Abstract
Leptin signaling in the hypothalamus is required for normal food intake and body weight homeostasis. Recent evidence suggests that besides the signal transducer and activator of transcription-3 (STAT3) pathway, several non-STAT3 pathways mediate leptin signaling in the hypothalamus. We have previously demonstrated that leptin stimulates phosphodiesterase-3B (PDE3B) activity in the hypothalamus, and PDE3 inhibitor cilostamide reverses anorectic and bodyweight reducing effects of leptin. To establish the physiological role of PDE3B signaling in the hypothalamus, we examined if leptin signaling through the PDE3B pathway is responsible for the activation of proopiomelanocortin (POMC) and neurotensin (NT) neurons, which are known to play a critical role in energy homeostasis. To this end, we assessed the effect of cilostamide on leptin-induced POMC and NT gene expression in the rat hypothalamus. Results showed that while central injection of leptin significantly increased both POMC and NT mRNA levels in the medial basal hypothalamus, cilostamide completely reversed this effect of leptin suggesting a PDE3B-activation dependent induction of POMC and NT gene expression by leptin. This result further suggests that the PDE3B pathway plays an important role in mediating leptin action in the hypothalamus.
Keywords: leptin, POMC, NT, hypothalamus, phosphodiesterase-3B
Introduction
Cumulative evidence suggests that leptin, a product of the obese gene (54), signals nutritional status to key regulatory centers in the hypothalamus and it has emerged as an important signal regulating energy homeostasis [16,18,19,47]. Leptin administration centrally or peripherally decreases food intake and body weight in a variety of animals [16, 49]. The deletion of leptin receptor (LEPR) in neurons leads to an obese phenotype [9], and transgenic supplementation of the LEPR in neurons of Leprdb/db mice results in an amelioration of the obese phenotype [27]. The early recognition of the LEPR as a member of the class 1 super-family of cytokine receptors resulted in prompt identification of the Janus-kinase 2 (JAK2)-signal transducer and activator of transcription-3 (STAT3) pathway as a major leptin-signaling pathway in the hypothalamus [5,18,19,50,51]. However, we have demonstrated that, in addition to the STAT3 pathway, leptin action in the hypothalamus is also mediated by an insulin-like signaling pathway involving stimulation of phosphatidylinositol 3-kinase (PI3K) and phosphodiesterase 3B (PDE3B) activities and reduction in cAMP levels in the hypothalamus [55]. In addition, cilostamide, a selective PDE3 inhibitor, reverses the anorectic and body weight reducing effect of leptin. While these results suggest a potential role of the PDE3B pathway in mediating leptin action in the hypothalamus, the physiological role of this pathway of leptin signaling in energy homeostasis remains unknown.
To demonstrate physiological role of the PDE3B pathway, it is important to demonstrate if this pathway were involved in mediating action of hypothalamic leptin sensitive neurons that play critical role in energy homeostasis. In this regard, proopiomelanocortin (POMC) producing neurons are known to play a significant role in energy homeostasis and in transducing leptin action in the hypothalamus [2,10,14,33,37,38,47]. In addition, several studies have reported neurotensin (NT) as an important centrally acting anorectic signal [29,31,48], which acts partially through histamine 1 receptor [35]. NT neurons are localized in the hypothalamus [24] and they are the targets of leptin signaling [38]. NT antagonist or antibody reverses the anorectic effect of leptin [45]. These results suggest that NT may play a role in mediating leptin action in the hypothalamus. Thus, to begin to establish physiological role of the PDE3B pathway of leptin signaling we tested the hypothesis that leptin action on POMC and NT neurons is mediated by activation of PDE3B pathway in the hypothalamus. To this end, we examined the effect of cilostamide, a selective PDE3 inhibitor, on leptin-induced POMC and NT gene expression in the hypothalamus.
Materials and methods
Adult male Sprague-Dawley rats, weighing ~250 g, obtained from Taconic Farms (Germantown, NY) were housed individually in a light (lights on 0500 h to 1900 h) and temperature (22 °C)-controlled room with food (pelleted Purina rat chow) and water available ad libitum. After 7 days of acclimatization, rats were subjected to the following experiments according to an approved Institutional Animal Care and Use Committee protocol.
Rats were implanted stereotaxically with 22-gauge stainless steel cannula into the third cerebroventricle under pentobarbital anesthesia [46]. After a recovery period of 14 days, rats were injected icv with cilostamide (10μg/1μl) in dimethyl sulfoxide (DMSO), or DMSO alone, and recombinant murine leptin (4 μg/2μl, Dr. A.F. Parlow, NHPP, Torrance, CA, USA) in artificial cerebrospinal fluid (aCSF, pH 7.4, Ref.20), or aCSF alone, at 1700–1800 hr, and food was withdrawn. One hour later, another injection of cilostamide or DMSO was given to the rats. After 24 hr, a similar injection protocol was used. Rats were killed by decapitation 15 hr after the last injection. Brains were removed immediately and the medial basal hypothalamus (MBH) were dissected out [36,40], frozen in liquid nitrogen, and kept at −80 C until processed for RNA extraction.
POMC and NT mRNA levels were measured by ribonuclease protection assay (RPA) [36]. Total RNA was isolated from MBH, using RNAzol (RNA STAT 60) followed by precipitation with isopropanol and ethanol washes according to the manufacturer’s instructions (TEL-TEST, Inc., Friendswood, TX). Rat POMC [23] and NT [25] cDNAs were kindly provided by Dr. J. L. Roberts (Mount Sinai School of Medicine, New York, NY) and P. R. Dobner (University of Massachusetts, Amherst, MA), respectively. A riboprobe generated from a plasmid containing a rat-specific β-actin cDNA fragment (Ambion Inc., Austin, TX) served as an internal control in all RPA. [α-32P]UTP-labeled antisense cRNA probes were synthesized using T7 RNA polymerases using a transcription kit (Ambion Inc., Austin, TX). Four μg of MBH RNA, 32P-labeled POMC and NT (150,000 cpm) and β-actin (20,000 cpm) cRNA probes, and 16 μg yeast tRNA (Boehringer Mannheim, Indianapolis, IN, USA) were allowed to hybridize in solution at 45 °C overnight, followed by combined RNAse A and T1 digestion of non-hybridized probe at 32 °C for 1 hour. Stable hybrids were extracted with phenol-chloroform followed by ethanol precipitation and then separated on 6% polyacrylamide-8M urea gels. The dried gels were exposed in a Bio-Rad Molecular Imaging Screen-K for 6 to 40 hours, and the image of each gel was acquired using a Molecular Imager FX (Bio-Rad Laboratories, Hercules, CA, USA). The volume analysis of each band was performed using Quantity One Software (Bio-Rad). POMC and NT mRNA values were first normalized with β-actin mRNA levels and then the values were expressed in relation to vehicle control.
All values are expressed as means ± standard error (SE). Statistical significance of differences was analyzed by randomized one-way analysis of variance followed by Student-Newman-Keuls multiple range test. Comparisons with p < 0.05 were considered to be significant.
Results
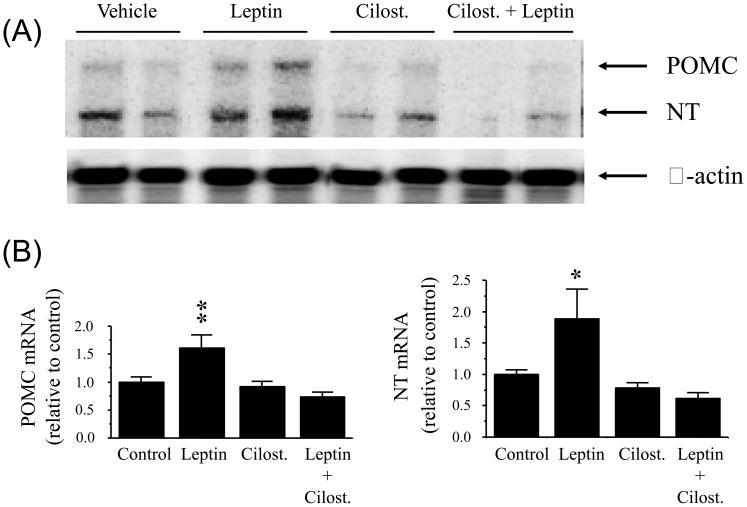
The changes in POMC and NT mRNA levels in the MBH are presented in Fig 1. The bands representing stable hybrids for POMC, NT or β-actin mRNA levels in the MBH extract from one of the RNAse protection assays are presented in Figure 1A. It is evident that intensity of the bands for POMC and NT mRNA was increased in the leptin treated group as compared to all other groups, while β-actin mRNA remained unchanged among the groups. Quantitative analysis showed that intra-cerebroventricular injection of leptin significantly increased both POMC and NT mRNA levels in the MBH when compared with vehicle (aCSF +DMSO) control group (p < 0.01 for POMC, p < 0.05 for NT). Cilostamide completely reversed the stimulating effect of leptin on POMC and NT mRNA levels in the MBH. However, cilostamide alone had no effect on POMC and NT mRNA levels.
Fig. 1.
Proopiomelanocortin (POMC) and neurotensin (NT) gene expression as determined by ribonuclease protection assay in the hypothalamus following intra-cerebroventricular injection of leptin alone or in combination with cilostamide (Cilost.), a selective PDE3 inhibitor. (A): representative phosphorimages showing the level of POMC mRNA, NT mRNA and β-actin mRNA in the hypothalamus. (B): results obtained by phosphor imaging showing the changes in POMC and NT mRNA levels. The values were first normalized to β-actin mRNA levels and then expressed as relative to vehicle (artificial cerebrospinal fluid + dimethyl sulfoxide) control. Values represent the mean ± SEM. Control: n = 4, leptin: n = 5, cilost. : n = 5, and leptin + cilost. : n = 7. * p < 0.05 and ** p < 0.01 vs. all other groups.
Discussion
The present study shows that POMC- and NT-producing neurons in the hypothalamus are activated by leptin, and PDE3 inhibition reverses this effect of leptin on POMC and NT gene expression. These results suggest that PDE3B activation plays a significant role in stimulation of POMC and NT neurons by leptin.
Leptin signaling in the hypothalamus is critical for normal energy homeostasis. The initial discovery of the leptin receptor as a member of the class 1 cytokine receptor super-family resulted in prompt identification of the JAK2-STAT3 pathway as a major pathway of leptin signaling in the hypothalamus [50,51]. Additionally, a defect in the STAT3 pathway has been identified in diet-induced obese (DIO) rats [28], and DIO mice [13] and brain-specific or leptin receptor- specific knockout of STAT3 causes obesity [4,17]. While the significance of the JAK2-STAT3 pathway is unequivocal, cumulative evidence strongly suggests that various non-STAT3 pathways including AMP-activated protein kinase (AMPK) [32], mammalian target of rapamycin (mTOR) [11], forkhead protein (FOXO1) [6,26], PI3K [34,55], and SHP2-GRB2-Ras-Raf-MAPK (mitogen-activated protein kinase) [3,7,8,53] pathways play significant role in transducing leptin action in the hypothalamus. We have demonstrated that leptin’s action in the hypothalamus is also mediated through an insulin-like signaling pathway involving induction of PI3K and PDE3B activities and a reduction of cAMP levels [55]. Furthermore, we have also demonstrated that the PDE3B pathway interacting with the STAT3 pathway constitutes a critical component of leptin signaling in the hypothalamus, in that PDE3 inhibition by cilostamide reversed the leptin-induced STAT3 activation in the hypothalamus [55]. While these findings clearly suggest a potential role of the PDE3B pathway in transducing leptin action in the hypothalamus, the physiological role of this pathway in energy homeostasis is still unknown.
The present study showed that leptin-induced stimulation of POMC and NT gene expression is dependent on PDE3B activation because cilostamide, a selective PDE3 inhibitor, completely reversed the stimulatory effect of leptin on these neurons. This is the first evidence suggesting a potential role of the PDE3B signaling pathway in mediating leptin action in these neurons. Because both POMC and NT neurons are implicated in energy homeostasis [9,26,28,41,43,44], demonstration of a role of PDE3B in mediation leptin action on these neurons provides further support in favor of a physiological role of this pathway in leptin signaling. In addition, PDE3B is co-localized in POMC neurons [30], and indirect evidence such as all Ob-Rb (long form of the leptin receptor) expressing neurons coexpress PDE3B [A. Sahu, unpublished], and PDE3B is expressed in those hypothalamic areas where NT and Ob-Rb are expressed [15,41], suggest that NT neurons may coexpress PDE3B. Thus, reversal of leptin-induced POMC and NT gene expression by cilostamide may suggest a direct role of PDE3B signaling in mediating leptin action in these neurons.
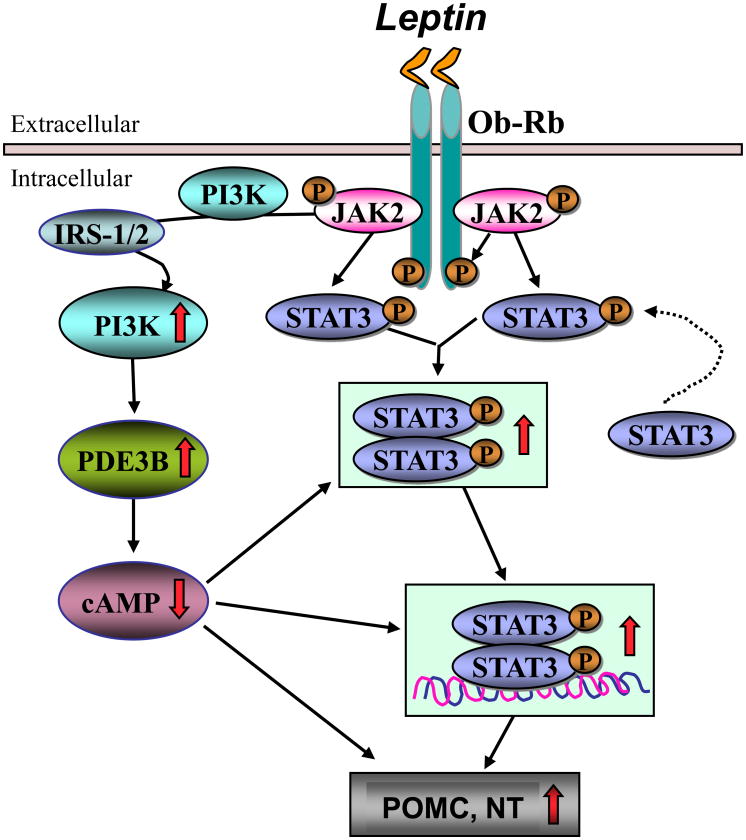
Whereas evidences such as leptin induces STAT3 in POMC neurons [22], disruption of long-form of leptin receptor (Ob-Rb)-STAT3 signaling by mutation of Tyr1138 in Ob-Rb results in reduction of POMC gene expression [4], gene expression of leptin-responsive POMC neurons in the hypothalamus requires STAT3 activation [33], selective deletion of STAT3 in POMC neurons causes mild obesity [52], and STAT3 plays a transcriptional role in the regulation of leptin-induced NT gene expression in N-39 neuronal cell line [12 ], suggest a role of STAT3 in mediating leptin action in these neurons, our study demonstrates that PDE3B pathway also mediates leptin’s action on POMC as well as NT neurons. Notably, we have previously shown that the development of leptin resistance in POMC and NT neurons following chronic central leptin infusion was associated with a defective leptin signaling through the PDE3B pathway without compromising the STAT3 pathway [36,42,44]. These studies taken together further suggest that leptin signaling through the PDE3B pathway plays an important role in mediating leptin action in POMC and NT neurons. Recent studies have also implicated PI3K signaling in POMC neurons to play a significant role in mediating leptin-mediated suppression of food intake [1, 21]. Additionally, PI3K is an upstream regulator of the PDE3B pathway of leptin signaling in the hypothalamus [43]. Thus, based on the available literature and current finding we hypothesize that both STAT3 and PDE3B pathways participate in transducing leptin action on POMC and NT neurons in the hypothalamus [Fig. 2].
Fig. 2.
Schematic of leptin signaling through STAT3 and PDE3B pathways in POMC and NT neurons in the hypothalamus. Leptin binding to it’s receptor (Ob-Rb) leads to activation of JAK2, receptor dimerization, JAK2-mediated Ob-Rb phosphorylation followed by phosphorylation and activation of STAT3. Activated STAT3 dimerizes, translocates to the nucleus, and trans-activate target genes including POMC and NT. Additionally, leptin activates PI3K and PDE3B, and decreases cAMP levels in the hypothalamus. Since PDE3 inhibition by cilostamide reverses the effect of leptin on STAT3 activation [Ref. 55] as well as leptin-induced POMC and NT gene expression (current study), it is possible that decrease in cAMP levels is necessary for STAT3 activation and subsequent stimulation of POMC and NT gene expression by leptin. Also, PDE3B-activation dependent decrease in cAMP levels may directly result in increased POMC and NT gene expression by leptin - a hypothesis need to be experimentally tested. IRS, insulin receptor substrate.
In summary, we have demonstrated that PDE3 inhibition reverses leptin-induced stimulation of POMC and NT gene expression. This study along with recent evidence of co-localization of PDE3B in POMC and other Ob-Rb expressing neurons suggest that leptin’s action on POMC and NT neurons is mediated, at least partly, through the activation of the PDE3B pathway.
Acknowledgments
This work was supported by NIH RO1 Grants DK61499 and DK78068. Thanks to A. F. Parlow and the NIDDK National Hormone & Pituitary Program, Torrance, CA, for supplying the recombinant murine leptin. Thanks are also due to Anantha S Metlakunta and Robert F Friedman for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJ, Batterham RL, Ashford ML, Vanhaesebroeck B, Withers DJ. Dominant role of the p110 isoform of PI3K over p110 in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 4.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 5.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 8.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 9.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 11.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 12.Cui H, Cai F, Belsham DD. Anorexigenic hormones leptin, insulin, and alpha-melanocyte-stimulating hormone directly induce neurotensin (NT) gene expression in novel NT-expressing cell models. J Neurosci. 2005;25:9497–9506. doi: 10.1523/JNEUROSCI.2269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 15.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 16.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 17.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 19.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci. 2001;21:2413–2424. doi: 10.1523/JNEUROSCI.21-07-02413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubowski M, Roberts JL. Multiplex solution hybridization-ribonuclease protection assay for quantification of different ribonucleic acid transcripts from snap- frozen neuroendocrine tissues of individual animals. J Neuroendocrinol. 1992;4:79–89. doi: 10.1111/j.1365-2826.1992.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 24.Kahn D, Abrams GM, Zimmerman EA, Carraway R, Leeman SE. Neurotensin neurons in the rat hypothalamus: an immunocytochemical study. Endocrinology. 1980;107:47–54. doi: 10.1210/endo-107-1-47. [DOI] [PubMed] [Google Scholar]
- 25.Kislauskis E, Bullock B, McNeil S, Dobner PR. The rat gene encoding neurotensin and neuromedin N. Structure, tissue-specific expression, and evolution of exon sequences. J Biol Chem. 1988;263:4963–4968. [PubMed] [Google Scholar]
- 26.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 27.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–435. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2004;286:R143–150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 29.Levine AS, Kneip J, Grace M, Morley JE. Effect of centrally administered neurotensin on multiple feeding paradigms. Pharmacol Biochem Behav. 1983;18:19–23. doi: 10.1016/0091-3057(83)90244-7. [DOI] [PubMed] [Google Scholar]
- 30.Litvin D, Sahu M, Koshinaka K, Sahu A. Evidence showing phosphodiesterase-3B (PDE3B) co-localization in proopiomelanocortin (POMC) and neuropeptide Y (NPY) expressing neurons in the hypothalamus: further insight into leptin signaling. 89th Annual Meeting of the Endocrine Society; Toronto, Canada. June 2–5, 2007; Abst# P4–4. [Google Scholar]
- 31.Luttinger D, King RA, Sheppard D, Strupp J, Nemeroff CB, Prange AJ., Jr The effect of neurotensin on food consumption in the rat. Eur J Pharmacol. 1982;81:499–503. doi: 10.1016/0014-2999(82)90116-9. [DOI] [PubMed] [Google Scholar]
- 32.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 33.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 34.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 35.Ohinata K, Shimano T, Yamauchi R, Sakurada S, Yanai K, Yoshikawa M. The anorectic effect of neurotensin is mediated via a histamine H1 receptor in mice. Peptides. 2004;25:2135–2138. doi: 10.1016/j.peptides.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Pal R, Sahu A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology. 2003;144:3789–3798. doi: 10.1210/en.2002-0148. [DOI] [PubMed] [Google Scholar]
- 37.Piper ML, Unger EK, Myers MG, Jr, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol. 2008;22:751–759. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahu A. Evidence suggesting that galanin (GAL), melanin-concentrating hormone (MCH), neurotensin (NT), proopiomelanocortin (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in the hypothalamus. Endocrinology. 1998;139:795–798. doi: 10.1210/endo.139.2.5909. [DOI] [PubMed] [Google Scholar]
- 39.Sahu A. Quantification of NPY mRNA by ribonuclease protection assay. Methods Mol Biol. 2000;153:219–230. doi: 10.1385/1-59259-042-X:219. [DOI] [PubMed] [Google Scholar]
- 40.Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 41.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Sahu A. Effects of chronic central leptin infusion on proopiomelanocortin and neurotensin gene expression in the rat hypothalamus. Neurosci Lett. 2008;440:125–129. doi: 10.1016/j.neulet.2008.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahu A, Metlakunta AS. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. Evidence suggesting phosphatidylinositol 3-Kinase as an upstream regulator of phosphodiesterase 3B mediated leptin signaling in the hypothalamus. Program No. 380.4. [Google Scholar]
- 44.Sahu A, Metlakunta AS. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J Neuroendocrinol. 2005;17:720–726. doi: 10.1111/j.1365-2826.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- 45.Sahu A, Carraway RE, Wang YP. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Res. 2001;888:343–347. doi: 10.1016/s0006-8993(00)03107-3. [DOI] [PubMed] [Google Scholar]
- 46.Sahu A, Crowley WR, Tatemoto K, Balasubramaniam A, Kalra SP. Effects of neuropeptide Y, NPY analog (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (ovx) and ovx estrogen, progesterone-treated rats. Peptides. 1987;8:921–926. doi: 10.1016/0196-9781(87)90081-7. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 48.Stanley BG, Hoebel BG, Leibowitz SF. Neurotensin: effects of hypothalamic and intravenous injections on eating and drinking in rats. Peptides. 1983;4:493–500. doi: 10.1016/0196-9781(83)90054-2. [DOI] [PubMed] [Google Scholar]
- 49.Tang-Christensen M, Havel PJ, Jacobs RR, Larsen PJ, Cameron JL. Central administration of leptin inhibits food intake and activates the sympathetic nervous system in rhesus macaques. J Clin Endocrinol Metab. 1999;84:711–717. doi: 10.1210/jcem.84.2.5458. [DOI] [PubMed] [Google Scholar]
- 50.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 51.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 52.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 53.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 55.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–728. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]