Abstract
Physical activity has been associated with a small improvement in quality of life (QOL) among those with multiple sclerosis (MS). This relationship may be indirect and operate through factors such as disability, fatigue, mood, pain, self-efficacy and social support. The present study examined variables that might account for the relationship between physical activity and QOL in a sample (N = 292) of individuals with a definite diagnosis of MS. The participants wore an accelerometer for 7 days and then completed self-report measures of physical activity, QOL, disability, fatigue, mood, pain, self-efficacy and social support. The data were analysed using covariance modelling in Mplus 3.0. The model provided an excellent fit for the data (χ2 = 51.33, df = 18, p < 0.001, standardised root mean squared residual = 0.03, comparative fit index = 0.98). Those who were more physically active reported lower levels of disability (γ = -0.50), depression (γ = -0.31), fatigue (γ = -0.46) and pain (γ = -0.19) and higher levels of social support (γ = 0.20), self-efficacy for managing MS (γ = 0.41), and self-efficacy for regular physical activity (γ = 0.49). In turn, those who reported lower levels of depression (β = -0.37), anxiety (β = -0.15), fatigue (β = -0.16) and pain (β = -0.08) and higher levels of social support (β = 0.26) and self-efficacy for controlling MS (β = 0.17) reported higher levels of QOL. The observed pattern of relationships supports the possibility that physical activity is indirectly associated with improved QOL in individuals with MS via depression, fatigue, pain, social support and self-efficacy for managing MS.
Keywords: physical activity, quality of life, mediators, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a prevalent neurological disease that affects approximately 400,000 adults in the United States (National Multiple Sclerosis Society, 2005). This chronic and progressive disease involves unpredictable episodes of inflammatory demyelination and axonal transection that result in lesions along axons of nerve fibres in the central nervous system (CNS). These lesions interfere with the smooth and rapid conduction of electrical potentials along neuronal pathways. The interference with neuronal conduction yields the many symptoms experienced by people with MS, and, ultimately, this disease process can result in functional limitations, disability and reduced quality of life (QOL).
There is an increased interest in the study of QOL in persons with MS (Benito-León, Morales, Rivera-Navarro, & Mitchell, 2003; Mitchell, Benito-León, Gonzalez, & Rivera-Navarro, 2005). QOL is an umbrella term that describes a number of outcomes that are considered important within an individual's life (Rejeski & Mihalko, 2001) and can include physical, social, psychological and spiritual dimensions of one's well-being (Benito-León et al., 2003; Mitchell et al., 2005). QOL represents a person's judgment about how well they are living based on a broad array of domains. Researchers have reported that individuals with MS have lower QOL than non-diseased and diseased populations (Benito-León et al., 2003; Mitchell et al., 2005). For example, individuals with MS had significantly lower overall and domain specific QOL than non-diseased controls in a recent cross-sectional study (Lobentanz et al., 2004). Other studies have reported that QOL in MS patients is reduced even when compared with those suffering from inflammatory bowel disease, ischaemic stroke and rheumatoid arthritis (Lankhorst et al., 1996; Naess, Beiske, & Myhr, 2008; Rudick, Miller, Clough, Gragg, & Farmer, 1992). Several features of MS likely contribute to reduced QOL, namely an onset during the productive years of one's life, an uncertain and unstable disease course, diffuse effects throughout the CNS and absence of a cure (Benito-León et al., 2003; Mitchell et al., 2005).
An important goal of researchers and clinicians involves improving the QOL of individual's with MS. This might be accomplished by understanding behavioural, psychological and social predictors of QOL in persons with MS (Benito-León et al., 2003; Mitchell et al., 2005). Physical activity is a potentially modifiable behaviour that has been associated with QOL in people with MS. Physical activity involves bodily movement produced by the contraction of skeletal muscles that increases energy expenditure above resting levels, and includes leisure-time physical activity, exercise, sport, occupational work and household chores (Bouchard & Shephard, 1994). Evidence from a recent meta-analysis of 13 studies with 109 effect sizes from 484 people with MS indicated that physical activity, in the form of exercise training, was associated with approximately one-fourth standard deviation improvement in QOL (g = 0.23, 95% CI = 0.15, 0.31; Motl & Gosney, 2008). An effective size of this magnitude is potentially clinically meaningful when compared, for example, with the overall effectiveness of disease modifying medications for reducing exacerbations in individuals with MS (d = 0.30; Filippini et al., 2003).
One core recommendation of the aforementioned meta-analysis involved future investigations of the factors that explain the improvement in QOL associated with physical activity in those with MS. This recommendation is based on the argument that the effect of physical activity on QOL may not be direct, but rather indirect and operate through intermediate factors (Stewart & King, 1991). Physical activity likely has its strongest effects on more proximal outcomes that are, in turn, related with the distal outcome of QOL. Emerging evidence has supported an indirect relationship between physical activity and QOL in older adults (Elavsky et al., 2005; McAuley, Konopack, Motl, Morris, Doerksen, & Rosengren, 2006), and two recent studies have demonstrated the possibility of an indirect relationship in persons with MS (Motl & Snook, 2008; Motl, Snook, McAuley, Scott, & Douglas, 2006). The first study indicated that physical activity was indirectly associated with QOL through a pathway that included exercise self-efficacy and functional limitations (Motl et al., 2006). The second study indicated that physical activity was related with QOL through a pathway that included self-efficacy for managing MS (Motl & Snook, 2008).
The primary limitation of that previous research includes the consideration of a relatively limited range of possible intermediate variables (i.e. self-efficacy and function) that might account for the relationship between physical activity and QOL in persons with MS. Indeed, a broader array of factors such as disability, fatigue, mood, pain, self-efficacy and social support might operate as intermediaries for explaining why physical activity is associated with improved QOL in MS; those variables all represent primary correlates of QOL in those with MS (Benito-León et al., 2003; Mitchell et al., 2005). Disability has been inversely correlated with overall QOL and physical health status in cross-sectional studies of patients with MS (Amato, Ponziani, Rossi, Liedl, Stefanile, & Rossi, 2001; Lobentanz, et al., 2004). Fatigue has been inversely correlated with aspects of QOL in cross-sectional studies of MS patients (Benedict et al., 2005; Lobentanz et al., 2004). The moods of anxiety and depression were significantly and inversely correlated with physical and mental aspects of health status in a cross-sectional study of patients with MS (Janssens et al., 2003). Pain was inversely correlated with aspects of QOL in two recent cross-sectional studies of individuals with MS (Kalia & O'Connor, 2005; Svendsen, Jensen, Hansen, & Bach, 2005). Self-efficacy was positively associated with physical and psychological health status in a cross-sectional study of MS patients (Riazi, Thompson, & Hobart, 2004). Both social support and self-efficacy were positively related to overall QOL in a cross-sectional sample of 786 persons with MS (Stuifbergen, Seraphine, & Roberts, 2000). Although such findings are consistent with the position that disability, anxiety, depression, fatigue, pain, self-efficacy and social support are correlates of QOL in MS (Benito-León et al., 2003; Mitchell et al., 2005), we are unaware of research that has considered these variables as intermediaries of the relationship between physical activity and QOL.
Overall, there has been accumulating evidence that physical activity is positively linked with QOL in MS (Motl & Gosney, 2008), and preliminary evidence that the effect of physical activity on QOL may be indirect (Motl, McAuley, Snook, Scott, 2006; Motl & Snook, 2008). The present study involved an examination of a broad array of variables for explaining the relationship between physical activity and QOL in persons with MS. We hypothesised that individuals with MS who are more physically active would have a higher QOL than those who are less physically active. We further expected that the positive relationship would be accounted for by the intermediate factors of disability, fatigue, mood, pain, self-efficacy and social support. Those factors have been identified as important influences of QOL in MS (Benito-León et al., 2003; Mitchell et al., 2005) and might be positively influenced by physical activity (Petajan & White, 1999; Schapiro, 2003; White & Dressendorfer, 2004), thereby providing additional explanations for the beneficial effect of physical activity on QOL in persons with MS.
Method
Participants
We recruited a convenience sample of individuals with MS who were members of the Greater Illinois, Gateway and Indiana chapters of the MS society. Recruitment was conducted through (a) research announcements mailed to past study participants, (b) advertisements placed in each chapter's MS Connection quarterly publication and (c) email messages that were distributed to all registered members of the chapters. Those interested in participation were asked to contact the research team through either e-mail or telephone, and collect calls were encouraged and accepted from those interested in participating and living outside the local calling area. This initial contact was followed-up by a phone call from a member of the research team who described the study and its procedures, answered all questions and conducted a brief screening interview. The screening criteria involved (a) having an established definite diagnosis of MS, (b) being relapse free in the last 30 days and (c) being ambulatory with minimal assistance. The definite diagnosis was confirmed based on completion of a form-letter by the participant's neurologist. Individuals were relapse free as we suspect that physical activity would be substantially reduced during and immediately after such an episode. Ambulatory with minimal assistance was defined as being able to walk with or without a cane, but excludes those using a wheelchair or motorised cart.
There were 511 individuals who were contacted about participation in this study, and 387 of those individuals underwent screening. Of those who were screened, there were 27 individuals who did not satisfy our inclusion criteria and 16 individuals who declined participation. We sent an informed consent document and verification letter to the remaining 344 individuals, and the forms were returned by 300 of the 344 individuals. Of those who returned the forms, eight did not continue with participation and reasons for lack of participation are unknown. The final convenience sample consisted of 292 individuals with MS. The sample consisted of 245 women and 47 men, and 246 were diagnosed with relapsing-remitting MS, 12 were diagnosed with primary progressive MS and 34 were diagnosed with secondary progressive MS. The mean age of the sample was 48.0 years (SD = 10.3, range = 20-69 years) and the mean duration, defined as time since definite diagnosis, of MS was 10.3 years (SD = 7.9, range = 1-35 years). The sample was mostly Caucasian (94%), married (68%), employed (53%) and educated (28% had some college education and 57.7% were college graduates) with a median annual household income of greater than $40,000 (67.7%).
Measures
Physical activity
Physical activity was measured by the Godin Leisure-Time Exercise Questionnaire (GLTEQ; Godin & Shephard, 1985) and the ActiGraph single-axis accelerometer (model 7164 version, Manufacturing Technology Incorporated, Fort Walton Beach, FL). The use of both self-report and objective measures has been recognised as ideal by experts (Dishman, Washburn, & Schoeller, 2001), and allowed for the modelling of physical activity as a latent variable in our data analyses. The GLTEQ is a self-administered two-part measure of usual physical activity; we only included the first part in this study consistent with previous research (Gosney, Scott, Snook, Motl, 2007; Motl et al., 2006). The first part has three items that measure the frequency of strenuous (e.g. jogging), moderate (e.g. fast walking) and mild (e.g. easy walking) exercise for periods of more than 15 minutes during one's free time in a typical week. The weekly frequencies of strenuous, moderate and mild activities are multiplied by nine, five and three metabolic equivalents, respectively, and summed to form a measure of total leisure activity. This study used the previous week as a time-frame for the GLTEQ, and participants completed the GLTEQ after wearing an accelerometer for the 7-day period.
The ActiGraph accelerometer contains a single, vertical axis piezoelectric bender element that generates an electrical signal proportional to the force acting on it. The acceleration/deceleration signal is digitised by an analogue-to-digital converter and numerically integrated over a pre-programmed epoch interval. At the end of each interval, the integrated value of movement counts is stored in RAM and the integrator is reset. The monitor is programmed for start time and data collection interval and data are retrieved for analysis via a PC interface and software provided with the unit. The downloaded data from the accelerometers are then entered into Microsoft Excel for data processing. In this study, the epoch was 1 minute, and the accelerometers were worn during the waking hours, except while showering, bathing and swimming, for a 7-day period. Waking hours was defined as the moment upon getting out of bed in the morning through the moment of getting into bed in the evening. The accelerometers were not worn during the night while the participants slept. The participants recorded the time that the accelerometer was worn on a log, and this was verified by inspection of the minute-by-minute accelerometer data. Regarding data processing, we summed the minute-by-minute counts across each of the 7 days and then averaged the total daily movement counts across the 7 days. This yielded accelerometer data in total movement counts per day with higher scores representing more physical activity. We have used both physical activity measures and the same procedures in our previous research on validity of physical activity measures and correlates of physical activity in MS patients (Gosney et al., 2007; Motl et al., 2006).
Quality of life
QOL was measured using the Leeds Multiple Sclerosis Quality of Life Scale (LMSQOL; Ford, Gerry, Tennant, Whalley, Haigh, & Johnson, 2001) and the Satisfaction With Life Scale (SWLS) (Diener, Emmons, Larsen, & Griffin, 1985; Pavot & Diener, 1993); this facilitated the formation of an overall QOL latent variable for our data analysis. The LMSQOL is an eight-item, unidimensional disease-specific measure of overall QOL. The LMSQOL has good internal consistency, test-retest reliability and evidence of score validity and virtually no floor or ceiling effects (Ford et al., 2001). Coefficient alpha for the LMSQOL was 0.82 in the present study. The SWLS is a five-item, unidimensional generic measure of overall QOL. The SWLS has good internal consistency, test-retest reliability and evidence of score validity (Diener et al., 1985; Pavot & Diener, 1993). Coefficient alpha for the SWLS was 0.89 in the present study.
Disability
Disability was measured using the Patient Determined Disease Steps (PDDS) scale (Hadjimichael, Kerns, Rizzo, Cutter, & Vollmer, 2007). The PDDS is a self-report questionnaire that contains a single item for measuring self-reported disability using an eight-level ordinal scale. Scores from the PDDS are linearly and strongly related with physician-administered EDSS scores (r = 0.93) (Hadjimichael et al., 2007).
Fatigue
Perceived fatigue was measured with the Fatigue Severity Scale (FSS) (Krupp, LaRocca, Muir-Nash, & Steinberg, 1989). The FSS has nine items that are combined to form an overall measure of a person's severity of fatigue symptoms. This scale has good evidence of internal consistency, test-retest reliability and score validity (Krupp et al., 1989). Coefficient alpha for the FSS was 0.93 in the present study.
Mood
The moods of anxiety and depression were measured by the Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, 1983). The HADS contains 14 items; seven items measure anxiety and seven items measure depression. This scale has been used in previous studies of QOL in MS (Janssens et al., 2003) and has good evidence of score reliability and validity (Zigmoid & Snaith, 1983). Coefficient alpha for the anxiety and depression components of the HADS were 0.83 and 0.82, respectively, in the present study.
Pain
Pain was measured with the short-form McGill Pain Questionnaire (SF-MPQ) (Melzack, 1987). This scale contains a 15-item adjective checklist that captures sensory and affective dimensions of pain. The scores from the items are summed to form a pain rating index. The SF-MPQ is internally consistent, reliable across time, and has evidence of score validity (Melzack, 1987). Coeffcient alpha for the SF-MPQ was 0.88 in the present study.
Self-efficacy
Self-efficacy was assessed by the Multiple Sclerosis Self-Efficacy Scale (MSSE) (Schwartz, Coulthard-Morris, Zeng, & Retzlaff, 1996) and the Exercise Self-Efficacy scale (EXSE) (McAuley, 1993). The MSSE has 18-items and measures two subscales of function and control. The function subscale measures confidence with functional abilities. The control subscale measures confidence with managing symptoms and coping with the demands of illness. The two subscales are combined for an overall measure of self-efficacy for managing MS. The EXSE scale has six items that assess an individual's beliefs in their ability to engage in 20 + minutes of moderate physical activity three times per week, in one month increments, across the next 6 months. Both scales are internally consistent and have evidence of score validity (McAuley, 1993; Schwartz et al., 1996). Coefficient alpha for MSSE and EXSE were 0.92 and 0.99, respectively, in the present study.
Social support
Perceptions of social support were measured by the 24-item Social Provisions Scale (SPS) (Cutrona & Russell, 1987). The SPS includes six subscales of reliable alliance, attachment, guidance, nurturance, social integration and reassurance of worth. Scores from the subscales are summed to form a single composite measure of social support. This scale has good internal consistency, test-retest reliability and evidence of score validity (Cutrona & Russell, 1987). Coefficient alpha for the SPS was 0.89 in the present study.
Procedure
After initial telephone contact and voluntary participation, an informed consent document and a form-letter for verifying the participant's diagnosis of MS were sent to all participants through the US postal service, along with pre-stamped and pre-addressed envelopes for return postal service. The researchers called to make sure the participants received the documents, understood the directions, and signed the informed consent. Once the informed consent was returned, a battery of questionnaires and an accelerometer were sent to all participants through the US postal service, along with a pre-stamped and pre-addressed envelope for return postal service. The researchers called to make sure the participants received the package and understood the directions. The participants wore the accelerometer for a 7-day period and then completed a battery of questionnaires that included the GLTEQ and measures of QOL, disability, mood, self-efficacy, social support, pain and fatigue on the 8th day. After wearing the accelerometer and completing the measures, participants returned the study materials through the US postal service. All questionnaires were checked for completeness. In the event of missing data, participants were contacted by a member of the research team to collect the data over the phone. All participants received $20 upon returning the study materials.
Data analysis
We tested models for describing (1) the relationship between physical activity and QOL and (2) the pattern of relationships among physical activity, disability, mood, self-efficacy, social support, pain, fatigue and QOL using covariance modelling and the Full-Information Maximum Likelihood (FIML) estimation in Mplus 3.0 (Muthén & Muthén, 1998-2003). The FIML estimator was selected because there were missing accelerometer data (4% missing data) and the FIML estimator is an optimal method for the treatment of missing data in covariance modelling that has yielded accurate fit indices with simulated missing data (Enders & Bandalos, 2001). Covariance modelling is a family of techniques that allow for testing the fit of hypothetical models that describe explanatory relationships among manifest and latent variables. Importantly, covariance modelling does not allow for inferences about causation based on covariances among variables, but instead allows for testing models that reflect hypotheses about causal processes that might underlie the data.
The first model that we tested is provided in Figure 1 and included a direct association between physical activity and QOL as latent variables. Physical activity was modelled as a latent variable using the GLTEQ scores and accelerometer counts as indicators. QOL was modelled as a latent variable using LMSQOL and SWLS overall scores as indicators. This model provided an indication of the strength of association between physical activity and QOL in the present study. The second model that we tested is provided in Figure 2 and included (a) direct associations between physical activity as a latent variable and disability, mood, self-efficacy, social support, pain and fatigue as manifest variables; (b) direct associations between disability, moods of anxiety and depression, self-efficacy for physical activity and managing MS, social support, pain and fatigue as manifest variables and QOL as a latent variable; and (c) bi-directional associations or correlations among disability, mood, self-efficacy, social support, pain and fatigue manifest variables. The later model provided an indication of the intermediate variables that might explain the association between physical activity and QOL.
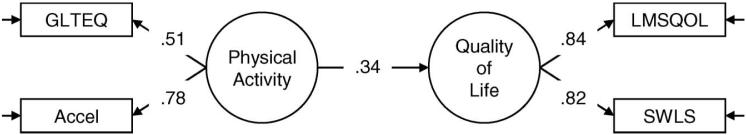
Figure 1.
Model that was tested for understanding the association between physical activity and quality of life in a sample of 292 individuals with multiple sclerosis. Note: The factor loadings and path coefficient are standardised estimates and statistically significant. GLTEQ, Godin Leisure-Time Exercise Questionnaire; Accel, accelerometer counts; LMSQOL, Leeds Multiple Sclerosis Quality of Life Scale; SLWS, Satisfaction With Life Scale.
Figure 2.
Model that was tested for understanding the associations among physical activity, disability, fatigue, mood, pain, self-efficacy, social support, and quality of life in a sample of 292 individuals with multiple sclerosis. Note: All coefficients are standardised estimates. Solid lines represent statistically significant paths, and dashed lines represent non-significant paths. GLTEQ, Godin Leisure-Time Exercise Questionnaire; Accel, accelerometer counts; Disability, Patient Determined Disease Steps Scale; Anxiety, Anxiety subscale of Hospital Anxiety and Depression Scale; Depression, Depression subscale of Hospital Anxiety and Depression Scale; Fatigue, Fatigue Severity Scale; Pain, Short-form of McGill Pain Questionnaire; Social Support, Social Provisions Scale; Self-efficacy for MS, Multiple Sclerosis Self-Efficacy Scale; Exercise Self-efficacy, Exercise Self-Efficacy Scale; LMSQOL, Leeds Multiple Sclerosis Quality of Life Scale; SLWS, Satisfaction With Life Scale.
Model fit was assessed using the χ2, standardised root mean squared residual (SRMR), and comparative fit index (CFI). The χ2 provides a simultaneous test that all residuals in the specified versus obtained variance/covariance matrices are zero (Bollen, 1989). The SRMR is the average of the standardised residuals between the specified and obtained variance/covariance matrices (Bollen, 1989). The CFI tests the proportionate improvement in fit by comparing the target model with the independence model (i.e. model with no correlations among observed variables). We based a good-model data fit on a non-significant chi-square value (Bollen, 1989) and combinatory rules of SRMR ≤ 0.08 and CFI ≥ 0.95 (Hu & Bentler, 1999).
Results
Descriptive statistics
The descriptive statistics for the variables are provided in Table 1 and the correlations among the variables are in Table 2. The correlations were all statistically significant (p < 0.05) with the exception of the correlations between anxiety with physical activity and disability.
Table 1.
Descriptive statistics for the measures in the sample of 292 individuals with multiple sclerosis.
| Measure | Mean score | Standard deviation | Range of scores |
|---|---|---|---|
| GLTEQ | 26.5 | 22.4 | 0-110 |
| Accelerometer | 220,006 | 121,252 | 32,011-760,721 |
| LMSQOL | 19.3 | 4.8 | 10-31 |
| SWLS | 21.8 | 8.0 | 5-35 |
| PDDS | 2.3 | 1.8 | 0-6 |
| HADS_A | 5.8 | 3.8 | 0-18 |
| HADS_D | 6.0 | 4.2 | 0-18 |
| FSS | 5.0 | 1.4 | 1-7 |
| MPQ | 10.7 | 7.8 | 0-33 |
| SPS | 76.9 | 10.7 | 41-96 |
| MSSE | 146.9 | 26.9 | 51-180 |
| EXSE | 72.1 | 32.9 | 0-100 |
Note: GLTEQ, Godin Leisure-Time Exercise Questionnaire; Accelerometer, accelerometer counts; LMSQOL, Leeds Multiple Sclerosis Quality of Life Scale; SLWS, Satisfaction with Life Scale; PDDS, Patient Determined Disease Steps Scale; HADS_A, Anxiety subscale of Hospital Anxiety and Depression Scale; HADS_D, Depression subscale of Hospital Anxiety and Depression Scale; FSS, Fatigue Severity Scale; MPQ, Short-form of McGill Pain Questionnaire; SPS, Social Provisions Scale; MSSE, Multiple Sclerosis Self-Efficacy Scale; EXSE, Exercise Self-Efficacy Scale.
Table 2.
Correlations among the latent and observed variables in the covariance modelling analysis for the sample of 292 individuals with multiple sclerosis.
| Latent/manifest variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Physical activity | - | |||||||||
| 2. Quality of life | 0.31 | - | ||||||||
| 3. Disability | -0.50 | -0.40 | - | |||||||
| 4. Anxiety | -0.05 | -0.62 | -0.02 | - | ||||||
| 5. Depression | -0.30 | -0.85 | 0.31 | 0.60 | - | |||||
| 6. Fatigue | -0.45 | -0.61 | 0.51 | 0.23 | 0.50 | - | ||||
| 7. Pain | -0.19 | -0.50 | 0.31 | 0.40 | 0.35 | 0.42 | - | |||
| 8. Social support | 0.20 | 0.71 | -0.20 | -0.45 | -0.61 | -0.32 | -0.27 | - | ||
| 9. Self-efficacy for MS | 0.41 | 0.73 | -0.55 | -0.39 | -0.62 | -0.58 | -0.48 | 0.45 | - | |
| 10. Exercise self-efficacy | 0.49 | 0.38 | -0.39 | -0.14 | -0.30 | -0.43 | -0.24 | 0.25 | 0.52 | - |
Note: All correlations are statistically significant (p < 0.05) with the exception of correlations between anxiety and physical activity and anxiety and disability. Disability, Patient Determined Disease Steps Scale; Anxiety, Anxiety subscale of Hospital Anxiety and Depression Scale; Depression, Depression subscale of Hospital Anxiety and Depression Scale; Fatigue, Fatigue Severity Scale; Pain, Short-form of McGill Pain Questionnaire; Social Support, Social Provisions Scale; Self-Efficacy for MS, Multiple Sclerosis Self-Efficacy Scale; Exercise Self-efficacy, Exercise Self-Efficacy Scale.
Model 1: Direct association between physical activity and QOL
The first model that we tested had a direct path between physical activity and QOL latent variables and it represented an excellent fit for the data (χ2 = 0.12, df = 1, p 0.73, SRMR = 0.00, CFI = 1.00). The factor loadings and path coefficient are provided Figure 1. The factor loadings for the indicators of physical activity (GLTEQ λ = 0.51; accelerometer λ= 0.78) and QOL (LMSQOL λ = 0.84; SLWS λ = 0.82) were statistically significant. The path coefficient between physical activity and QOL was statistically significant (γ = 0.34) and indicated that those who were more physically active reported higher levels of QOL.
Model 2: Indirect association between physical activity and QOL
The second model that we tested included (1) physical activity and QOL as latent variables and (2) disability, mood, self-efficacy, social support, pain and fatigue as manifest intermediate variables. This model represented an acceptable fit for the data (χ2 = 51.33, df = 18, p < 0.001, SRMR = 0.03, CFI 0.98). The statistically significant path coefficients are provided in Figure 2 and indicated that those who were more physically active reported lower levels of disability (γ = -0.50), depression (γ = -0.31), fatigue (γ = -0.46) and pain (γ = -0.19) and higher levels of social support (γ = 0.20), self-efficacy for managing MS (γ = 0.41) and self-efficacy for regular physical activity (γ = 0.49). The path coefficients further indicated that those who reported lower levels of depression (β = -0.37), anxiety (β = -0.15), fatigue (β = -0.16), and pain (β = -0.08) and higher levels of social support (β = 0.26) and self-efficacy for controlling MS (β = 0.17) reported higher levels of QOL. Therefore, the relationship between physical activity and QOL was indirect and accounted for by depression (γβ = 0.11), fatigue (γβ = 0.07), pain (γβ = 0.02), social support (γβ = 0.05) and self-efficacy for managing MS (γβ = 0.07).
Discussion
MS is associated with compromised QOL (Benito-León et al., 2003; Mitchell et al., 2005) and physical activity in the form of exercise training has yielded a one fourth standard deviation improvement in QOL in this population (Motl & Gosney, 2008). The present study examined disability, fatigue, mood, pain, self-efficacy and social support as intermediate variables that might explain the association between physical activity and QOL in individuals with MS. As expected, the initial analysis indicated that physical activity was positively correlated with QOL. The subsequent analysis demonstrated that the relationship between physical activity and QOL followed an indirect pathway through depression, fatigue, pain, social support and self-efficacy for managing MS. Those with MS who were more physically active reported lower depression, fatigue and pain and higher social support and self-efficacy for managing MS, and lower depression, fatigue and pain and higher social support and self-efficacy for managing MS were associated with better QOL. Therefore, physical activity programmes might be more strongly linked with improved QOL under conditions that maximise improvements in depression, fatigue, pain, social support and self-efficacy for managing MS. Longitudinal and experimental studies are necessary for further identifying those variables as the key mediators of the relationship between physical activity and QOL in MS.
Our findings have implications for conceptualising and examining physical activity in relationship with QOL in those with MS and possibly other populations. Indeed, the association between physical activity and QOL is likely indirect rather than direct (Courneya & Friedenreich, 1999; Elavsky et al., 2005; McAuley et al., 2006; Rejeski & Mihalko, 2001) based on the position that physical activity influences proximal or intermediate variables, and, in turn, those intermediate variables influence the distal outcome of QOL (Stewart & King, 1991). We are aware of two previous studies that supported such a hypothesis in persons with MS (Motl et al., 2006; Motl & Snook, 2008) and this hypothesis has received support from older adults (e.g. Elavsky et al., 2005; McAuley et al., 2006). The present study further supported an indirect relationship between physical activity and QOL based on an expanded number of the intermediate variables including depression, fatigue, pain, social support and self-efficacy for managing MS. We encourage additional efforts that examine a broad array of psychological, social, biological and physical variables as intermediates of the relationship between physical activity and QOL in many diverse populations including persons with MS.
We observed that the moods of anxiety and depression as well as fatigue, pain, social support and self-efficacy for managing MS were independent correlates of QOL. Those who reported higher levels of depression, anxiety, fatigue and pain and lower levels of social support and self-efficacy for controlling MS reported worse QOL. Our results are largely consistent with previous literature reviews (Benito-León et al., 2003; Mitchell et al., 2005) and research examining influences of QOL in MS (Amato et al., 2001; Benedict et al., 2005; Janssens et al., 2003; Kalia & O'Connor, 2005; Lobentanz et al., 2004; Riazi et al., 2004; Stuifbergen et al., 2000; Svendson et al., 2005). The totality of research therefore supports those psychological and social variables as moderate and strong predictors of QOL in persons with MS (Mitchell et al., 2005). Interestingly, fewer biological or physical variables have been identified as predictors of QOL in MS (Mitchell et al., 2005), and the existing biological or physical variables such as progressive disease course and brain lesion load and atrophy have been weaker predictors. Perhaps psychological and social variables are more proximal influences of QOL in MS, whereas biological and physical variables are more distal influences (Mitchell et al., 2005). Another possibility is that individuals weigh psychological and social variables more heavily than physical and biological variables in personal judgments about QOL.
This study is not without limitations that can be overcome in future research. Notably, the cross-sectional nature of the data precludes conclusions and inferences about the causal and directional relationships among variables (Weinstein, 2007). We do recognise the possibility of alternative models for explaining the associations among physical activity and QOL. Accordingly, future research should consider other intermediate variables, alternative models and longitudinal and experimental research designs when examining the relationship between physical activity and QOL in persons with MS. Another limitation is that individuals who were already regular exercisers may have had more interest in participating in this study based on our recruitment of individuals interested in a study of physical activity and QOL through MS society chapters. Future research should consider methods of recruiting a less physically active sample perhaps through a “blinded” recruitment focus whereby participants were recruited for a study on non-exercise influences of QOL such as self-efficacy, social support, or fatigue. We further note that the sample primarily consisted of Caucasian women with relapsing-remitting MS and this is generally consistent with the demographics of MS (National Multiple Sclerosis Society, 2005). We fully acknowledge that future researchers should consider using a more diverse sample. This would allow for a broader generalisation of the findings among less representative groups of individuals with MS such as men and persons who are not Caucasian.
The consistent evidence of a positive relationship between physical activity and QOL in those with MS has implications for health-care professionals including clinicians, physical therapists and exercise specialists. Those professionals might consider focusing on physical activity behaviour as a method of mitigating reductions in QOL that are often observed in individuals with MS. This is particularly important given that individuals with MS are less physically active than the general population (Motl, McAuley, & Snook, 2005). Although encouraging physical activity is potentially challenging given mobility issues associated with MS, possible options include developing self-efficacy, focusing on an enjoyable experience, establishing an environment in which people feel comfortable and developing social support both in and out of the physical activity environment (Motl et al., 2006; Petajan & White, 1999). Continued cross-sectional, prospective and experimental research will advance our understanding of methods of increasing physical activity and maximising the QOL benefits in individuals with MS.
Acknowledgement
This work was funded by the National Institute of Neurological Diseases and Stroke (NS054050).
References
- Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Multiple Sclerosis. 2001;7(5):340–344. doi: 10.1177/135245850100700511. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Wahlig E, Bakshi R, Fishman I, Munschauer F, Zivadinov R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. Journal of the Neurological Sciences. 2005;231(1-2):29–34. doi: 10.1016/j.jns.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Morales JM, Rivera-Navarro J, Mitchell AJ. A review about the impact of multiple sclerosis on health-related quality of life. Disability and Rehabilitation. 2003;25:1291–1303. doi: 10.1080/09638280310001608591. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. John Wiley; New York: 1989. [Google Scholar]
- Bouchard C, Shephard RJ. Physical activity, fitness and health: the model and key concepts. In: Bouchard C, Shephard RJ, Stephens T, editors. Physical activity, fitness, and health: international proceedings and consensus statement. Human Kinetics; Champaign, IL: 1994. pp. 77–88. [Google Scholar]
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999;21:171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- Cutrona C, Russell D. The provisions of social relationships and adaptation to stress. Advances in Personal Relationships. 1987;1:37–67. [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Washburn RA, Schoeller DA. Measurement of physical activity. Quest. 2001;53:295–309. [Google Scholar]
- Elavsky S, McAuley E, Motl RW, Konopack JF, Marquez DX, Hu L, et al. Physical activity enhances long-term quality of life in older adults: efficacy, esteem, and affective influences. Annals of Behavioral Medicine. 2005;30(2):138–145. doi: 10.1207/s15324796abm3002_6. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. [PubMed] [Google Scholar]
- Filippini G, Munari L, Incorvaia B, Ebers GC, Polman C, D'Amico R, et al. Interferons in relapsing remitting multiple sclerosis: a systematic review. Lancet. 2003;361:545–552. doi: 10.1016/S0140-6736(03)12512-3. [DOI] [PubMed] [Google Scholar]
- Ford HL, Gerry E, Tennant A, Whalley D, Haigh R, Johnson MH. Developing a disease-specific quality of life measure for people with multiple sclerosis. Clinical Rehabilitation. 2001;15(3):247–258. doi: 10.1191/026921501673658108. [DOI] [PubMed] [Google Scholar]
- Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Sciences. 1985;10:141–146. [PubMed] [Google Scholar]
- Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis: validity of self-report and objective measures. Family and Community Health. 2007;30:144–150. doi: 10.1097/01.fch.0000264411.20766.0c. [DOI] [PubMed] [Google Scholar]
- Hadjimichael O, Kerns RB, Rizzo MA, Cutter G, Vollmer T. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain. 2007;127:35–41. doi: 10.1016/j.pain.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Janssens AC, van Doorn PA, de Boer JB, Kalkers NF, van der Meche FG, Passchier J, et al. Anxiety and depression influence the relation between disability status and quality of life in multiple sclerosis. Multiple Sclerosis. 2003;9(4):397–403. doi: 10.1191/1352458503ms930oa. [DOI] [PubMed] [Google Scholar]
- Kalia LV, O'Connor PW. Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Multiple Sclerosis. 2005;11(3):322–327. doi: 10.1191/1352458505ms1168oa. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Lankhorst GJ, Jelles F, Smits RC, Polman CH, Kuik DJ, Pfennings LE, et al. Quality of life in multiple sclerosis: the disability and impact profile (DIP) Journal of Neurology. 1996;243(6):469–474. doi: 10.1007/BF00900502. [DOI] [PubMed] [Google Scholar]
- Lobentanz IS, Asenbaum S, Vass K, Sauter C, Klosch G, Kristoferitsch W, et al. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurologica Scandinavica. 2004;110(1):6–13. doi: 10.1111/j.1600-0404.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. Journal of Behavioral Medicine. 1993;16:103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- McAuley E, Konopack JF, Motl RW, Morris KS, Doerksen SE, Rosengren KR. Physical activity and quality of life in older adults: influence of health status and self-efficacy. Annals of Behavioral Medicine. 2006;31(1):99–103. doi: 10.1207/s15324796abm3101_14. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Benito-León J, González JM, Rivera-Navarro J. Quality of life and its assessment in multiple sclerosis: integrating physical and psychological components of well-being. Lancet Neurology. 2005;4:556–566. doi: 10.1016/S1474-4422(05)70166-6. [DOI] [PubMed] [Google Scholar]
- Motl RW, Gosney JL. Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Multiple Sclerosis. 2008;14:129–135. doi: 10.1177/1352458507080464. [DOI] [PubMed] [Google Scholar]
- Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Multiple Sclerosis. 2005;11(4):459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- Motl RW, McAuley E, Snook EM, Scott JA. Validity of physical activity measures in ambulatory individuals with multiple sclerosis. Disability and Rehabilitation. 2006;28:1151–1156. doi: 10.1080/09638280600551476. [DOI] [PubMed] [Google Scholar]
- Motl RW, Snook EM. Physical activity, self-efficacy, and quality of life in multiple sclerosis. Annals of Behavioral Medicine. 2008;35:111–115. doi: 10.1007/s12160-007-9006-7. [DOI] [PubMed] [Google Scholar]
- Motl RW, Snook EM, McAuley E, Scott JA, Douglas ML. Correlates of physical activity among individuals with MS. Annals of Behavioral Medicine. 2006;32:152–161. doi: 10.1207/s15324796abm3202_13. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. Muthén & Muthén; Los Angeles: 1998-2003. [Google Scholar]
- Naess H, Beiske AG, Myhr KM. Quality of life among young patients with ischaemic stroke compared with patients with multiple sclerosis. Acta Neurologica Scandinavica. 2008;117:181–185. doi: 10.1111/j.1600-0404.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- National Multiple Sclerosis Society . Multiple sclerosis information sourcebook. Information Resource Center and Library of the National Multiple Sclerosis Society; New York: 2005. [Google Scholar]
- Pavot W, Diener E. Review of the satisfaction with life scale. Psychological Assessment. 1993;5:164–172. [Google Scholar]
- Petajan JH, White AT. Recommendations for physical activity in patients with multiple sclerosis. Sports Medicine. 1999;27(3):179–191. doi: 10.2165/00007256-199927030-00004. [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. Journals of Gerontology Series A: Biological Sciences Medical Sciences. 2001;56:23–35. doi: 10.1093/gerona/56.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- Riazi A, Thompson AJ, Hobart JC. Self-efficacy predicts self-reported health status in multiple sclerosis. Multiple Sclerosis. 2004;10(1):61–66. doi: 10.1191/1352458504ms986oa. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Miller D, Clough JD, Gragg LA, Farmer RG. Quality of life in multiple sclerosis. Comparison with inflammatory bowel disease and rheumatoid arthritis. Archives of Neurology. 1992;49(12):1237–1242. doi: 10.1001/archneur.1992.00530360035014. [DOI] [PubMed] [Google Scholar]
- Schapiro RT. Managing the symptoms of multiple sclerosis. 4th ed. Demos Medical Publishing; New York: 2003. [Google Scholar]
- Schwartz CE, Coulthard-Morris L, Zeng Q, Retzlaff P. Measuring self-efficacy in people with multiple sclerosis: a validation study. Archives of Physical Medicine and Rehabilitation. 1996;77:394–398. doi: 10.1016/s0003-9993(96)90091-x. [DOI] [PubMed] [Google Scholar]
- Stewart AL, King AC. Evaluating the efficacy of physical activity for influencing quality-of-life outcomes in older adults. Annals of Behavioral Medicine. 1991;13:108–116. [Google Scholar]
- Stuifbergen AK, Seraphine A, Roberts G. An explanatory model of health promotion and quality of life in chronic disabling conditions. Nursing Research. 2000;49(3):122–129. doi: 10.1097/00006199-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Svendsen KB, Jensen TS, Hansen HJ, Bach FW. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain. 2005;114(3):473–481. doi: 10.1016/j.pain.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Weinstein ND. Misleading tests of health behavior theories. Annals of Behavioral Medicine. 2007;33:1–10. doi: 10.1207/s15324796abm3301_1. [DOI] [PubMed] [Google Scholar]
- White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Medicine. 2004;34(15):1077–1100. doi: 10.2165/00007256-200434150-00005. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]