mol%) |
temp (°C) /time (h) |
yield (%)b |
| 1 | 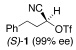 |
2 | 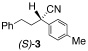 |
A | 40/20 | 91 (98% ee) |
| 2 | A | 40/24 | 93 (94% ee) |
|||
| 3 | 1 | B | 21/20 | 74 | ||
| 4 | 1 | A | 21/48 | 82 | ||
| 5 | 1 | A | 40/20 | 51c | ||
| 6 | 1 | A | 40/20 | 76 | ||
| 7 | 1 | B | 21/20 | 68 | ||
| 8 | 1 | A | 40/20 | 94 | ||
| 9 | 2 | B | 21/20 | 31 | ||
| 10 | 2 | B | 21/20 | 78 | ||
| 11 | 1 | A | 40/36 | 21 | ||
| 12 | 1 | 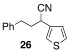 |
A | 40/20 | 56 | |
| 13 | 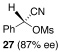 |
9 | 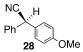 |
C | 40/24 | 84 (80% ee) |
Reaction conditions: triflate/mesylate (0.15 mmol), boronic acid (0.3 mmol), Pd catalyst (5 mol%), and KF (0.6 mmol) in toluene (2 mL)/H2O (10 µL).
Enantiomeric excess determined by chiral HPLC.
Using K3PO4·H2O (0.6 mmol) instead of KF; same conditions using KF (0.6 mmol) gave 33% yield.
To determine the stereospecificity of the cross-coupling, the absolute configuration of adduct 6 (Table 2, entry 2) was established via hydrolysis to the known carboxylic acid 29 (eq 3). Comparison of the optical rotation of 29 with literature values10 showed a total inversion of the configuration during the coupling. Since both the transmetalation and the reductive elimination are known to occur with retention,1 this is consistent with studies on the oxidative addition of secondary alkyl halides.6,11
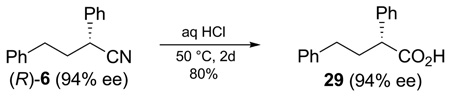 |
(3) |
In summary, we introduce a valuable variant of the traditional Suzuki cross-coupling that stereospecifically transfers a functionalized, sp3-hybridized stereogenic carbon to aryl, heteroaryl, and vinyl boronic acids using commercially available palladium catalysts under mild conditions. The coupling proceeds with complete inversion of configuration. The resultant nitrile can be easily converted to a variety of alternative functional groups of value in organic synthesis and thus achieves a higher level of molecular complexity than traditional Suzuki reactions. Efforts to utilize other electrophiles and nucleophiles are underway.
Supplementary Material
Acknowledgment
Financial support from the Robert A. Welch Foundation and NIH (GM31278, DK38226).
Footnotes
Supporting Information Available: Experimental procedures and characterization/spectra of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Second Completely Revised and Enlarged Edition. Volumn 1. Weinheim: Wiley-VCH; 2004. p. 478. [Google Scholar]; (b) Diederich F, Stang PJ, editors. Metal-Catalyzed Cross-coupling Reactions. Weinheim: Wiley-VCH; 1998. p. 517. [Google Scholar]
- 2.(a) Corbet J-P, Mignani G. Chem. Rev. 2006;106:2651–2710. doi: 10.1021/cr0505268. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Bulger PG, Sarlah D. D. Angew. Chem. Int. Ed. 2005;44:4442–4489. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 3.Recent reviews: Crudden CM, Glasspoole BW, Lata CJ. Chem. Commun. 2009:6704–6716. doi: 10.1039/b911537d. Rudolph A, Lautens M. Angew. Chem, Int. Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611. Frisch AC, Beller M. Angew. Chem, Int. Ed. 2005;48:674–688. doi: 10.1002/anie.200461432. Netherton MR, Fu GC. Adv. Synth. Catal. 2004;346:1525–1532.
- 4.Suzuki Zhou J, Fu GC. J. Am. Chem. Soc. 2004;126:1340–1341. doi: 10.1021/ja039889k. Gonzalez-Bobes F, Fu GC. J. Am. Chem. Soc. 2006;128:5360–5361. doi: 10.1021/ja0613761. Saito B, Fu GC. J. Am. Chem. Soc. 2007;129:9602–9603. doi: 10.1021/ja074008l. Saito B, Fu GC. J. Am. Chem. Soc. 2008;130:6694–6695. doi: 10.1021/ja8013677. Stille: Powell DA, Maki T, Fu GC. J. Am. Chem. Soc. 2005;127:510–511. doi: 10.1021/ja0436300. Hiyama: Strotman NA, Sommer S, Fu GC. Angew. Chem., Int. Ed. 2007;46:3556–3558. doi: 10.1002/anie.200700440. Dai X, Strotman NA, Fu GC. J. Am. Chem. Soc. 2008;130:3302–3303. doi: 10.1021/ja8009428. Negishi: Fischer C, Fu GC. J. Am. Chem. Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509. Arp FO, Fu GC. J. Am. Chem. Soc. 2005;127:10482–10483. doi: 10.1021/ja053751f. Son S, Fu GC. J. Am. Chem. Soc. 2008;130:2756–2757. doi: 10.1021/ja800103z.
- 5.Studte C, Breit B. Angew. Chem, Int. Ed. 2008;47:5451–5455. doi: 10.1002/anie.200800733. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez N, de Arellano CR, Asensio G, Medio-Simon M. Chem. Eur. J. 2007;13:4223–4229. doi: 10.1002/chem.200601488. [DOI] [PubMed] [Google Scholar]
- 7.For syntheses and stereospecific cross-couplings of non-racemic α-hydroxy stannanes, see Ye J, Bhatt RK, Falck JR. J. Am. Chem. Soc. 1994;116:1–5. Falck JR, Bhatt RK, Ye J. J. Am. Chem. Soc. 1995;117:5973–5982. Falck JR, Patel PK, Bandyopadhyay A. J. Am. Chem. Soc. 2007;129:790–793. doi: 10.1021/ja064948q. He A, Falck JR. Angew. Chem, Int. Ed. 2008;47:6586–6589. doi: 10.1002/anie.200802313.
- 8.Recent reviews: Holt J, Hanefeld U. Curr. Org. Synth. 2009;6:15–37. North M, Usanov DL, Young C. Chem. Rev. 2008;108:5146–5226. doi: 10.1021/cr800255k. Khan NH, Kureshy RI, Abdi SHR, Agrawal S, Jasra RV. Coord. Chem. Rev. 2008;252:593–623. Chen F-X, Feng X. Curr. Org. Synth. 2006;3:77–97.
- 9.Netherton MR, Dai C, Neuschutz K, Fu GC. J. Am. Chem. Soc. 2001;123:10099–10100. doi: 10.1021/ja011306o. [DOI] [PubMed] [Google Scholar]
- 10.[α]D = −56.8 (c 24.9, CHCl3) for (R)-29: Kozikowski AP, Tückmantel W, George C. J. Org. Chem. 2000;65:5371–5381. doi: 10.1021/jo000485+.
- 11.(a) Lau KSY, Fries RW, Stille JK. J. Am. Chem. Soc. 1974;96:4983–4986. [Google Scholar]; (b) Stille JK, Lau KSY. J. Am. Chem. Soc. 1976;98:5841–5849. [Google Scholar]; (c) Netherton MR, Fu GC. Angew. Chem. Int. Ed. 2002;41:3910–3912. doi: 10.1002/1521-3773(20021018)41:20<3910::AID-ANIE3910>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]; (d) Hills ID, Netherton MR, Fu GC. Angew. Chem. Int. Ed. 2003;42:5749–5752. doi: 10.1002/anie.200352858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.