Abstract
The modulation of cytoplasmic Ca2+ concentration by release from internal stores through the inositol trisphosphate receptor (InsP3R) Ca2+ release channel is a ubiquitous signaling system involved in the regulation of numerous processes. Because of its ubiquitous expression and roles in regulating diverse cell physiological processes, it is not surprising that the InsP3R has been implicated in a number of disease states. However, relatively few mutations in InsP3R genes have been identified to date. Here, I will discuss mutations in the type 1 InsP3R that have been discovered by analyses of human patients and mice with neurological disorders. In addition, I will highlight diseases caused by mutations in other genes, including Huntington's and Alzheimer's diseases and some spinocerebellar ataxias, where the mutant proteins have been found to exert strong influences on InsP3R function that may link InsP3R to disease pathogenesis.
Keywords: IP3, Disease, Neurodegeneration, Calcium, Ion channel
Introduction
The modulation of [Ca2+]i is a ubiquitous signaling system involved in the regulation of numerous processes, including transepithelial transport, learning and memory, muscle contraction, synaptic transmission, secretion, motility, membrane trafficking, excitability, gene expression, and cell division. Activation of phospholipases Cβ and Cγ by ligand interaction with G-protein- or tyrosine kinase-linked receptors, respectively, results in the hydrolysis of phosphatidlyinositol 4,5 bisphosphate, generating inositol 1,4,5-trisphosphate (InsP3). InsP3 binds to its receptor (InsP3R), a ligand-gated Ca2+ release channel in the endoplasmic reticulum (ER). Analyses of InsP3-mediated [Ca2+]i signals in single cells have revealed them to be unexpectedly complex. In the temporal domain, this complexity is manifested as repetitive spikes or oscillations, with frequencies often tuned to levels of stimulation, suggesting that [Ca2+]i signals may be transduced by amplitude as well as frequency encoding. In the spatial domain, [Ca2+]i signals may initiate at specific locations and remain highly localized or propagate as waves [2, 11]. Thus, InsP3-mediated [Ca2+]i signals are often organized to provide different signals to discrete parts of the cell.
Three genes and alternatively spliced isoforms have identified a family of InsP3Rs in mammalian cells, including humans [15]. The three full-length sequences are 60–80% homologous. The InsP3R is ubiquitously expressed, perhaps in all cell types [15, 68]. The isoforms have distinct and overlapping patterns of expression with most cells expressing more than one, and expression levels can be modified during differentiation and by use-dependent degradation [15]. This impressive diversity of expression suggests that cells require distinct InsP3Rs to regulate specific functions. Nevertheless, the functional implications of this diversity, at the single channel, cellular, and organ level, remain largely unappreciated.
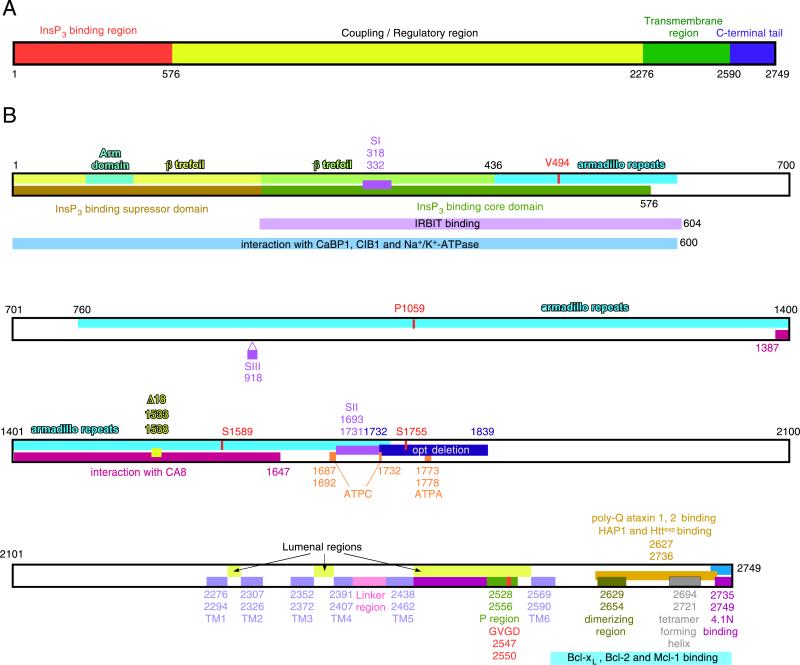
The InsP3Rs are ~2,700–2,800 amino acid intracellular membrane proteins that exist as homo- or hetero-tetramers [15, 32]. Structurally, the proteins contain a cytoplasmic N-terminus comprising ~85% of the protein, a hydrophobic region predicted to contain six membrane-spanning helices, and a relatively short cytoplasmic C-terminus (Fig. 1a). Functionally, the N-terminal domain can be divided into one comprising an N-terminal InsP3 binding domain and a more distal ‘regulatory’/‘coupling’ domain (Fig. 1b). InsP3 binding to the InsP3R is stoichiometric and localized by mutagenesis and an X-ray structure to a region within residues 226–578 [5, 80, 81]. The InsP3R is itself a ligand-gated ion channel. The basic six-transmembrane (TM)-domain topology of InsP3Rs is shared with other cation channels (Fig. 2). By analogy, putative TM helices 5 and 6 and the intervening intra-luminal loop likely constitute the permeation pathway [4, 53]. Binding of InsP3 gates the channel open, modulated by the linker region that contains consensus sequences for phosphorylation, proteolytic cleavage, and binding by proteins and ATP that integrate other signaling pathways or metabolic states with the function of the InsP3R.
Fig. 1.
Structural determinants of the InsP3R. a Overall domain structure. The InsP3R molecule depicted as a linear amino acid sequence, with the amino terminal InsP3 binding region (red), coupling region (yellow), transmembrane region (green), and carboxyl tail (blue) depicted. b Linear amino acid sequence. Residues are numbered according to the rat type 1 SI+, SII+, SIII– sequence (protein accession number 121838). The structural features shown are: Arm sub-domain and β-trefoil in the InsP3-binding suppressor domain; β-trefoil and armadillo repeats in InsP3 binding core-domain; armadillo repeats in the coupling/regulatory domain; alternative splicing regions SI, SII, and SIII for type 1 InsP3R; opt deletion in type 1 InsP3Rmutant; Δ18 deletion in type 1 InsP3R; ATP-binding site ATPA; transmembrane helices TM1–6, and pore-forming P region with selectivity filter; dimerizing region; tetramer forming region; S1589 and S1755 are PKA/PKG phosphorylation sites; mutation of Pro1059 to Leu in type 1 InsP3R associated with SCA15; mutation of Val494 to Ile in type 1 InsP3R associated with SCA15. The sequences involved in the interaction of InsP3R channel with the following proteins are also depicted: CaBP1; IRBIT; CIB1; Na+/K+-ATPase; HAP1 and Httexp; poly-glutamine (poly-Q)-expanded ataxins 1 and 2; protein 4.1N; CA8; Bcl-2, Bcl-xL, and Mcl-1. Modified from [15] with permission from the American Physiological Society
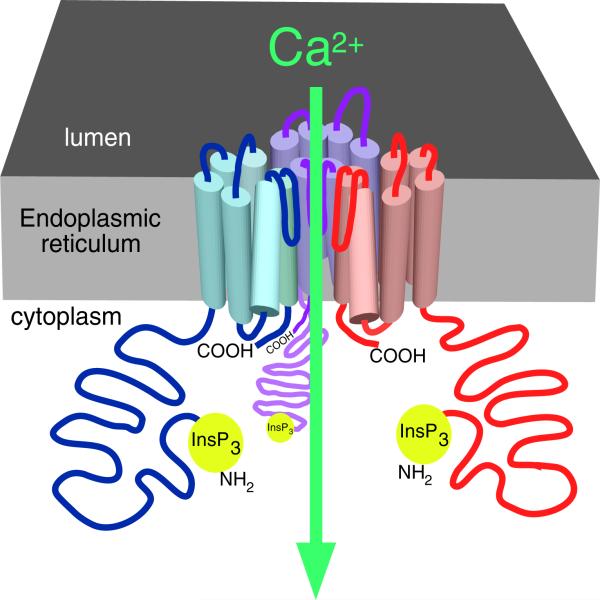
Fig. 2.
The InsP3R Ca2+ release channel. Cartoon depicting three of four InsP3R molecules (in different colors) in a single tetrameric channel structure. Part of the lumenal loop connecting transmembrane helices 5 and 6 of each monomer dips into the fourfold symmetrical axis, creating the permeation pathway for Ca2+ efflux from the lumen of the endoplasmic reticulum. Reproduced from [15] with permission from the American Physiological Society
Because of its ubiquitous expression and roles in regulating diverse cell physiological processes in so many cell types, it is perhaps not surprising that the InsP3R has been implicated in a number of disease states, including polycystic kidney disease [41], cholestasis [55], cardiac arrythmias [36], and inflammation [51] among others. It is surprising, however, that relatively few mutations in InsP3R genes have been identified to date. Many disease-causing mutations have been discovered in the other major and related family of intracellular Ca2+ release. It seems likely that the relative dearth of identified disease-causing InsP3R mutations reflects a widespread functional redundancy of multiple InsP3R isoforms expressed in most cells. In this review, I will discuss mutations in the type 1 InsP3Rthat have been discovered by analyses of human patients and mice with neurological disorders. In addition, I will highlight diseases caused by mutations in other genes, including Huntington's and Alzheimer's diseases (ADs) and some spinocerebellar ataxias (SCAs), where the mutant proteins have been found to exert strong influences on InsP3R function that may link InsP3R to disease pathogenesis.
Role of InsP3R in spinocerebellar ataxias 15 and 16
To date, the only known diseases that have been definitively linked to a mutation(s) in an InsP3R gene are spinocerebellar ataxias 15 and 16. The SCAs are a heterogeneous group of genetic disorders with clinically distinct features. Both SCA15 and SCA16 are uncommon, autosomal, dominant, pure cerebellar ataxias with adult onset, slow progression, and pronounced cerebellar atrophy. Heterozygous deletions of the 5′ part of the type 1 InsP3R gene, encompassing exons 1–10, 1–40, and 1–44 (of 59 exons), were identified in three human SCA15 families [73] and a heterozygous complete deletion of the gene was found in another [21]. Heterozygous deletion of exons 1–48 was identified in an individual affected by SCA16 [30]. That the diseases were associated with mutations in the type 1 channel isoform is consistent with the high level of InsP3R-1 protein expression in the cerebellum, particularly in the Purkinje neurons [16, 44, 47]. SCA15 was linked to a complete deletion of the InsP3R-1 [21], and SCA16 revealed deletions in the InsP3R-1 gene only, without involvement of the adjacent SUMF1 gene [30], demonstrating that the diseases are caused by InsP3R-1 loss of function. Western blot analysis of immortalized lymphoblasts from SCA15-affected family members with exons 1–10 deleted had strongly reduced InsP3R-1 expression [73]. Haplo-insufficiency of InsP3R-1 may account for the delayed onset and slow progression of the disease. In contrast, knockout of the mouse InsP3R-1 results in ataxia and seizures within a couple of weeks of birth, with death by 4 weeks of age [46]. The absence of epilepsy or reduced life span in the SCA15 and SCA16 patients may suggest that the InsP3R-1 expression from one allele provides a protection against a more severe phenotype. In agreement, heterozygote InsP3R-1 knockout mice suffer only from some defects in motor coordination [50].
Two heterozygous missense mutations in InsP3R-1 have also been identified in SCA15-affected individuals. In one family, patients had valine at position 494 in the amino acid sequence replaced by isoleucine (V494I) [17]. This residue is located within the InsP3 binding domain (Fig. 1b). The core InsP3 binding domain consists of a proximal β-trefoil domain linked to an α-helical-rich armadillo repeat domain. InsP3 binding is coordinated by residues contributed by both domains [5, 15]. Val494 is located in the armadillo repeat domain at the end of a loop that connects the first two alpha helices. It is located far from the InsP3R binding pocket, and it is not particularly conserved among species. In the Xenopus InsP3R-1, the residue is an isoleucine. Thus, an association of the disease with the V494I mutation in SCA15 patients is perhaps surprising. Based on the gene deletion phenotypes, if the mutation is indeed responsible for the SCA15 disease, it must represent a loss of function. This region of the protein also binds proteins, including CaBP1 [77, 79], CIB1 [77], and IRBIT [1] (Fig. 1b), although the role of this particular residue in their interactions with the InsP3R has not been studied.
In another family, patients had proline at position 1059 in the amino acid sequence replaced by leucine (P1059L; (21)). The proline is conserved in type 1 InsP3R isoforms among species, although it is alanine in the human types 2 and 3 isoforms. Nothing is known regarding the role of this residue in the ion channel function of the InsP3R-1. This residue is localized in the coupling domain (Fig. 1b), with the surrounding region not known to be involved in protein interactions. It is possible that the missense mutation does not cause disease, although this was felt to be unlikely [21]. Again, if the mutation is indeed responsible for the SCA15 disease, it must represent a loss of function. In a preliminary report, expression of recombinant rat InsP3R-1 containing the corresponding mutation formed a functional channel [49]. As discussed below, an InsP3R-1 mutation in the opistotonus (opt) mouse is phenotypically equivalent to that of the knockout [57], whereas the recombinant channel is functional [70]. Thus, the mechanisms that account for the loss-of-function phenotype remain to be determined.
InsP3R mutant mice
The opt mouse
The opistotonus mouse was identified as having a naturally occurring deletion of exons 43 and 44 in the type 1 InsP3R channel that results in an in-frame deletion of residues 1732–1839 in the regulatory domain immediately after the SII splice region [57] (Fig. 1b). The phenotype of the homozygous opt mouse is similar to that of the type 1 InsP3R homozygous knock-out mouse. Both mice are smaller than their normal littermates at birth, lack normal locomotor behaviors, display seizures at about 2 weeks of life, and then die by 3–4 weeks of age [46, 57]. Metabotropic glutamate receptor-mediated Ca2+ release was only moderately diminished in Purkinje cell soma in P4 cerebellar slices from homozygous opt mice [57]. A reconstituted recombinant opt InsP3R-1 was functional, although it had apparent diminished ATP sensitivity compared with wild-type (WT) channels [70]. The opt-deleted region of the InsP3R contains a putative ATP binding site (Fig. 1b) that may account for reduced ATP responsiveness, and it also contains a PKA phosphorylation site. The mutant protein is expressed at lower levels than the wild-type protein [57]. We have confirmed that InsP3R-1 protein level is reduced by ~50% in the heterozygote opt brain and is nearly undetectable in brain lysates from homozygous opt mice (our unpublished results). It seems most likely that the major deficit in opt mice is reduced InsP3R-1 protein expression, consistent with the nearly identical phenotypes of opt and InsP3R-1 knock-out mice. However, the Ca2+ imaging results may not be easily reconciled with this conclusion. The mechanisms that account for the reduced channel expression are unknown. The fact that the recombinant channel behaves relatively normally suggests that the 108-residue deletion does not prevent normal oligomerization, ligand binding, permeation, or gating. Persistent activation of Gαq induces down-regulation of InsP3R protein levels in some cells [78]. However, the published single channel studies do not indicate that the opt channel is hyper-active, suggesting that this mechanism is not responsible. It is possible that cellular quality control mechanisms recognize the channel as defective and quickly degrade it.
The Δ18 mouse
The Δ18 mouse was identified as having an in-frame deletion of 18 base pairs within exon 36 of InsP3R-1 that results in the deletion of six residues (residues 1533–1538; Glu-Ser-Cys-Ile-Arg-Val) in the regulatory domain [73] (Fig. 1b). It was observed as a severe autosomal recessive progressive movement disorder, with a survival time of approximately 4 weeks, phenotypes reminiscent of both InsP3R-1 knock-out and opt mice. The six residues are not particularly conserved across the three channel isoforms and species, although the Cys and a basic amino acid 2 residue downstream appear to be. The functional significance of these residues is unknown. In a preliminary study, it was reported that the recombinant rat channel with the six-residue deletion formed a functional channel [49]. Nevertheless, as in the opt mouse, the deletion appears to be associated with a pronounced reduction of InsP3R-1 protein levels, measured by immunostaining in cerebellar Purkinje neurons and Western blotting of whole brain lysates [73]. It is likely therefore that the disease phenotype is caused by a lack of InsP3R-1 protein expression as a consequence of the deletion.
Hints of defective InsP3R roles in other ataxias
CA8
A homozygous mutation in the CA8 gene was discovered in a consanguineous family as the cause of a syndrome of ataxia and mild mental retardation and ambulation on all four extremities (quadrupedal gait) [72]. CA8 encodes for the carbonic anhydrase-related protein VIII, a catalytically inactive carbonic anhydrase with strong expression in cerebellar Purkinje neurons. In affected humans, serine at position 298 was replaced by proline. In cell culture, the mutation resulted in severely reduced CA8 protein expression, which was in part restored by inhibition of the proteasome [72]. It was therefore speculated that the mutation destabilizes the protein and causes disease as a result of a loss of CA8 function. Loss of function of CA8 was previously identified as the autosomal recessive deficit in the waddles (wdl) mouse [31], a spontaneous model that has a 19-base-pair deletion in CA8 that results in a lack of mRNA and protein in the homozygote. Thus, it, too, is a CA8 loss of function. The mouse has ataxia and appendicular dystonia that produces nearly straight limbs and a “waddling” side-to-side gait during ambulation [31]. Homozygous mice have normal gross cerebellar morphology with abnormalities of parallel fiber–Purkinje cell synapses and defects in excitatory transmission [24].
The only reported function of CA8 is to inhibit InsP3 binding to the InsP3R-1 [26]. CA8 was discovered in a yeast two-hybrid screen as an interactor with the regulatory domain of the channel within residues 1387–1647 (Fig. 1b). CA8 and InsP3R-1 co-localized extensively in isolated cerebellar Purkinje cells. Binding of CA8 reduced the apparent affinity of the channel for [3H]InsP3 binding [26]. Since the binding region is distinct from the InsP3 binding domain, this reflects an allosteric effect, suggesting that CA8 binding induces conformational changes in the protein. However, nothing else is known regarding the functional consequences of CA8 binding to the InsP3Ror whether the binding is related to the phenotypes described above. Lack of interaction of CA8 with the InsP3R in the CA8 patients or wdl mice would not be expected, a priori, to result in loss of InsP3R function. As discussed below, polyglutamine-expanded huntingtin and ataxins 1 and 2, the latter that result in cerebellar ataxias, all bind to the InsP3R and enhance its sensitivity to InsP3. Furthermore, mutant presenilins that cause Alzheimer's disease bind to the InsP3R and increase its activity (below). It is conceivable therefore that InsP3R-1 is more sensitive to InsP3 in CA8 patients and wdl mice, and that the disease is a result of gain of function of the channel. Further studies are necessary to establish a role of the InsP3Rin CA8 patients and wdl mice.
Spinocerebellar ataxias 2 and 3
Abnormal Ca2+ release through the InsP3R has been implicated in two spinocerebellar ataxias in addition to SCAs 15 and 16. Following their observations of the interaction of mutant huntingtin protein with the carboxyl terminus of the InsP3R-1 (below), the Bezprozvanny group explored whether other disease-causing polyglutamine repeat proteins similarly bind there and affect the channel activity [8, 42]. SCA2 and SCA3 are caused by polyglutamine expansions in ataxin2 and ataxin3, respectively [35, 38]. It was discovered in pull-down and co-immunoprecipitation assays that the polyglutamine expanded forms, but not the normal proteins, and each interacted with the same region of the InsP3R-1 carboxyl terminus (residues 2627–2749) [8, 42] (Fig. 1b). To explore the functional consequences of these interactions, either the wild-type or mutant ataxin proteins were co-expressed with rat InsP3R-1 in Sf9 cells, and microsomes purified from the infected cells were reconstituted into lipid planar bilayers. In each case, reconstituted channels from the cells expressing the mutant ataxins were more sensitive to activation by lower concentrations of InsP3. In cells in culture, medium spiny neurons in the case of SCA2 and Purkinje neurons in the case of SCA3, metabotropic glutamate receptor stimulation caused higher-peak cytoplasmic Ca2+ responses in cells from the mutant mice. It was speculated that these Ca2+ responses may underlie cellular toxicity because enhanced cell death induced by prolonged glutamate exposure in the disease neurons was diminished by treatment with dantrolene, a RyR Ca2+ release channel inhibitor. It was suggested that dantrolene provided this protection by inhibiting Ca2+ signals that emanate from InsP3-induced Ca2+ release that is amplified by CICR by the RyR. Dantrolene feeding provided protection against age-dependent disease-associated morphological and behavioral deficits. The authors concluded that abnormal neuronal Ca2+ signaling through the InsP3R-1 may play a role in the pathogenesis of many polyglutamine expansion disorders [8, 42].
The role of InsP3R in Huntington's disease
Huntington's disease (HD) is a dominantly inherited neurodegenerative disorder caused by amino-terminal polyglutamine expansions in huntingtin (Htt), a large ubiquitously expressed protein [20, 75, 82]. The disease is associated with movement disorders, cognitive decline, and psychiatric symptoms that progress over 15–20 years before death. Brain neurons in HD have characteristic cytoplasmic and nuclear aggregates containing htt and other proteins [82]. HD pathogenesis is due to a toxic gain of function of mutant htt that results in neuronal loss in the cortex and striatum, although the molecular mechanisms that underlie pathogenesis and the selective vulnerability of particular neuronal populations are still debated on [69, 75, 82]. The physiological function of wild-type htt is unknown. Its sequence indicates that it possesses many HEAT repeats, protein interaction domains that suggest that it may serve as a molecular scaffold [43].
The type 1 InsP3R was identified in a comprehensive screen of protein interactors with htt [33]. Importantly, it behaved as a genetic modifier of HD-associated neurodegeneration. In a Drosophila model, in which a polyglutamine-expanded human Htt transgene expression in the eye caused retinal degeneration, InsP3R expression modified the eye phenotype: reduced expression of the channel suppressed it, whereas enhanced expression made it more severe [33]. In a separate screen, huntingtin-associated protein 1 (HAP1), a protein that also interacts with Htt, particularly the polyglutamine expanded forms (Httexp) [13], was also identified to interact with InsP3R-1. The interaction was with the carboxyl terminus of the channel within residues 2627–2736 [65] (Fig. 1b). This channel construct also directly bound to Httexp and to a lesser extent to Htt. Both interactions were strengthened by the presence of HAP1, suggesting that the three proteins might exist in a complex [65]. Whereas the addition of recombinant HAP1 to the cytoplasmic aspect of reconstituted InsP3R-1 was without effect on channel Po evoked by a sub-saturating [InsP3], subsequent additions of amino-terminal fragments of Htt or Httexp, or application of premixed HAP1–Htt/Httexp, increased channel activity [65, 66]. Full-length Httexp but not Htt increased Po in response to sub-saturating [InsP3] without HAP1 pre-exposure [65]. Consistent with these effects, over-expression of full-length Httexp, but not Htt, increased InsP3-dependent Ca2+ release in cultured medium spiny neurons in response to the threshold levels of the agonist. Enhanced Ca2+ release causedbyHttexp was partially dependent on HAP1, as the effect was less robust in HAP1–/– cells [66]. It was suggested that the enhanced InsP3 sensitivity of the Htt/HAP-bound InsP3R-1 may contribute to the progression of HD by exaggerated Ca2+ release-dependent neuronal apoptosis [64].
Expression in vitro of a GFP-fused carboxyl-terminal interacting region in medium spiny neurons from an HD transgenic mouse normalized exaggerated cytoplasmic Ca2+ responses to glutaminergic stimulation and provided protection from glutamate excitotoxicity [63]. Long-term expression of the construct in the striatum of virally injected mice reduced the biochemical interaction of InsP3R-1 and httexp and reduced motor coordination deficits and loss of medium spiny neurons. Remarkably, the fusion protein also reduced the load of aggregated Httexp [63]. Although many questions remain, these studies indicate a strong involvement of the InsP3R in HD pathogenesis and suggest novel therapeutic targets and strategies.
The role of InsP3R in Alzheimer's disease
Alzheimer's disease is a common form of dementia involving slowly developing and ultimately fatal neurodegeneration. The etiology of AD is debated on, with age being the main risk factor but with major molecular mechanisms remaining unclear. A hallmark feature of AD is accumulation of extracellular β amyloid (Aβ) plaques, intracellular neurofibrillary tangles, and neuronal loss [14]. Mutations in presenilins (PS1 and PS2) and amyloid precursor protein (APP) cause most early-onset, autosomal dominant familial cases of the disease (FAD) [67]. Presenilins are components of a protein complex that proteolytically processes APP into Aβ peptides [54]. In the “amyloid hypothesis” of AD, an accumulation of Aβ due to defective processing and clearance leads to pathological sequelae associated with the disease [22]. The identification of three components in FAD—PS1, PS2, and APP—that are linked in a biochemical pathway that impinges on Aβ production has strongly influenced the acceptance of the amyloid hypothesis [22]. Nevertheless, much evidence suggests that altered Ca2+ signaling is associated with expression of FAD mutant PS in symptomatic or pre-symptomatic patient cells and in brain neurons in AD mouse models long before the appearances of plaques or tangles and in a variety of heterologous expression systems [3, 19, 37, 61]. Before the molecular identification of PS, it was shown that fibroblast lines from AD patients (later shown to harbor a FAD mutation in PS1) generated exaggerated [Ca2+]i responses to sub-maximal concentrations of two G-protein coupled receptor agonists that activate PLC [29]. Subsequent studies have confirmed that FAD PS expression is associated with an exaggerated ER Ca2+ release in several cell systems. However, these studies have not led to consensus regarding the molecular mechanisms involved (reviewed in 37, 58]. Exaggerated ER Ca2+ release has been ascribed to the enhanced loading of the ER lumen [58] due either to enhanced SERCA Ca2+ pump activity [18] or to disruption of a putative Ca2+ channel function of wild-type PS [48, 71]. Alternately, exaggerated Ca2+ release has been accounted for by enhanced Ca2+ liberation from normal stores through InsP3R[40, 58] or RyR [6, 56, 60]Ca2+ release channels, both in vivo [6, 56, 59, 60] and in vitro [12, 25, 29, 39], either as a consequence of enhanced channel expression [6, 7, 34, 61] or, in the case of the InsP3R, of enhanced activity in response to its ligand InsP3 [10, 29].
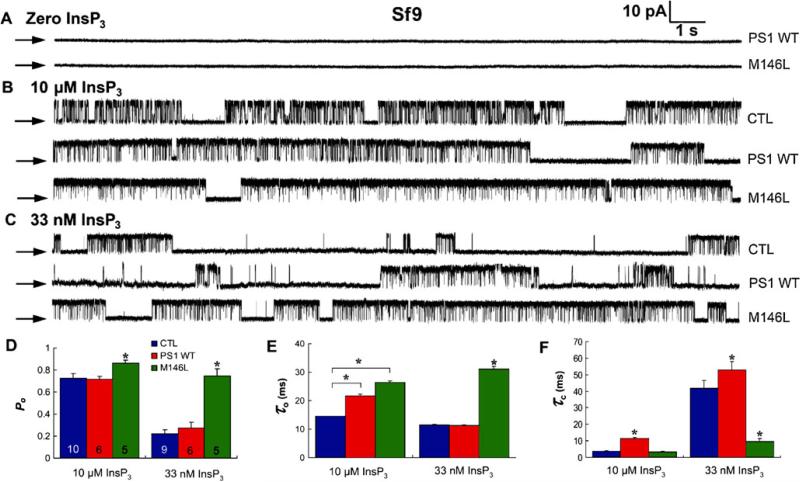
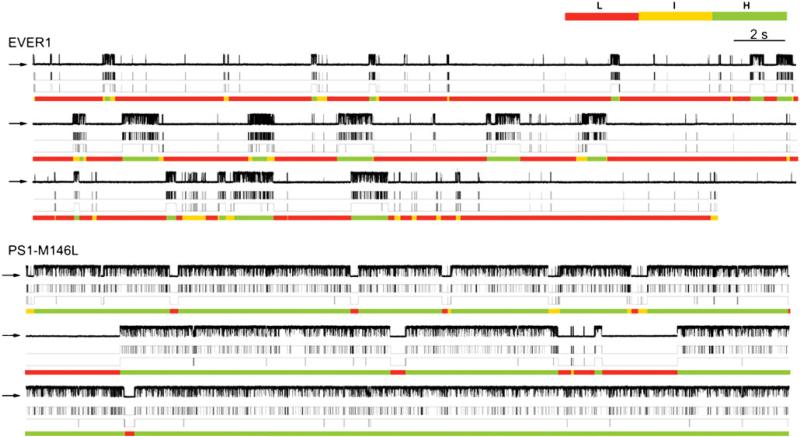
A biochemical interaction of WT and FAD mutant PS1 and 2 with the InsP3R was identified. Functionally, FAD PS specifically had gain-of-function consequences for the activity of the channel, which in turn was associated with exaggerated Ca2+ signaling in intact cells [9, 10]. An example is shown in (Fig. 3). FAD and WT-PS1 were expressed in insect Sf9 cells, and patch clamp experiments were performed on isolated nuclei to record InsP3R ion channels in their native membrane [45]. The InsP3 and Ca2+ regulation of the Sf9 channel, the type 1 isoform, is similar to the dominant neuronal type in the mammalian brain [27]. In conditions optimal for channel activity, InsP3R channels in control nuclei had a high Po (Fig. 3b). In nuclei from either M146L-PS1 or WT-PS1-expressing cells, no novel ion channels were detected (Fig. 3a), nor were channels observed in the absence of InsP3 (Fig. 3a) or in the presence of InsP3 and its competitive inhibitor heparin. Activated InsP3R channels in WT-PS1-infected cells had Po similar to control cells, whereas Po was elevated significantly in FAD M146L-PS1-infected cells (Fig. 3b, d). With sub-saturating [InsP3], Po was elevated approximately threefold in nuclei from FAD PS1-expressing cells compared with control and WT-PS1 expressing cells, to a degree comparable to that observed in saturating [InsP3] (Fig. 3c, d). Similar results were obtained with an FAD PS2 mutant (N141I) [9, 10].
Fig. 3.
Effects of PS1 expression on InsP3R single channel activity in Sf9 cells. a–c Representative current recordings in isolated nuclei from Sf9 cells infected with PS1 WT or M146L baculoviruses in the absence (a) or presence of saturating (10 μM; b) or sub-saturating (33 nM; c) InsP3 in pipette solution. Channel activity was not evoked by PS1 alone in the absence of InsP3 (a), whereas InsP3R channels were activated in the presence of InsP3 (b, c). Pipette [Ca2+] was 1 μM; arrows, zero current level. Summary of effects of PS1 expression on InsP3R channel open probability Po (d) mean open time (τo) (e), and mean closed time (τc) (f). Asterisks, p<0.01, unpaired t-test. From [10] with permission from Elsevier
Enhanced InsP3R channel activity appears to be a conserved feature of FAD PS-expressing cells, since similar results were observed in cells expressing other FAD mutant PS [9]. γ-Secretase-dead mutants also significantly enhanced the InsP3R channel activity, although to a lesser extent than the FAD mutants, indicating that the secretase activity of PS is not required for its effects on InsP3R gating [9]. Interestingly, the Po of channels recorded from cells infected with frontotemporal dementia-associated mutant PS1 was not different from controls [9]. Thus, several FAD-mutant PS have similar effects on InsP3R gating, and these effects appear not to be recapitulated in PS mutants associated with a different neurological disease.
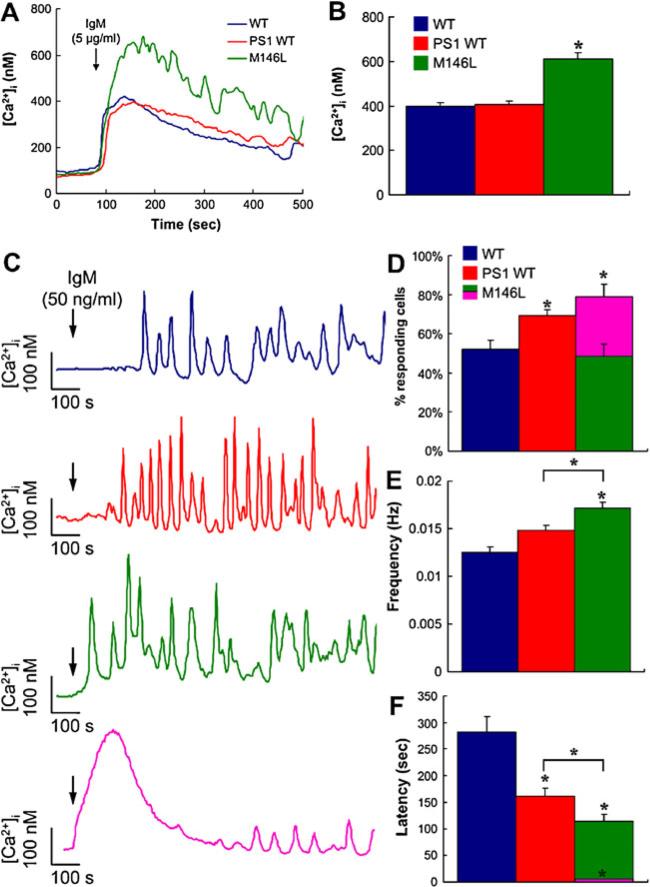
These effects of FAD PS on InsP3R gating appear to be remarkably robust, since they have now been observed in five different cellular systems: Sf9 cells, chicken DT40 B cells, human FAD patient B cell-derived lymphoblasts and fibroblasts, and neurons from FAD-PS AD mice, in the absence of and preceding disease pathology [9, 10]. The strikingly similar effects of mutant PS expression of InsP3R channels in such diverse cellular systems from different species suggest that the effects of mutant PS on channel activity is a robust one in all cell types and InsP3R isoforms. The results at the single-channel level are consistent with the observations of exaggerated InsP3-mediated [Ca2+]i signals in FAD patient fibroblasts [29] and other cells with mutant PS expressed. An example in DT40 B cells, where the electrophysiological effects of FAD PS have been demonstrated [10], is shown in Fig. 4. [Ca2+]i signals mediated by InsP3R were elicited by cross-linking the B cell receptor (BCR). Weak stimulation triggered repetitive Ca2+ oscillations and spiking in ~50% of control cells (Fig. 4c, d), due to periodic release from ER through InsP3R, because they are absent in DT40-KO cells in which all three isoforms of the InsP3R were genetically deleted [62][76]. In cells expressing FAD PS1, oscillation frequency and number of cells responding was increased, and the latency between application of agonist and first response decreased (Fig. 4e, f). In a subset (30%) of cells expressing M146L-PS1, the latency was nearly abolished (Fig. 4c,d,f), a response that was reminiscent of that of normal cells to strong stimulation [76]. These effects demonstrate that FAD PS1 generates exaggerated InsP3R-mediated [Ca2+]i responses, as observed in other cell types, and they suggest, consistent with the single channel studies, that FAD PS expression enhances InsP3R sensitivity to InsP3.
Fig. 4.
Exaggerated [Ca2+]i signaling in mutant PS-expressing DT40 cells. a, b Responses to strong stimulation by BCR antibody of DT40 cell [Ca2+]i. a Representative single-cell responses to 5 μg/ml anti-IgM (added at arrow) in untransfected (blue) and PS1-WT (red) and PS1-M146L (green) stably transfected DT40 cells. b Summary of peak [Ca2+]i responses triggered by 5 μg/ml anti-IgM (n=90). Asterisk, p<0.01 compared with WT and PS1-WT (c–f). Responses to weak stimulation by BCR antibody of DT40 cell [Ca2+]i. c Representative single cell [Ca2+]i responses to 50 ng/ml anti-IgM (IgM; added at arrow) stimulation of BCR in control (blue), PS1-WT (red), and PS1-M146L (green and pink) stably transfected DT40 cells. d Summary of percentage of cells responding to 50 ng/ml anti-IgM (n=90). Of PS1-M146L-expressing cells, ~30% (purple) exhibited a different, exaggerated [Ca2+]i response. e [Ca2+]i oscillation frequency triggered by anti-IgM in WT DT40, PS1-WT- and PS1-M146L-expressing cells. f Summary of latencies to first response in WT DT40, PS1-WT- and PS1-M146L-expressing cells. The 30% of PS1-M146L-expressing cells that exhibited the exaggerated response had nearly no latency (purple). Asterisks, p<0.01 compared with WT DT40 cells. Asterisks with bars, p<0.01 PS1-Wt vs PS1-M146L. From [10] with permission from Elsevier
The electrophysiological studies suggest that FAD PS stimulate InsP3R gating by a mechanism that involves PS-mediated effective sensitization of the channel to InsP3, most likely through an allosteric mechanism. Nevertheless, this conclusion is somewhat tentative, as are those regarding of the effects of polyglutamine-expanded protein interaction effects on InsP3R gating discussed earlier, since the effects of PS have in each case only been examined at a single [Ca2+]i. InsP3 and Ca2+ regulate the channel in a complicated manner, with InsP3 affecting gating through modulation of Ca2+ inhibition. The relationship between channel Po and [InsP3] and [Ca2+]i cannot be adequately characterized by determining Po at different [InsP3] at just one [Ca2+]i. Depending on the [Ca2+]i used, different apparent functional affinities for InsP3 can be observed [15]. Thus, it will be important to extend these studies to examine the effects over physiologically relevant ranges on both [InsP3] and [Ca2+]i. With this caveat in mind, modal gating analysis suggested that the FAD mutant PS regulates channel activity by impinging upon the normal ligand activation mechanisms. Ligand regulation of InsP3R gating is largely mediated by altering the propensity of the channel to gate in particular modes [28]. Strongly activated channels gate in a high Po H mode characterized by long bursting activities: an intermediate Po I mode is characterized by fast channel openings and closings and a low Po L mode is characterized by long closings with brief openings. In nuclei isolated from control cells expressing an irrelevant protein or from Sf9 cells infected with WT-PS1 or PS2, similar modal distributions were observed (Fig. 5). In contrast, the H mode was the dominant gating mode of InsP3R recorded from FAD PS-expressing cells (Fig. 5). FAD PS therefore enhances InsP3R gating by mode switching, causing the channel to spend more time in the H mode. Modal gating regulation may have important functional consequences. The channel open time in the L mode (~10 ms) is short enough that it may not increase local [Ca2+] sufficiently to recruit additional InsP3R- or RyR-mediated CICR. In contrast, the much longer activity bursts in the H mode (>200 ms) can provide a sufficiently large Ca2+ flux to enable a normally local Ca2+ signaltobeamplified andpropagatedbyCICR [15]. It was suggested [9] that, because InsP3 R and RyR are clustered and spatially organized to provide local [Ca2+]i signals as a critical element of physiological specificity, mode-shifting by FAD PS may result not only in exaggerated local Ca2+ signaling but also in the disruption of spatial specificity by enabling CICR to transmit signals more globally [15, 28]. Mode switching by FAD PS of InsP3R gating may account for observations InsP3-dependent, exaggerated RyR-mediated Ca2+ signals in neurons (e.g., 60, 61).
Fig. 5.
Modal gating analyses of InsP3R channels under the influence of FAD-linked mutant PS. Distinct single channel InsP3R gating behaviors from EVER1 (control) vs PS1-M146L-expressing Sf9 cells. Each section consists of a set of four traces of the same single channel current record: (top) unprocessed current trace, (second) idealized current trace generated using Qub software (third), idealized current trace after burst analysis (closing events <10 ms were filtered), and (bottom) modal assignment by analyzing channel burst (tb) and gap (tg) durations [28]. In EVER1-infected cells, low Po is associated with prevalence of L gating mode. In PS1-M146L infected cells, enhanced Po is manifested by increased tb and decreased tg. Channel occupancy of H mode is dominant, whereas occupancy of L gating mode is significantly decreased. From [9] with permission from the American Association for the Advancement of Science
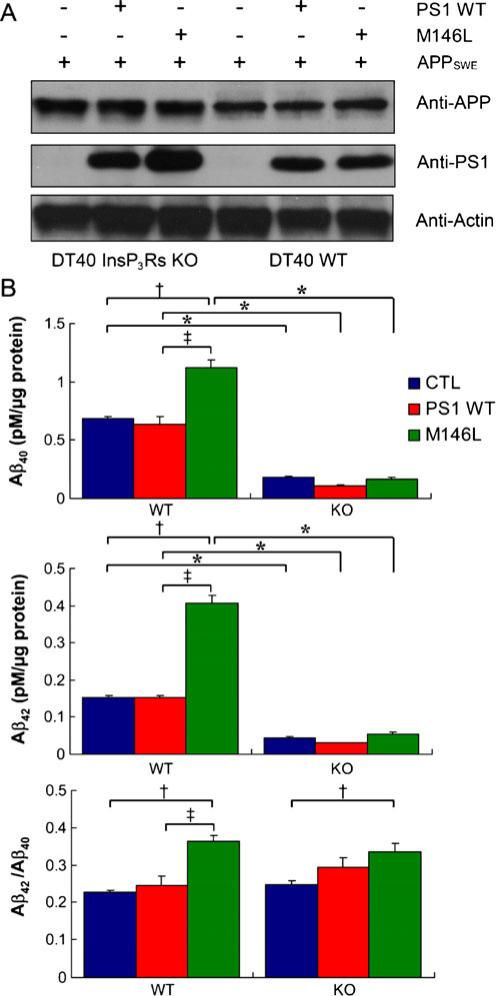
InsP3-mediated [Ca2+]i signals regulate many cell physiological processes. A major question is whether the effects of mutant PS on InsP3R-mediated Ca2+ signaling impinge on disease pathogenesis. It is possible that exaggerated Ca2+ signals in AD may influence reactive oxygen species generation, mitochondrial function, gene transcription, and Aβ production [52], features associated with AD. The possible role of InsP3RinAβ production was examined in DT40 cells that were engineered to stably express APP harboring Swedish mutations (APPSWE) that enhance Aβ production, together with either PS1-WT or PS1-M146L (Fig. 6, top). PS1-M146L specifically enhanced Aβ40 and Aβ42 secretion by approximately two and threefold, respectively, compared with control cells. Of note, the Aβ42/Aβ40 ratio was enhanced in mutant PS1-expressing cells (Fig. 6), as observed in AD patients. To determine the role of the InsP3R in PS1-dependent APP processing, APP and PS1-expressing cells were generated in the DT40 cells that lacked expression of InsP3R (InsP3R-KO). Notably, mutant PS1 enhancement of Aβ secretion observed in InsP3R-expressing cells was abolished. Furthermore, the absolute levels of Aβ peptides detected were strongly reduced in all control and PS1-expressing InsP3R-KO lines (Fig. 6, bottom). These results suggest that APP processing by FAD PS1 has a strong dependence on InsP3R activity (Fig. 7).
Fig. 6.
APP processing is dependent on InsP3R. a Stable expression of PS1-WT and PS1-M146L proteins in wild-type (WT) and InsP3R-deficient (KO) DT40 cell lines that stably expressed APPSWE. Actin probed as loading control. b ELISA measurements of Aβ40 (top), Aβ42 (middle), and Aβ42/Aβ40 ratio (bottom) secreted over 48 h by InsP3R-expressing wild-type (WT; left) or InsP3R-deficient (KO DT40 cells; right) DT40 cells stably expressing APPSWE alone (blue) or APPSWE with PS1-WT (red) or PS1-M146L (green). Asterisks, p< 0.01 compared with control WT cells. Cross, p<0.01 compared with control WT cells; double cross, p<0.01 compared with PS1-WT cells. From [10] with permission from Elsevier
Fig. 7.
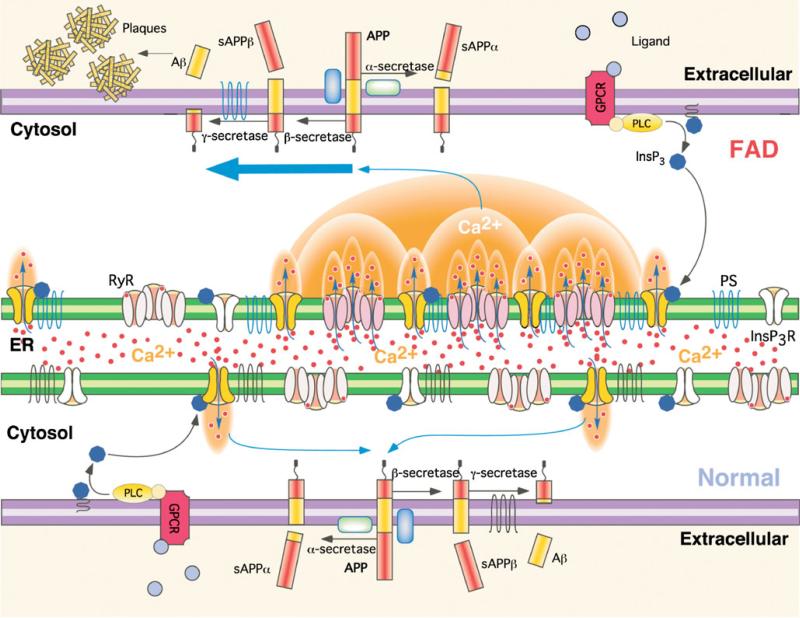
Hypothetical molecular mechanism of enhanced Aβ production due to Ca2+ disruption in FAD PS cells. APP is processed by either α-secretase or β-secretase, the latter leading to Aβ generation after subsequent cleavage by γ-secretase. Stimulation of G-protein coupled receptors or other cell surface receptors by extracellular ligands activates phospholipase C (PLC), which cleaves phosphatidylinositol bisphosphate to produce InsP3. InsP3 binds to and activates the InsP3R to release Ca2+ from ER stores, increasing cytoplasmic Ca2+ concentration. In normal cells, these Ca2+ signals are tightly regulated in time, space, and amplitude. In FAD cells, mutant PS exerts stimulatory effects on InsP3R gating by modal switching to the H mode associated with prolonged channel openings. H mode gating generates exaggerated Ca2+ signaling by promoting additional release channel recruitment by CICR. Increased cytoplasmic Ca2+ concentration promotes β-secretase activity [23] and Aβ production [19, 52] which, together with mutant PS-enhanced production of amyloidogenic Aβ, results in plaque formation. From [9] with permission from the American Association for the Advancement of Science
Conclusions
Human and mouse genetics have provided the strongest evidence that InsP3R mutations can result in disease. However, the number of mutations and diseases identified are limited. To date, only one channel isoform, the type 1, has been implicated in these studies. Furthermore, the diseases have all been neurological and predominantly cerebellar. Cerebellar Purkinje neurons, with the highest InsP3R-1 protein expression levels in the body, are the final integrators of sensory inputs and provide cerebellar cortex outputs that control motor coordination. The genetics has suggested that the lack of two functional InsP3R-1 alleles disrupts cerebellar function. It is interesting that the recombinant expression of several of the InsP3R-1 mutant proteins produces functional ion channels, including opt, Δ18, and P1059L, but appear to cause a disease due to diminished expression, perhaps due to rapid degradation by cellular quality control mechanisms. It will be interesting, in future studies, to examine the effects of these mutations not only on ion channel properties but also on the kinetics of channel biogenesis and turnover. RyR1 and RyR2 are the predominant RyR isoforms in skeletal and cardiac muscle, respectively. Many mutations in these isoforms have been identified because of the pronounced muscle phenotypes they cause. The reliance in the cerebellum on one particular InsP3R isoform seems to be the exception in the human body. It seems likely that most cell types and tissues have functional InsP3R redundancy that renders InsP3R mutations phenotypically non-penetrant. Nevertheless, it seems likely that other diseases will be discovered that have mutations in InsP3R genes as their basis. A recent genome-wide association study linked an intronic single nucleotide polymorphism in the InsP3R-2 gene to risk of amyotrophic lateral sclerosis, a motor neuron disease [74].
Acknowledgements
Acknowledgement is made to the donors of ADR, a program of the American Health Assistance Foundation (A2008-137 to J.K.F.) and the National Institutes of Health (MH059937 and GM056328) for support of this research.
Footnotes
This article is published as part of the special issue on channelopathies
References
- 1.Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003;278:10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Lipp P. Calcium—a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 3.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehning D, Mak D-OD, Foskett JK, Joseph SK. Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J Biol Chem. 2001;276:13509–13512. doi: 10.1074/jbc.C100094200. [DOI] [PubMed] [Google Scholar]
- 5.Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 6.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Tang TS, Tu H, Nelson O, Pook M, Hammer R, Nukina N, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28:12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung KH, Mei L, Mak D-OD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of InsP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 12.Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi RE, Alkon DL. Calcium responses in fibroblasts from asymptomatic members of Alzheimer's disease families. Neurobiol Dis. 1998;5:37–45. doi: 10.1006/nbdi.1998.0176. [DOI] [PubMed] [Google Scholar]
- 13.Feigin A, Zgaljardic D. Recent advances in Huntington's disease: implications for experimental therapeutics. Curr Opin Neurol. 2002;15:483–489. doi: 10.1097/00019052-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 15.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuichi T, Simon-Chazottes D, Fujino I, Yamada N, Hasegawa M, Miyawaki A, Yoshikawa S, Guenet JL, Mikoshiba K. Widespread expression of inositol 1,4,5-trisphosphate receptor type 1 gene (Insp3r1) in the mouse central nervous system. Receptors Channels. 1993;1:11–24. [PubMed] [Google Scholar]
- 17.Ganesamoorthy D, Bruno DL, Schoumans J, Storey E, Delatycki MB, Zhu D, Wei MK, Nicholson GA, McKinlay Gardner RJ, Slater HR. Development of a multiplex ligation-dependent probe amplification assay for diagnosis and estimation of the frequency of spinocerebellar ataxia type 15. Clin Chem. 2009;55:1415–1418. doi: 10.1373/clinchem.2009.124958. [DOI] [PubMed] [Google Scholar]
- 18.Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer's disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Group THsDCR A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 21.Hara K, Shiga A, Nozaki H, Mitsui J, Takahashi Y, Ishiguro H, Yomono H, Kurisaki H, Goto J, Ikeuchi T, Tsuji S, Nishizawa M, Onodera O. Total deletion and a missense mutation of ITPR1 in Japanese SCA15 families. Neurology. 2008;71:547–551. doi: 10.1212/01.wnl.0000311277.71046.a0. [DOI] [PubMed] [Google Scholar]
- 22.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 23.Hayley M, Perspicace S, Schulthess T, Seelig J. Calcium enhances the proteolytic activity of BACE1: an in vitro biophysical and biochemical characterization of the BACE1–calcium interaction. Biochim Biophys Acta. 2009;1788:1933–1938. doi: 10.1016/j.bbamem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Hirasawa M, Xu X, Trask RB, Maddatu TP, Johnson BA, Naggert JK, Nishina PM, Ikeda A. Carbonic anhydrase related protein 8 mutation results in aberrant synaptic morphology and excitatory synaptic function in the cerebellum. Mol Cell Neurosci. 2007;35:161–170. doi: 10.1016/j.mcn.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirashima N, Etcheberrigaray R, Bergamaschi S, Racchi M, Battaini F, Binetti G, Govoni S, Alkon DL. Calcium responses in human fibroblasts: a diagnostic molecular profile for Alzheimer's disease. Neurobiol Aging. 1996;17:549–555. doi: 10.1016/0197-4580(96)00074-7. [DOI] [PubMed] [Google Scholar]
- 26.Hirota J, Ando H, Hamada K, Mikoshiba K. Carbonic anhydrase-related protein is a novel binding protein for inositol 1,4,5-trisphosphate receptor type 1. Biochem J. 2003;372:435–441. doi: 10.1042/BJ20030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ionescu L, Cheung KH, Vais H, Mak DO, White C, Foskett JK. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ionescu L, White C, Cheung KH, Shuai J, Parker I, Pearson JE, Foskett JK, Mak DO. Mode switching is the major mechanism of ligand regulation of InsP3 receptor calcium release channels. J Gen Physiol. 2007;130:631–645. doi: 10.1085/jgp.200709859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwaki A, Kawano Y, Miura S, Shibata H, Matsuse D, Li W, Furuya H, Ohyagi Y, Taniwaki T, Kira J, Fukumaki Y. Heterozygous deletion of ITPR1, but not SUMF1, in spinocerebellar ataxia type 16. J Med Genet. 2008;45:32–35. doi: 10.1136/jmg.2007.053942. [DOI] [PubMed] [Google Scholar]
- 31.Jiao Y, Yan J, Zhao Y, Donahue LR, Beamer WG, Li X, Roe BA, Ledoux MS, Gu W. Carbonic anhydrase-related protein VIII deficiency is associated with a distinctive lifelong gait disorder in waddles mice. Genetics. 2005;171:1239–1246. doi: 10.1534/genetics.105.044487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph SK, Bokkala S, Boehning D, Zeigler S. Factors determining the composition of inositol trisphosphate receptor hetero-oligomers expressed in COS cells. J Biol Chem. 2000;275:16084–16090. doi: 10.1074/jbc.M000506200. [DOI] [PubMed] [Google Scholar]
- 33.Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, Cha GH, Ukani L, Chepanoske CL, Zhen Y, Sahasrabudhe S, Olson J, Kurschner C, Ellerby LM, Peltier JM, Botas J, Hughes RE. Huntingtin interacting proteins are genetic modifiers of neuro-degeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasri NN, Kocks SL, Verbert L, Hebert SS, Callewaert G, Parys JB, Missiaen L, De Smedt H. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knockout cells. Cell Calcium. 2006;40:41–51. doi: 10.1016/j.ceca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I. CAG expansions in a novel gene for Machado–Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- 36.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 38.Lastres-Becker I, Rub U, Auburger G. Spinocerebellar ataxia 2 (SCA2). Cerebellum. 2008;7:115–124. doi: 10.1007/s12311-008-0019-y. [DOI] [PubMed] [Google Scholar]
- 39.Leissring MA, LaFerla FM, Callamaras N, Parker I. Subcellular mechanisms of presenilin-mediated enhancement of calcium signaling. Neurobiol Dis. 2001;8:469–478. doi: 10.1006/nbdi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 40.Leissring MA, Paul BA, Parker I, Cotman CW, LaFerla FM. Alzheimer's presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29:9148–9162. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald ME. Huntingtin: alive and well and working in middle management. Sci STKE. 2003;2003:pe48. doi: 10.1126/stke.2003.207.pe48. [DOI] [PubMed] [Google Scholar]
- 44.Maeda N, Niinobe M, Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5- trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. Embo J. 1990;9:61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mak D-OD, White C, Ionescu L, Foskett JK. Nuclear patch clamp electrophysiology of inositol trisphosphate receptor Ca2+ release channels. In: Putney JW Jr, editor. Methods in calcium signaling research. Boca Raton; CRC: 2005. [Google Scholar]
- 46.Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, Yoneshima H, Miyawaki A, Fukuuchi Y, Furuichi T, Okano H, Mikoshiba K, Noda T. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5- trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 47.Nakanishi S, Maeda N, Mikoshiba K. Immunohistochemical localization of an inositol 1,4,5-trisphosphate receptor, P400, in neural tissue: studies in developing and adult mouse brain. J Neurosci. 1991;11:2075–2086. doi: 10.1523/JNEUROSCI.11-07-02075.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nienaber T, Betzenhauser MJ, Wagner LE, Yule DI. An analysis of the activity of two disease associated mutations of the inositol 1,4,5-trisphosphate receptor type-1. Biophysical Journal 2010 Biophysical Society Meeting. 2010 Abstracts :2660-Pos/B2389. [Google Scholar]
- 50.Ogura H, Matsumoto M, Mikoshiba K. Motor discoordination in mutant mice heterozygous for the type 1 inositol 1,4,5-trisphosphate receptor. Behav Brain Res. 2001;122:215–219. doi: 10.1016/s0166-4328(01)00187-5. [DOI] [PubMed] [Google Scholar]
- 51.Park KM, Yule DI, Bowers WJ. Tumor necrosis factor-alpha potentiates intraneuronal Ca2+ signaling via regulation of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2008;283:33069–33079. doi: 10.1074/jbc.M802209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Querfurth HW, Selkoe DJ. Calcium ionophore increases amyloid beta peptide production by cultured cells. Biochemistry. 1994;33:4550–4561. doi: 10.1021/bi00181a016. [DOI] [PubMed] [Google Scholar]
- 53.Ramos-Franco J, Galvan D, Mignery GA, Fill M. Location of the permeation pathway in the recombinant type 1 inositol 1,4,5-trisphosphate receptor. J Gen Physiol. 1999;114:243–250. doi: 10.1085/jgp.114.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selkoe DJ. The genetics and molecular pathology of Alzheimer's disease: roles of amyloid and the presenilins. Neurol Clin. 2000;18:903–922. doi: 10.1016/s0733-8619(05)70232-2. [DOI] [PubMed] [Google Scholar]
- 55.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer's disease. J Neurochem. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 57.Street VA, Bosma MM, Demas VP, Regan MR, Lin DD, Robinson LC, Agnew WS, Tempel BL. The type 1 inositol 1,4,5-trisphosphate receptor gene is altered in the opisthotonos mouse. J Neurosci. 1997;17:635–645. doi: 10.1523/JNEUROSCI.17-02-00635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stutzmann GE. Calcium dysregulation, IP3 signaling, and Alzheimer's disease. Neuroscientist. 2005;11:110–115. doi: 10.1177/1073858404270899. [DOI] [PubMed] [Google Scholar]
- 59.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stutzmann GE, Smith I, Caccamo A, Oddo S, Parker I, Laferla F. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer's mouse models. Ann N Y Acad Sci. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- 62.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. Embo Journal. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang TS, Guo C, Wang H, Chen X, Bezprozvanny I. Neuroprotective effects of inositol 1,4,5-trisphosphate receptor C-terminal fragment in a Huntington's disease mouse model. J Neurosci. 2009;29:1257–1266. doi: 10.1523/JNEUROSCI.4411-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci U S A. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang TS, Tu H, Orban PC, Chan EY, Hayden MR, Bezprozvanny I. HAP1 facilitates effects of mutant huntingtin on inositol 1,4,5-trisphosphate-induced Ca release in primary culture of striatal medium spiny neurons. Eur J NeuroSci. 2004;20:1779–1787. doi: 10.1111/j.1460-9568.2004.03633.x. [DOI] [PubMed] [Google Scholar]
- 67.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- 69.Tobin AJ, Signer ER. Huntington's disease: the challenge for cell biologists. Trends Cell Biol. 2000;10:531–536. doi: 10.1016/s0962-8924(00)01853-5. [DOI] [PubMed] [Google Scholar]
- 70.Tu H, Miyakawa T, Wang Z, Glouchankova L, Iino M, Bezprozvanny I. Functional characterization of the type 1 inositol 1,4,5-trisphosphate receptor coupling domain SII(+/-) splice variants and the opisthotonos mutant form. Biophys J. 2002;82:1995–2004. doi: 10.1016/S0006-3495(02)75548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turkmen S, Guo G, Garshasbi M, Hoffmann K, Alshalah AJ, Mischung C, Kuss A, Humphrey N, Mundlos S, Robinson PN. CA8 mutations cause a novel syndrome characterized by ataxia and mild mental retardation with predisposition to quadrupedal gait. PLoS Genet. 2009;5:e1000487. doi: 10.1371/journal.pgen.1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van de Leemput J, Chandran J, Knight MA, Holtzclaw LA, Scholz S, Cookson MR, Houlden H, Gwinn-Hardy K, Fung HC, Lin X, Hernandez D, Simon-Sanchez J, Wood NW, Giunti P, Rafferty I, Hardy J, Storey E, Gardner RJ, Forrest SM, Fisher EM, Russell JT, Cai H, Singleton AB. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. doi: 10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Es MA, Van Vught PW, Blauw HM, Franke L, Saris CG, Andersen PM, Van Den Bosch L, de Jong SW, van 't Slot R, Birve A, Lemmens R, de Jong V, Baas F, Schelhaas HJ, Sleegers K, Van Broeckhoven C, Wokke JH, Wijmenga C, Robberecht W, Veldink JH, Ophoff RA, van den Berg LH. ITPR2 as a susceptibility gene in sporadic amyotrophic lateral sclerosis: a genome-wide association study. Lancet Neurol. 2007;6:869–877. doi: 10.1016/S1474-4422(07)70222-3. [DOI] [PubMed] [Google Scholar]
- 75.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 76.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White C, Yang J, Monteiro MJ, Foskett JK. CIB1, a ubiquitously expressed Ca2+-binding protein ligand of the InsP3 receptor Ca2+ release channel. J Biol Chem. 2006;281:20825–20833. doi: 10.1074/jbc.M602175200. [DOI] [PubMed] [Google Scholar]
- 78.Wojcikiewicz RJ, Pearce MM, Sliter DA, Wang Y. When worlds collide: IP(3) receptors and the ERAD pathway. Cell Calcium. 2009;46:147–153. doi: 10.1016/j.ceca.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, McBride S, Mak DO, Vardi N, Palczewski K, Haeseleer F, Foskett JK. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc Natl Acad Sci U S A. 2002;99:7711–7716. doi: 10.1073/pnas.102006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshikawa F, Iwasaki H, Michikawa T, Furuichi T, Mikoshiba K. Cooperative formation of the ligand-binding site of the inositol 1,4,5- trisphosphate receptor by two separable domains. J Biol Chem. 1999;274:328–334. doi: 10.1074/jbc.274.1.328. [DOI] [PubMed] [Google Scholar]
- 81.Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- 82.Young AB. Huntingtin in health and disease. J Clin Invest. 2003;111:299–302. doi: 10.1172/JCI17742. [DOI] [PMC free article] [PubMed] [Google Scholar]