Abstract
Significant advances in therapy for lysosomal storage disorders have occurred with an accelerating pace over the past decade. Although enzyme replacement therapy has improved the outcome of lysosomal storage disorders, antibody responses have occurred and sometimes prevented efficacy, especially in cross-reacting immune material negative patients with Pompe disease. Preclinical gene therapy experiments have revealed the relevance of immune responses to long-term efficacy. The choice of regulatory cassette played a critical role in evading humoral and cellular immune responses to gene therapy in knockout mouse models, at least in adult animals. Liver-specific regulatory cassettes prevented antibody formation and enhanced the efficacy of gene therapy. Regulatory T cells prevented transgene directed immune responses, as shown by adoptive transfer of antigen-specific immune tolerance to enzyme therapy. Immunomodulatory gene therapy with a very low vector dose could enhance the efficacy of enzyme therapy in Pompe disease and other lysosomal storage disorders.
INTRODUCTION
Lysosomal storage disorders can be effectively treated, if biochemical correction can be achieved prior to the onset of irreversible complications. Pompe disease stands out among lysosomal storage disorders due to the lack of devastating central nervous system involvement, rendering it much more amenable to treatment even later in life. The deficiency of GAA (acid maltase; EC 3.2.1.20) causes Pompe disease. GAA normally functions as an acid hydrolase that metabolizes lysosomal glycogen, and GAA deficiency causes lysosomal glycogen accumulation in virtually all tissues [1]. However, Pompe disease affects the heart and skeletal muscle primarily, and infants with Pompe disease develop profound weakness and hypotonia. Infantile-onset Pompe disease (glycogen storage disease type II; MIM 232300) causes death early in childhood from cardiorespiratory failure related to an underlying hypertrophic cardiomyopathy, if enzyme replacement therapy (ERT) is delayed or the patient fails to respond sustainably to ERT [2; 3]. Late-onset forms of Pompe disease feature progressive weakness without significant cardiomyopathy, and patients with juvenile-onset Pompe disease typically become ventilator-dependent due to respiratory muscle involvement [2].
ERT with GAA, like other lysosomal enzymes, has been feasible because it is taken up via mannose-6-phophate receptor mediated uptake in organs other than the brain [4]. The blood-brain barrier prevents effective uptake of circulating lysosomal enzymes by the central nervous system. The availability of ERT with recombinant human (h) GAA has prolonged survival and ameliorated the cardiomyopathy of infantile Pompe disease [5]; however, the extent to which ERT will be efficacious in late-onset Pompe disease is still being studied. Documented limitations of ERT in Pompe disease include the requirement for frequent intravenous infusions of high levels of GAA to achieve efficacy, degree of pre-ERT muscle damage, and the possibility of humoral immunity [4]. The recombinant hGAA doses were markedly higher than doses required for ERT in other lysosomal storage disorders, reflecting the high threshold for correction of GAA deficiency in the skeletal muscle of Pompe disease patients [4; 5].
The cellular basis for low response to ERT by skeletal muscle has been very thoroughly evaluated in liver-transgenic, immune tolerant Pompe disease mice. High level, liver-specific expression of hGAA demonstrated the facile correction of glycogen storage in the heart, as compared to skeletal muscle [6; 7]. The resistance of type II myofibers to correction, in comparison with type I myofibers, explained the resistance of the quadriceps and gastrocnemius to over-expression from the liver-specific transgene [8]. ERT was only partially efficacious in Pompe disease mice carrying a low activity liver-specific transgene; moreover, increasing the dose of recombinant hGAA from 20 mg/kg/week to 100 mg/kg/week increased glycogen clearance from <50% to only 75% in skeletal muscle [9]. The basis for inadequate correction of type II myofibers is abnormal autophagy of glycogen, accompanied by the accumulation of poorly acidified late endosyomes/lysosomes [10]. Furthermore, hGAA trafficking and processing was impaired by abnormal autophagy [10; 11]. Poor uptake of recombinant hGAA by skeletal muscle was also linked to the low abundance of the cation-independent mannose-6-phosphate receptor in skeletal muscle compared to heart [9; 12; 13].
Early gene therapy experiments revealed the Pompe disease might be responsive to gene therapy, although antibody responses to GAA replacement complicated these experiments. Intravenous administration of adenovirus (Ad) vectors encoding GAA demonstrated generalized correction of glycogen storage in the GAA-null mouse model for Pompe disease [14; 15], although glycogen gradually re-accumulated in the months following vector administration [16]. Similarly, a transgene containing a ubiquitously active cytomegalovirus enhancer/chicken β-actin promoter regulatory cassette demonstrated the secretion of hGAA when delivered within pseudotyped adeno-associated virus (AAV) vectors in immunodeficient, GAA-null mice; however this strategy was not successful in immunocompetent GAA-null mice due to anti-hGAA antibody production that prevented efficacy [17; 18]. The immunodeficient GAA-null mice were bred to avoid the neutralizing antibody response to hGAA introduced with an AAV or Ad vector [16; 17]. Even neonatal administration of GAA did not consistently induce tolerance to GAA expression with an AAV vector in GAA-null mice, because a subset of those mice still formed anti-GAA antibodies in response to administration of an AAV vector encoding hGAA [19].
Cross-reacting immune material (CRIM)-negative status associated with a poor response to ERT
Pilot studies of ERT with rhGAA improved cardiomyopathy and prolonged all subjects’ survival beyond one year [4]. Patients with Pompe disease who lack any residual GAA protein are deemed CRIM-negative. CRIM-negative subjects with Pompe disease in these clinical trials formed very high, sustained anti-hGAA antibodies and demonstrated markedly reduced efficacy from ERT [4; 5; 20]. In the first pilot study of ERT in Pompe disease using Chinese hamster ovary cell-derived recombinant hGAA, the two patients who were CRIM-negative produced higher titers of anti-hGAA antibodies than the third patient who was CRIM-positive. This corresponded with a markedly reduced efficacy of the treatment in the CRIM-negative patients [4]. Tolerization therapy, including administration of high dose rhGAA with immune suppressant drugs, failed to improve the clinical response to ERT in those CRIM-negative subjects. Indeed, high dose hGAA therapy precipitated nephrotic syndrome in one of the CRIM-negative subjects, possibly related to effects of antibody complexes upon the glomerular basement membrane [21]. Taken together, these data suggest that immune tolerance to ERT is absent in CRIM-negative patients, and that high titer antibody formation reduced any clinical benefit from ERT. At present there is no proven immune modulation or tolerization protocol for patients that maintained the efficacy of ERT following the formation of anti-GAA antibodies, and protocols involving immunosuppressive drug therapy are complicated by the risk for immune deficiencies and organ toxicity.
The antibody response to ERT has been remarkably similar to inhibitory antibody formation in hemophilia [22]. Hemophilia B is like Pompe disease, in that CRIM-negative patients frequently mount high-titer IgG antibody responses to protein replacement therapy with coagulation factor IX (FIX) that interferes with efficacy. Furthermore, attempts to induce immune tolerance through immunosuppression and high level replacement frequently fail to suppress antibodies sustainably. Another risk stems from cytotoxicity related to these agents in patients with infantile Pompe disease, a very fragile population with underlying cardiomyopathy. Finally, these attempts at immunomodulatory therapy often precipitate nephritic syndrome. Taken together, these data suggest that immune tolerance to ERT or coagulation factor therapy is absent in CRIM-negative patients, and once antibody formation occurs subsequent protein therapy is likely to fail.
The relevance of antibody responses to ERT in lysosomal storage disorders has often been downplayed, although CRIM-negative subjects often formed antibodies and responded poorly to ERT A retrospective analysis of CRIM-negative Pompe disease patients clearly demonstrated an attenuated response to enzyme in all outcome measures compared to CRIM positive patients: significantly decreased survival, invasive ventilation free survival, less improvement in cardiac response, and a regression of motor milestones [4; 5; 20; 23]. In Fabry disease, hemizygous male patients mounted an antibody response to ERT much more frequently than females, possibly because males tend to be CRIM-negative [24; 25]. Recently, the presence of high titer IgG was associated with increased storage of globotriasylceramide in capillary endothelial cells, indicating poorer efficacy from ERT [26]. Antibody formation against ERT in canine mucopolysaccharidosis type I reduced efficacy [27]. Mucopolysaccharidosis type I patients who formed high titer antibodies responded poorly to ERT in clinical trials [28; 29]. Thus, antibody formation reduced the efficacy of ERT in three lysosomal storage disorders, suggesting that immunomodulatory therapies should be developed at least for CRIM-negative patients.
Animal studies have demonstrated that humoral immunity against the infused enzyme presents an obstacle to successful therapy. ERT has encountered antibody and hypersensitivity reactions in rodents, which demonstrated immunity to repeated administration of the therapeutic protein. Brooks et al. published classic example of the impact of antibody formation upon ERT [30]. When 4-sulphatase was administered to normal rats, high titer antibodies inactivated the enzyme in reticuloendothelial tissues. The inactivation of 4-sulphatase could be saturated by increasing the enzyme dose, but upon re-administration of the enzyme those rats developed hypersensitivity reactions with poor recovery. Similarly, when GAA-null mice were treated with ERT, fatal hypersensitivity reactions occurred [9]. The formation of anti-GAA antibodies and associated infusion reactions prevented continuation of ERT beyond 3 weeks in GAA-null mice. Like CRIM-negative patients with Pompe disease, GAA-null mice lack immune tolerance to hGAA and ERT has no efficacy, even provoking fatal anaphylaxis [9]. Only by the generation of liver-transgenic tolerant Pompe disease mice through the insertion of a low-expressing liver-specific transgene, could long-term ERT be tested in a Pompe disease mouse model [9]. The similarity of the antibody response between GAA-null mice and CRIM-negative Pompe disease patients could be related to the complete lack of residual GAA protein expression. If GAA deficiency is caused by two underlying null mutation(s), the immune system is likely to react to recombinant hGAA by forming antibodies during ERT.
Modeling immune responses to hGAA with AAV vectors in Pompe disease mice
The potential advantages of gene therapy over ERT have become clear in GAA-null mouse experiments. Fortunately, vector-mediated hGAA expression has avoided hypersensitivity reactions, although antibodies against hGAA were sometimes formed. However, the efficacy of gene therapy in these experiments was inversely related to the presence of antibody responses. Intravenous administration of a second generation Ad vector encoding GAA initially corrected glycogen accumulations in the heart and skeletal muscle, but glycogen gradually re-accumulated in the months following vector administration coincident with the appearance of anti-GAA antibodies [16]. Furthermore, the appearance of anti-GAA antibodies correlated with the disappearance of secreted hGAA precursor from the plasma and a lack of efficacy from viral vectors [16; 31].
Tissue-specific hGAA expression has evaded immune responses and enhanced efficacy from AAV vector-mediated gene therapy in mice with Pompe disease or Fabry disease (Table). Key features of the AAV vectors evaluated in GAA-null mice included the following: 1) muscle-specific expression achieved efficacy despite the presence of anti-hGAA antibodies; 2) ubiquitously expressed hGAA provoked cellular and humoral responses; 3) liver-specific expression evaded immune responses, correcting the heart and skeletal muscles; and 4) low-level liver-specific expression prevented the antibody response without cross-correcting muscles, and enhanced the efficacy of simultaneous ERT. Each of these features is described in detail below.
Table.
Impact of regulatory cassette upon transgene-directed immune responses
| Disease model |
Regulatory cassette |
Efficacy | Antibody response |
CTL | Pseudotype |
|---|---|---|---|---|---|
| Fabry | CMV (nonspecific) |
No | Present | Present | AAV2 [36] |
| Fabry | DC190 (liver- specific) |
Yes | Absent | Absent | AAV2 [36] |
| Gaucher | D170 (liver- specific) |
Yes | Absent | Absent | AAV2/8 [39] |
| Niemann-Pick | DC190 (liver- specific) |
Yes | Absent | Absent | AAV2/8 [37] |
| Niemann-Pick | DC190 (liver- specific) and CB (nonspecific) |
Yes | Absent for combination; present for CB- containing vector alone |
Absent | AAV2/8 (DC- 190) and AAV2 (CB) [38] |
| Pompe | CB (nonspecific) |
No | Present | Absent | AAV2/6 [33] or AAV2/8 [31] |
| Pompe* | CMV (nonspecific) |
Yes | Low level, declining |
Absent | AAV2/1 [40; 41] |
| Pompe | MCK, MHCK7 (muscle- specific) |
Yes | Present | Absent | AAV2/8 [33; 34] |
| Pompe* | Duck hepatitis B virus (nonspecific) |
Yes, if seronegative |
Sporadic | Absent | AAV2/8 hGAA [19]. |
| Pompe | LSP (liver- specific) |
Yes | Absent | Absent | AAV2/8 [31; 33] |
| Pompe | DC190 (liver- specific) |
Yes | Absent | Absent | AAV2/8 adjuvant [44] |
Neonatal
The availability of novel AAV serotypes has advanced gene therapy by improving the tropism of vectors for target tissues including the liver and striated muscle, when pseudotyped as AAV7 (AAV2/7) or AAV8 (AAV2/8) [32]. Therapeutic levels of hGAA were expressed with AAV2/8 vectors that delivered genes to the liver approximately 100-fold more efficiently in mice, including GAA-null mice, in comparison with traditional AAV2 vectors [18; 32]. Additionally, muscle-restricted hGAA expression with either an AAV2/7 or AAV2/8 vector containing the abbreviated muscle-specific creatine kinase (MCK) promoter/enhancer to drive hGAA expression resulted in long-term correction of glycogen content in the heart and skeletal muscle [33; 34]. Incorporating an α-myosin heavy chain enhancer within the MCK promoter/enhancer, in the regulatory cassette MHCK7, increased transgene activity markedly in the heart and skeletal muscle [35]. The MHCK7 cassette in AAV-MHCK7hGAApA expressed hGAA at high levels 18 weeks after intramuscular administration, although high-titer anti-GAA antibodies were formed [34]. The presence of antibodies did not prevent the correction of glycogen content when hGAA was expressed specifically in the skeletal muscle of GAA-null mice, presumably due to the stable transduction of individual myofibers.
Ziegler and colleagues demonstrated the significance of liver-specific transgene expression of lysosomal enzymes in both Fabry and Pompe disease mice (Table). When an AAV2 vector containing a liver-specific regulatory cassette to drive α-galactosidase was administered to Fabry mice, prolonged biochemical correction was accompanied by immune tolerance to α-galactosidase [36]. Immune tolerance was demonstrated by the lack of anti-α-galactosidase antibodies following an immune challenge with adjuvant and recombinant human α-galactosidase. In contrast, an AAV2 vector containing a CMV promoter/enhancer provoked antibody formation in Fabry mice and failed to achieve biochemical correction [36].
The induction of immune tolerance has been demonstrated following administration of an AAV vector containing a liver-specific promoter in Niemann-Pick mice (Table). An AAV2/8 vector containing a liver-specific promoter to drive acid sphingomyelinase expression cleared accumulations of sphingomyelin from lysosomes, reducing hepatosplenomegaly and pulmonary inflammation in Niemann-Pick mice [37]. No antibodies against acid sphingomyelinase were provoked by this therapeutic transgene expression. In a later study the benefit of immune tolerance to acid sphingomyelinase was demonstrated by a subsequent experiment involving the injection of two vectors via different routes, one systemically and the other to the brain. Co-administration of the aforementioned vector, injected intravenously, with another vector containing the CB promoter injected in the brain achieved a higher degree of efficacy [38]. The induction of immune tolerance to acid sphingomyelinase actually increased the efficacy from the brain injection, putatively by preventing antibody formation. The vector containing the liver-specific promoter failed to correct the brain pathology by itself, confirming that the added benefit was indirect and associated with a lack of antibody formation.
The benefit of immune tolerance was further demonstrated in Gaucher disease mice. An AAV2/8 vector containing a liver-specific regulatory cassette secreted human glucocerebrosidase continuously, which prevented glucosylceramide accumulations and the appearance of Gaucher cells in the liver, spleen, and lungs [39]. A challenge with recombinant glucocerebrosidase failed to provoke antibody formation in vector-treated mice, whereas mock-treated mice formed high-titer antibodies in response to enzyme administration. This experiment suggested the possibility that gene therapy might actually improve the benefit of ERT.
Experiments in Pompe disease mice confirmed the importance of liver-specific transgene expression, in contrast with nonspecific expression, with regard to evading immune responses against the therapeutic protein. An AAV2/8 vector containing the ubiquitously active CB regulatory cassette provoked antibody and cytotoxic T cell responses (CTL) responses in response to GAA expression in GAA-null mice [31; 33]. Immunocompetent GAA-null mice produced high titer anti hGAA IgG in response to a ubiquitously expressing vector, packaged as either AAV2/6. Cellular responses hGAA included lymphocytic infiltrates and activation of CD4+ and CD8+ lymphocytes in injected skeletal muscle and in the liver following intravenous administration [31; 33]. When an AAV2/1 vector containing the CMV promoter/enhancer driving GAA was administered to neonatal GAA-null mice, anti-GAA antibody titers waned and transgene expression persisted for up to a year, indicating that partial immune tolerance to GAA could be achieved by vector administration very early in life [40; 41].
The possibility that liver-restricted expression of GAA with an AAV2/8 vector would prevent the formation of anti-hGAA antibodies in GAA-null mice and achieve efficacy was demonstrated with a vector containing a liver-specific regulatory cassette (LSP). The aforementioned AAV vector contained a liver-specific regulatory cassette that also diminished antibody responses to a therapeutic protein expressed in hemophilia B mice and dogs [42; 43]. When pseudotyped as AAV2/8, AAV-LSPhGAA, was administered intravenously (1 × 1011 or 5 × 1011 vp) to 3 month-old GAA-null mice and compared with the administration of the ubiquitously expressing AAV-CBhGAA vector (5 × 1011 vp). The administration of the AAV-LSPhGAA vector led to sustained levels of the 110 kDa precursor of hGAA in plasma for more than 12 weeks [31]. This was in contrast to the levels following administration of AAV-CBhGAA vector which were undetectable after 14 days [31]. ELISA performed 6 weeks following vector administration demonstrated no detectable anti-GAA antibodies in response to liver-specific hGAA expression with AAV-LSPhGAA in GAA-null mice (<1:200), whereas constitutive hGAA expression with AAV-CBhGAA provoked a vigorous antibody response starting 6 weeks post-injection. An AAV2/8 vector containing a different liver-specific regulatory cassette, DC-190, to drive hGAA expression also achieved long-term biochemical correction in GAA-null, Pompe disease mice [44]. These data suggested that liver-restricted, high-level expression of hGAA induced immune tolerance in Pompe disease mice, similarly to previous reports in hemophilia and Fabry disease mice [42; 45].
Immunomodulatory gene therapy enhances the efficacy of ERT in murine Pompe disease
The possibility that gene therapy could be used as an immunomodulatory therapy was further explored in Pompe disease mice. Liver-specific expression had prevented the formation of antibodies against hGAA in GAA-null mice, which was associated with sustained elevation of hGAA in plasma (Figure 1), as well as the correction of glycogen storage in the striated muscles [31; 44; 46]. The biochemical correction of striated muscle was associated with functional improvement in the Rotarod test [31; 44; 46] and in the wire hanging test [44]. The lowest number of vector particles to establish immune tolerance to hGAA was <1×1011 and >1×1010 vp for AAV-DC190-GAA, when that AAV2/8 vector was administered prior to an immune challenge with rhGAA and adjuvant [44]. The vector dose for AAV-LSPhGAA that was used to achieve immune tolerance was 2×1010 vp/mouse, and the lower threshold for the establishment of immune tolerance has not been established [47].
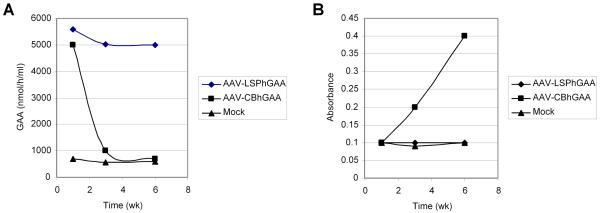
Figure 1. Antibody formation was associated with reduced plasma hGAA in vector-treated GAA-null mice.
The choice of promoter influenced antibody formation following AAV2/8 vector administration [31]. Idealized curves showing hGAA in plasma (A) and antibodies detected by ELISA (B). AAV-LSPhGAA, containing the liver-specific regulatory cassette, prevented antibody formation and produced sustained plasma hGAA elevation. AAV-CBhGAA, containing the ubiquitously active CB regulatory cassette, provoked antibody formation and produced transient plasma hGAA elevation.
A strategy for immunomodulatory gene therapy was developed in GAA-null mice by administering a low number of AAV-LSPhGAA vector particles (2×1010 vector particles (vp)) prior to the initiation of ERT, thereby inducing immune tolerance to rhGAA and enhancing the efficacy of ERT [47]. The combination of vector administration and ERT resulted in enhanced biochemical correction of the heart and improved Rotarod performance, and antibody formation and hypersensitivity reactions were prevented. AAV-LSPhGAAPreventing antibody formation enhanced the efficacy of ERT, whereas neither vector administration nor ERT alone achieved efficacy. An immunomodulatory gene therapy strategy could be an important adjunct to ERT in CRIM-negative Pompe disease patients. The efficacy of ERT would be enhanced by preventing or suppressing antibody responses, and safety would be enhanced by the low number of vector particles needed to induce immune tolerance [47].
CD4+CD25+ Treg cells mediate immune tolerance therapeutic proteins
Immune tolerance in gene therapy models has correlated with high level, liver-specific expression, which has prevented antibody responses against therapeutic proteins. CD4+CD25+ Treg cells that express FoxP3 maintain immune tolerance, based upon their ability to transfer immune tolerance and the loss of tolerance if Treg cells are depleted [48]. Depletion of Treg cells by thymectomy induced autoimmune diseases, which could be reversed by transfer of CD25+CD4+ Treg cells [49]. When CD25+ T cells were depleted from CD4+ cells prior to inoculation of athymic nude mice, those mice developed autoimmune diseases which were reversible by CD4+CD25+ T cell inoculation [50]. The role of Treg cells in modulating immune responses to gene therapy for hemophilia B was demonstrated several years ago. Acquisition of tolerance to FIX required induction of regulatory CD4+ T cells, most likely CD4+CD25+ Treg cells, which suppressed neutralizing antibody formation [51].
Recently the involvement of CD25+ T cells in immune tolerance to hGAA was demonstrated in GAA-null mice, when depletion of CD25+ cells prevented the maintenance of immune tolerance to hGAA [52]. The role of CD4+CD25+ Treg cells in immune tolerance has been evaluated more thoroughly in other genetic disease models. Adoptive transfer of CD4+CD25+ cells to naïve recipient mice, following administration of an AAV vector to donor mice, prevented antibody formation in response to an immune challenge with human FIX in hemophilia B mice [53]. Similarly, an AAV2/8 vector containing a liver-specific regulatory cassette induced tolerance to α-galactosidase in Fabry disease mice, and the transfer of splenocytes from vector-treated mice prevented the antibody response against an α-galactosidase challenge in recipient Fabry mice [45]. Taken together, these data strongly support the ability of an AAV vector containing a liver-specific regulatory cassette to induce immune tolerance to an introduced foreign protein. The enhanced efficacy of AAV vectors in knockout mice relies upon the induction of Treg-mediated immune tolerance by liver-restricted, transgene expression.
The mechanisms for inducing immune tolerance to a peptide antigen through oral or nasal administration have been investigated, and provide a framework for understanding of how liver-specific transgene expression might induce immune tolerance. During the induction of immune tolerance a shift from Th1 and Th2 to Th3 and Tr1 responses occurs, resulting in decreased CTL and antibody responses (Figure) [54]. The secretion of IL10 and TGF-β correlates with these changes and stimulates Treg cells involved in suppression. One mechanism by which Treg cells induce immune tolerance is by interacting with antigen presenting cells to reduce CD4+ helper T cells, thus suppressing antibody production by B cells and impairing CTL (Figure 2). These mechanisms for inducing tolerance have been demonstrated in a mouse model for hemophilia B. Following nasal administration of a FIX-derived peptide antigen, IL10 and TGF-β levels increased and Treg cells were shown to suppress antibody formation [55]. Isolation of CD4+CD25+ Tregs from tolerant donor mice and transfer to naïve recipients resulted in a transfer of immune tolerance. These results further supported the role of Treg cells in the induction of immune tolerance to human FIX [56].
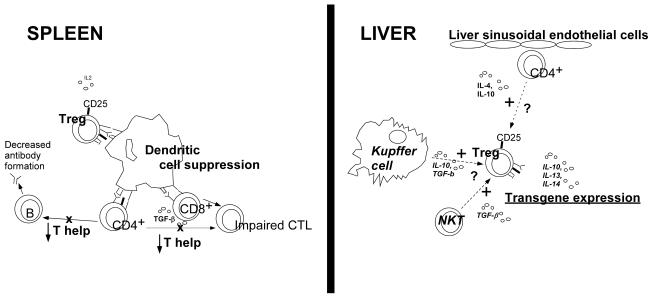
Figure 2. Treg cell mediated induction of immune tolerance in response to stimulation by NKT cells or by Kupffer cells.
Treg cells induce immune tolerance through suppression of activated dendritic cells to reduce CD4+ lymphocyte help, thereby impairing CTL and reducing antibody responses. Naïve CD4+ T cells interact with liver sinusoidal endothelial cells, and could assume an anti-inflammatory phenotype [57]. Similarly, Kupffer cells and/or NKT cells might stimulate Treg cells in the liver to induce immune tolerance, resulting in the suppression of dendritic cell activation in the spleen [59; 60].
A model for immunomodulatory gene therapy recognizes the central role of Treg cells and hepatic transgene expression (Figure 2). The precise mechanism of Treg cell stimulation has not been elucidated. It has been proposed that naïve CD4+ T cells interact with liver sinusoidal endothelial cells to differentiate to an anti-inflammatory phenotype, secreting IL-4 and IL-10 [57]. Kuppfer cells and natural killer T (NKT) cells also might be involved in stimulating Treg cells (Figure 2). Previously the depletion of Kupffer cells prevented tolerance induction via portal vein administration of an antigen [58]. More recently, stimulation of Kupffer cells was associated with Treg activation in response to hepatic gene expression [59], and this mechanism could play a role in immunomodulatory gene therapy (Figure 2). Additionally, NKT cells may also play a role in stimulating Treg cells (Figure 2), because these cells can be active in immune suppression and their numbers are increased in liver [57; 60].
SUMMARY
Clinical trials of ERT and preclinical experiments have revealed the significance of immune responses to hGAA in Pompe disease, particularly antibody responses. The highest titer anti-hGAA antibodies occurred in CRIM-negative Pompe disease patients, and corresponded with reduced efficacy of ERT in those subjects (clinical decline). Whereas ubiquitous expression of therapeutic proteins provoked antibody formation and CTL, liver-specific promoters have induced immune tolerance to therapeutic proteins and enhanced efficacy in mouse models for hemophilia B, Fabry disease, and Pompe disease. CD4+CD25+FoxP3+ Treg cells are critical for the development of immune tolerance to hGAA and other therapeutic proteins in the relevant knockout mouse models. Immune tolerance to the transgene could be achieved by liver-specific expression with AAV vectors in Pompe disease, other lysosomal storage disorders, and other conditions that respond to protein replacement therapy, thereby enhancing efficacy. An exciting possibility for translation to clinical trials of immunomodulatory gene therapy will justify further preclinical experiments in these disease models, and the consideration of early phase clinical trials to address unmet medical needs in these disorders.
ACKNOWLEDGEMENTS
DDK was supported by NIH Grants R01 HL081122-01A1 from the National Heart, Lung, and Blood Institute and 1R01HD054795-01A2 from the National Institute of Child Health and Human Development.
Footnotes
DISCLOSURE STATEMENT
SYP and DDK have received research/grant support from Genzyme Corporation. PSK has received research/grant support from Genzyme Corporation and is a member of the Pompe Disease Advisory Board for Genzyme Corporation. The clinical trials with rhGAA have been supported by a grant from Genzyme Corporation at the various sites that patients were treated. rhGAA, in the form of Genzyme’s product, Myozyme™, has now been approved by the US FDA and the European Union as therapy for Pompe disease. Duke University and inventors for the method of treatment and predecessors of the cell lines used to generate rhGAA may benefit financially pursuant to the University’s Policy on Inventions, Patents and Technology Transfer, even if those cell lines are not used in the commercialized therapy.
REFERENCES
- [1].Ponce E, Witte DP, Hirschhorn R, Huie ML, Grabowski GA. Murine acid alpha-glucosidase: cell-specific mRNA differential expression during development and maturation. Am.J.Pathol. 1999;154:1089–1096. doi: 10.1016/s0002-9440(10)65361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hirschhorn R, Reuser AJJ. Glycogen Storage Disease Type II: Acid α-Glucosidase (Acid Maltase) Deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis for Inherited Disease. McGraw-Hill; New York: 2001. pp. 3389–3419. [Google Scholar]
- [3].Kishnani PS, Hwu WL, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J.Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- [4].Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet.Med. 2001;3:132–138. [PubMed] [Google Scholar]
- [5].Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid {alpha}-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- [6].Raben N, Lu N, Nagaraju K, et al. Conditional tissue-specific expression of the acid alpha-glucosidase (GAA) gene in the GAA knockout mice: implications for therapy. Hum.Mol.Genet. 2001;10:2039–2047. doi: 10.1093/hmg/10.19.2039. [DOI] [PubMed] [Google Scholar]
- [7].Raben N, Jatkar T, Lee A, et al. Glycogen stored in skeletal but not in cardiac muscle in acid alpha-glucosidase mutant (Pompe) mice is highly resistant to transgene-encoded human enzyme. Mol.Ther. 2002;6:601–608. [PubMed] [Google Scholar]
- [8].Raben N, Fukuda T, Gilbert AL, et al. Replacing acid alpha-glucosidase in Pompe disease: Recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol. Ther. 2005;11:48–56. doi: 10.1016/j.ymthe.2004.09.017. [DOI] [PubMed] [Google Scholar]
- [9].Raben N, Danon M, Gilbert AL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol. Genet. Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- [10].Fukuda T, Ewan L, Bauer M, et al. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann. Neurol. 2006;59:700–708. doi: 10.1002/ana.20807. [DOI] [PubMed] [Google Scholar]
- [11].Fukuda T, Ahearn M, Roberts A, et al. Autophagy and mistargeting of therapeutic enzyme in skeletal muscle in pompe disease. Mol.Ther. 2006;14:831–839. doi: 10.1016/j.ymthe.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Winkel LPF, Van den Hout JMP, Kamphoven JHJ, et al. Enzyme replacement therapy in late-onset Pompe’s disease: A three-year follow-up. Ann. Neurol. 2004;55:495–502. doi: 10.1002/ana.20019. [DOI] [PubMed] [Google Scholar]
- [13].Zhu YX, Li XM, Kyazike J, et al. Conjugation of mannose 6-phosphate-containing oligosaccharides to acid alpha-glucosidase improves the clearance of glycogen in Pompe mice. J. Biol. Chem. 2004;279:50336–50341. doi: 10.1074/jbc.M409676200. [DOI] [PubMed] [Google Scholar]
- [14].Amalfitano A, McVie-Wylie AJ, Hu H, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc.Natl.Acad.Sci.U.S.A. 1999;96:8861–8866. doi: 10.1073/pnas.96.16.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pauly DF, Fraites TJ, Toma C, et al. Intercellular transfer of the virally derived precursor form of acid alpha-glucosidase corrects the enzyme deficiency in inherited cardioskeletal myopathy Pompe disease. Hum.Gene Ther. 2001;12:527–538. doi: 10.1089/104303401300042447. [DOI] [PubMed] [Google Scholar]
- [16].Ding EY, Hodges BL, Hu H, et al. Long-term efficacy after [E1-, polymerase-] adenovirus-mediated transfer of human acid-alpha-glucosidase gene into glycogen storage disease type II knockout mice. Hum.Gene Ther. 2001;12:955–965. doi: 10.1089/104303401750195917. [DOI] [PubMed] [Google Scholar]
- [17].Sun B, Chen YT, Bird A, et al. Packaging of an AAV vector encoding human acid alpha-glucosidase for gene therapy in glycogen storage disease type II with a modified hybrid adenovirus-AAV vector. Mol.Ther. 2003;7:467–477. doi: 10.1016/s1525-0016(03)00022-4. [DOI] [PubMed] [Google Scholar]
- [18].Sun B, Zhang H, Franco LM, et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol.Ther. 2005;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- [19].Cresawn KO, Fraites TJ, Wasserfall C, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum.Gene Ther. 2005;16:68–80. doi: 10.1089/hum.2005.16.68. [DOI] [PubMed] [Google Scholar]
- [20].Kishnani PS, Nicolino M, Voit T, et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J.Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hunley TE, Corzo D, Dudek M, et al. Nephrotic syndrome complicating alpha-glucosidase replacement therapy for Pompe disease. Pediatrics. 2004;114:e532–e535. doi: 10.1542/peds.2003-0988-L. [DOI] [PubMed] [Google Scholar]
- [22].Dimichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br.J.Haematol. 2007;138:305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- [23].Kishnani PS, Goldenberg PC, Dearmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol.Genet.Metab. 2009 doi: 10.1016/j.ymgme.2009.08.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schiffmann R, Kopp JB, Austin HA, III, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- [25].Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A--replacement therapy in Fabry’s disease. N Engl.J.Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- [26].Ohashi T, Iizuka S, Ida H, Eto Y. Reduced alpha-Gal A enzyme activity in Fabry fibroblast cells and Fabry mice tissues induced by serum from antibody positive patients with Fabry disease. Mol.Genet.Metab. 2008;94:313–318. doi: 10.1016/j.ymgme.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [27].Dickson P, Peinovich M, McEntee M, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J.Clin Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wraith JE, Beck M, Lane R, et al. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human alpha-L-iduronidase (laronidase) Pediatrics. 2007;120:e37–e46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]
- [29].Kakavanos R, Turner CT, Hopwood JJ, Kakkis ED, Brooks DA. Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I. Lancet. 2003;361:1608–1613. doi: 10.1016/S0140-6736(03)13311-9. [DOI] [PubMed] [Google Scholar]
- [30].Brooks DA, Hopwood JJ, King BM. Immune response to enzyme replacement therapy: clinical signs of hypersensitivity reactions and altered enzyme distribution in a high titre rat model. Biochim.Biophys.Acta. 1998;1407:163–172. doi: 10.1016/s0925-4439(98)00034-9. [DOI] [PubMed] [Google Scholar]
- [31].Franco LM, Sun B, Yang X, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol.Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- [32].Gao GP, Alvira MR, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc.Natl.Acad.Sci.U.S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun B, Zhang H, Franco LM, et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol.Ther. 2005;11:889–898. doi: 10.1016/j.ymthe.2005.01.012. [DOI] [PubMed] [Google Scholar]
- [34].Sun B, Young SP, Li P, et al. Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol.Ther. 2008;16:1366–1371. doi: 10.1038/mt.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Salva MZ, Himeda CL, Tai PW, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol.Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- [36].Ziegler RJ, Lonning SM, Armentano D, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol. Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- [37].Barbon CM, Ziegler RJ, Li C, et al. AAV8-mediated hepatic expression of acid sphingomyelinase corrects the metabolic defect in the visceral organs of a mouse model of Niemann-Pick disease. Mol.Ther. 2005;12:431–440. doi: 10.1016/j.ymthe.2005.03.011. [DOI] [PubMed] [Google Scholar]
- [38].Passini MA, Bu J, Fidler JA, et al. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc.Natl.Acad.Sci.U.S.A. 2007;104:9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McEachern KA, Nietupski JB, Chuang WL, et al. AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J.Gene Med. 2006;8:719–729. doi: 10.1002/jgm.901. [DOI] [PubMed] [Google Scholar]
- [40].Mah C, Cresawn KO, Fraites TJ, et al. Sustained correction of glycogen storage disease type II using adeno-associated virus serotype 1 vectors. Gene Ther. 2005;12:1405–1409. doi: 10.1038/sj.gt.3302550. [DOI] [PubMed] [Google Scholar]
- [41].Mah C, Pacak CA, Cresawn KO, et al. Physiological Correction of Pompe Disease by Systemic Delivery of Adeno-associated Virus Serotype 1 Vectors. Mol.Ther. 2007;15:501–507. doi: 10.1038/sj.mt.6300100. [DOI] [PubMed] [Google Scholar]
- [42].Wang L, Takabe K, Bidlingmaier SM, Ill CR, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc.Natl.Acad.Sci.U.S A. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang L, Nichols TC, Read MS, Bellinger DA, Verma IM. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol.Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- [44].Ziegler RJ, Bercury SD, Fidler J, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum.Gene Ther. 2008;19:609–621. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
- [45].Ziegler RJ, Cherry M, Barbon CM, et al. Correction of the Biochemical and Functional Deficits in Fabry Mice Following AAV8-mediated Hepatic Expression of alpha-galactosidase A. Mol.Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]
- [46].Sun B, Zhang H, Benjamin DK, Jr., et al. Enhanced Efficacy of an AAV Vector Encoding Chimeric, Highly Secreted Acid alpha-Glucosidase in Glycogen Storage Disease Type II. Mol.Ther. 2006;14:822–830. doi: 10.1016/j.ymthe.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sun B, Bird A, Young SP, et al. Enhanced response to enzyme replacement therapy in pompe disease after the induction of immune tolerance. Am.J.Hum.Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sakaguchi S. Naturally arising Foxp3-expressing CD25(+) CD4(+) regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- [49].Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunological Self-Tolerance Maintained by Activated T-Cells Expressing Il-2 Receptor Alpha-Chains (Cd25) - Breakdown of A Single Mechanism of Self-Tolerance Causes Various Autoimmune-Diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- [51].Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sun B, Kulis MD, Young SP, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine Pompe Disease. Mol.Ther. 2009 doi: 10.1038/mt.2009.195. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cao O, Dobrzynski E, Wang L, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Faria AM, Weiner HL. Oral tolerance. Immunol.Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cao O, Armstrong E, Schlachterman A, et al. Immune deviation by mucosal antigen administration suppresses gene-transfer-induced inhibitor formation to factor IX. Blood. 2006;108:480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cao O, Furlan-Freguia C, Arruda VR, Herzog RW. Emerging role of regulatory T cells in gene transfer. Curr.Gene Ther. 2007;7:381–390. doi: 10.2174/156652307782151506. [DOI] [PubMed] [Google Scholar]
- [57].Crispe IN. Hepatic T cells and liver tolerance. Nat.Rev.Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- [58].Roland CR, Mangino MJ, Duffy BF, Flye MW. Lymphocyte suppression by Kupffer cells prevents portal venous tolerance induction: a study of macrophage function after intravenous gadolinium. Transplantation. 1993;55:1151–1158. doi: 10.1097/00007890-199305000-00041. [DOI] [PubMed] [Google Scholar]
- [59].You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen YG, Choisy-Rossi CM, Holl TM, et al. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J.Immunol. 2005;174:1196–1204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]