Abstract
This study demonstrates the capabilities of MR imaging in the assessment of cardiac pacing induced ventricular dyssynchrony, and findings support the need for employing more physiological pacing. A human donor heart deemed non-viable for transplantation, was reanimated using an MR compatible, four-chamber working perfusion system. The heart was imaged using a 1.5T MR scanner while being paced from the right ventricular apex (RVA) via an epicardial placed lead. Four-chamber, short-axis, and tagged short-axis cines were acquired in order track wall motion and intramyocardial strain during pacing. The results of this study revealed that the activation patterns of the left ventricle (LV) during RVA pacing demonstrated intraventricular dyssynchrony; as the left ventricular mechanical activation proceeded from the septum and anterior wall to the lateral wall, with the posterior wall being activated last. As such, the time difference to peak contraction between the septum and lateral wall was ∼125 ms. Likewise, interventricular dyssynchrony was demonstrated from the four-chamber cine as the time difference between the peak LV and RV free wall motion was 180 ms. With the ongoing development of MR safe and MR compatible pacing systems, we can expect MRI to be added to the list of imaging modalities used to optimize cardiac resynchronization therapy and/or alternate site pacing.
Keywords: Cardiac pacing, dyssynchrony, magnetic resonance imaging, myocardial contraction
Inroduction
The right ventricular apex (RVA) has been the standard site for intracardiac pacing lead implantation for decades; i.e., due to both the relative ease of implantation and associated lead stability. Yet more recently, through imaging modalities such as echocardiography, pacing at the RVA has been clinically shown to result in both mechanical dyssynchrony and deleterious ventricular remodeling (1,2). Nevertheless, it has also been recently considered that cardiac MR (CMR) has several advantages over current echocardiographic methods for the assessment of mechanical dyssynchrony, including: (1) the ability to quantify transmural circumferential strain and myocardial scar burden and distribution, and/or (2) image acquisition is relatively independent of operator skill and thus has fewer technical issues, resulting in more accurate and reproducible results (3). More specifically, myocardial tagging has been employed to quantify mechanical dyssynchrony as a MRI predictor of responses to biventricular pacing (4). However, to date the clinical use of MRI for the assessment of mechanical dyssynchrony during cardiac pacing is nonexistent: as having an implanted pacing system remains a contraindication for CMR (5). Here we uniquely made use of MR imaging to visualize and quantify RVA pacing induced interventricular and intraventricular dyssynchrony in an experimentally isolated functional human heart.
Materials and Methods
A human donor heart, deemed not viable for transplant, was reanimated using a custom built, MR safe and compatible, four-chamber working perfusion system adapted from previously described Visible Heart methodologies (6, 7). After the individual cannulation of the superior vena cava, aorta, pulmonary artery, and left pulmonary vein, the heart was perfused with a clear, Krebs-Henseleit buffer solution which was used as a blood substitute. All CMR images were acquired with a 1.5T Siemens Avanto scanner (Siemens, Erlangen, Germany). For the pacing study, a temporary bipolar pacing lead (6495 STREAMLINE, Medtronic, Inc.) was epicardially placed at the RVA and activated using a pacing system analyzer (5311, Medtronic, Inc.); thus used to control the heart rate and stimulus amplitude (Figure 1A). Following defibrillation, and the elicitation of a native sinus rhythm, the heart was paced at a rate of 80 beats/minute and three surface electrodes were placed on the ventricles for image gating. Subsequently, a flexible phased-array receiver coil was placed over the heart and the patient bed was positioned such that the heart was located at the isocenter of the magnet (Figure 1B). Four-chamber, short-axis, and tagged short-axis cines were acquired for the tracking of wall motion and intramyocardial strain in order to quantify and visualize pacing induced dyssynchrony. A retro-gated steady state free precession (True-FISP) cine imaging sequence was used with the following parameters: TR/TE = 3.41 ms/1.18 ms, slice thickness = 6 mm, pixel spacing = 1.4 × 1.4 mm2, number of phases = 25, FOV = 360 mm × 292 mm, flip angle = 67°. A fast gradient-echo (FLASH) sequence was used for tagged cine imaging: TR/TE = 4.49 ms/2.32 ms effective, slice thickness = 8 mm, pixel spacing = 0.94 × 0.94 mm2, number of phases = 20, tag separation = 6 mm, field of view = 240 × 240 mm, flip angle = 10°. Analyses of the cine images were performed using the MASS software system (version 4.2, Medis, Leiden, The Netherlands) in order to segment the RV and LV endocardial borders and compute the regional wall motions throughout all cardiac phases. In order to quantify interventricular dyssynchrony, wall motions were measured in the four-chamber cine at each time point as the average displacement of 50 equally distributed points along the RV and LV endocardium toward the central long-axis of each ventricle (Figure 2). A harmonic phase analysis software package, HARP (Diagnosoft, Maryland, USA), was employed to calculate intramyocardial circumferential strain and to map the sequence of contraction of the LV (8).
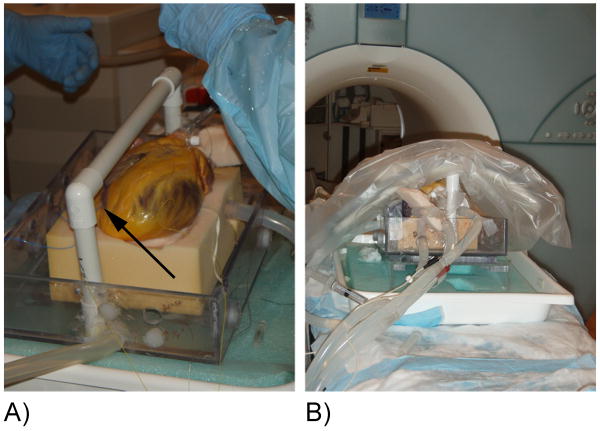
Figure 1.
(A) The human heart is shown with the location of the temporary pacing lead indicated (arrow). (B) Placement of the heart and receiver coil on the patient bed prior to imaging.
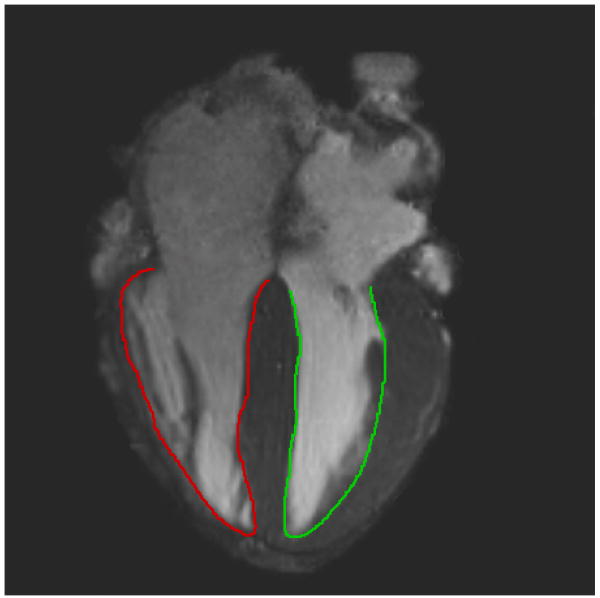
Figure 2.
Long-axis cine image used to measure endocardial excursion of the LV (green) and RV (red).
Results
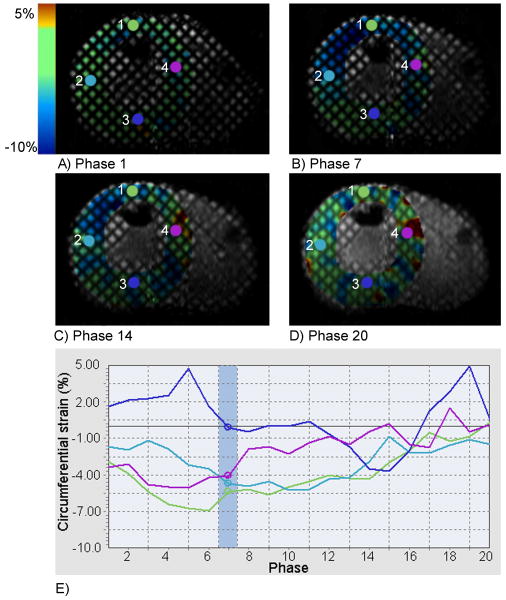
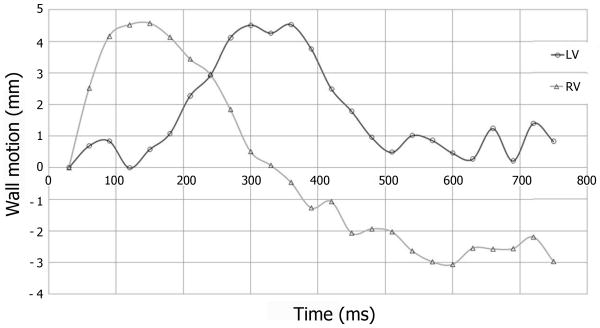
A color map of circumferential strain in the left ventricular (LV) short-axis at the papillary level during RVA pacing is shown in Figure 3. The activation pattern of the LV during RVA pacing demonstrated pacing induced intraventricular dyssynchrony; as the mechanical activation proceeded from the septum and anterior wall (Figure 3, probe locations 4 and 1 respectively) to the LV lateral wall (probe 2) with the posterior wall (probe 3) being activated last. Furthermore, the septum, anterior wall, and lateral wall were relaxing as the posterior wall was contracting (Figure 3, Phase 14), where the time difference to peak contraction between the septum and lateral wall was approximately 125 ms. Figure 3 can also be viewed as a cine in Data Supplemental Movie 1. Pacing induced dyssynchrony could also be visualized in the short-axis cine without tagging (Data Supplemental Movie 2), where pre-excitation of the RV, and LV asynchronous mechanical activation could easily be discerned. Interventricular dyssynchrony was evident from the analyses of RV and LV wall motions plotted in Figure 4, as the RV free wall attained maximal displacement approximately 180 ms before the LV free wall. The four-chamber cine used to calculate wall motion can be viewed in real-time in Data Supplemental Movie 3, where interventricular dyssynchrony or pre-excitation of the RV was clearly demonstrated.
Figure 3.
Circumferential strain superimposed on the LV short-axis at beginning-systole (A), mid-systole (B), end-systole (C), and end-diastole (D). The circumferential strain values are plotted throughout the cardiac cycle (E) for the given probe locations (1-4) indicated in the short-axis images, where the phase at mid-systole is highlighted (Phase 7).
Figure 4.
Average endocardial excursion of the RV and LV measured from the four-chamber long-axis cine.
Discussion
To our knowledge, this is the first demonstration of pacing induced dyssynchrony in an experimentally isolated human heart using MRI. This unique characterization of RVA pacing induced dyssynchrony further emphasizes the need for clinical approaches to induce more physiological pacing, and also demonstrates the capabilities of MRI in the assessment of pacing induced ventricular dyssynchrony.
It should be noted that, the MR measurement of ventricular dyssynchrony is not solely limited to myocardial tagging techniques. For example, MR phase velocity mapping has been successfully used to quantify myocardial dyssynchrony in patients with LV dysfunction and conduction system disease scheduled for CRT (9). More specifically, dyssynchrony parameters (peak myocardial velocities and time to peak velocities) assessed with MR phase velocity mapping correlated well with measurements obtained using tissue Doppler imaging within the same group of patients. Furthermore, MR phase velocity mapping has several advantages over TDI, including the ability to acquire multidirectional velocity information (TDI can only assess velocities for trajectories moving directly towards and away from the transducer) and is not limited by echocardiographic windows across the chest. As such, MR phase velocity imaging would also be a useful tool in the assessment of CRT responses or pacing induced dyssynchrony, as well as strain and displacement derived MR assessments which were utilized here.
Furthermore, our group has previously reported that this in vitro model offers similar working physiologic function as demonstrated in swine (10). Yet, it should be clearly noted that this experimental approach is not without limitations. Fortunately, the time from cross-clamping of the aorta and subsequent administration of cardioplegia, to reanimation of the heart within the MRI scanner was only ∼3 hours. Nonetheless, ischemic time results in injury to the myocardium, even if optimal procedures for protection are used, and therefore the viability of the heart will become affected (it should be noted that for heart transplantation, tissue is considered viable for ∼4-6 hours after cardioplegia is initially administered). In addition, we have previously noted that in vitro cardiac function gradually declines after approximately 1 hour of reanimation; for this study, all images were acquired within 1 hour of reanimation in an attempt to elicit maximum viability (10).
To date, MRI for patients with pacemakers remains a contraindication due to the risks of lead heating (e.g., which can lead to tissue damage), gradient induced stimulation (e.g., which can cause unintended cardiac stimulation and induce arrhythmias), and electromagnetic compatibility (5). For these reasons, device manufacturers have been developing new MRI safe pacemakers, and recently the findings in one clinical trial, for one such device, has shown that there was no evidence of MRI related adverse events (11,12).
In conclusion, with the ongoing development of MR safe and MR compatible pacing systems, and the ability of MRI to quantify regional circumferential strain, MRI will likely be used as a clinical tool to accurately characterize the effects of cardiac resynchronization therapies, alternate site lead placements, and/or the optimization of any such pacing therapies.
Supplementary Material
Acknowledgments
This research was conducted using a human organ donated by a generous family and was supported by LifeSource. This family agreed to this donation for research, so to be used for the advancement of medical and scientific outcomes. In addition, we acknowledge Phil Matta for data collection and Gary Williams, BS, for movie file editing.
Grant support: This work was funded by the NIH grant T32AR007612.
Footnotes
Disclosures: None.
References
- 1.Tops LF, Schalij MJ, Holman ER, et al. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol. 2006;48(8):1642–1648. doi: 10.1016/j.jacc.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 2.Thambo JB, Bordachar P, Garrigue S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004;110(25):3766–3772. doi: 10.1161/01.CIR.0000150336.86033.8D. [DOI] [PubMed] [Google Scholar]
- 3.Helm RH, Lardo AC. Cardiac magnetic resonance assessment of mechanical dyssynchrony. Current opinion in cardiology. 2008;23(5):440–446. doi: 10.1097/HCO.0b013e32830b3865. [DOI] [PubMed] [Google Scholar]
- 4.Russel IK, Zwanenburg JJ, Germans T, et al. Mechanical dyssynchrony or myocardial shortening as MRI predictor of response to biventricular pacing? J Magn Reson Imaging. 2007;26(6):1452–1460. doi: 10.1002/jmri.21133. [DOI] [PubMed] [Google Scholar]
- 5.Faris OP, Shein MJ. Government viewpoint: U.S. Food & Drug Administration: Pacemakers, ICDs and MRI. Pacing Clin Electrophysiol. 2005;28(4):268–269. doi: 10.1111/j.1540-8159.2005.50035.x. [DOI] [PubMed] [Google Scholar]
- 6.Eggen M, Swingen C, Matta P, et al. Design of a Novel Perfusion System to Perform MR Imaging of an Isolated Beating Heart. J Med Devices. 2009;3(2):027536. [Google Scholar]
- 7.Hill AJ, Laske TG, Coles JA, Jr, et al. In vitro studies of human hearts. Ann Thorac Surg. 2005;79:168–177. doi: 10.1016/j.athoracsur.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 8.Osman NF, Prince JL. Visualizing myocardial function using HARP MRI. Phys Med Biol. 2000;45(6):1665–1682. doi: 10.1088/0031-9155/45/6/318. [DOI] [PubMed] [Google Scholar]
- 9.Delfino JG, Bhasin M, Cole R, et al. Comparison of myocardial velocities obtained with magnetic resonance phase velocity mapping and tissue Doppler imaging in normal subjects and patients with left ventricular dyssynchrony. J Magn Reson Imaging. 2006;24(2):304–311. doi: 10.1002/jmri.20641. [DOI] [PubMed] [Google Scholar]
- 10.Chinchoy E, Soule CL, Houlton AJ, et al. Isolated four-chamber working swine heart model. Ann Thorac Surg. 2000;70(5):1607–1614. doi: 10.1016/s0003-4975(00)01977-9. [DOI] [PubMed] [Google Scholar]
- 11.Sutton R, Kanal E, Wilkoff BL, et al. Safety of magnetic resonance imaging of patients with a new Medtronic EnRhythm MRI SureScan pacing system: clinical study design. Trials. 2008;9:68. doi: 10.1186/1745-6215-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkoff BL, Sommer T, Taborsky M. Heart Rhythm Society Scientific Sessions. Late-Breaking Clinical Trials; Boston, MA: May 14th, 2009. Worldwide randomized clinical trial to evaluate new pacemaker system designed for use during magnetic resonance imaging. Abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.