Abstract
Background
To assess trends in cancer, we evaluated the risk of one generation compared to that 25 years earlier (generational risk) for three groupings of cancers: those for which a substantial proportion is related to tobacco; those reflecting advances in screening or treatment; and a residual category of all other cancers.
Methods
In persons 20-84 years of age, we used age-period-cohort models to summarize time trends in terms of generational risk and average annual percent change for U.S. cancer incidence (1975-2004) and mortality (1970-2004) rates associated with these three cancer groupings.
Results
Adult white men today developed 16% fewer tobacco-related cancers and had 21% fewer deaths due to those cancers than their fathers’ generation, while adult white women experienced increases of 28% and 19%, respectively, relative to their mothers. Incidence of commonly screened cancers rose 74% in men and 10% in women, while mortality fell 25% in men and 31% in women. For cancers not known to be chiefly linked to tobacco or screening, incidence was 34% and 23% higher in white men and women, respectively, than in their parents’ generation 25 years earlier. Mortality in this residual category decreased 14% in men and 18% in women. Results among blacks were qualitatively similar to those among whites.
Conclusions
Despite declining overall cancer death rates, adults are experiencing increased incidence of cancer not associated with tobacco or screening relative to their parents. Future research should examine whether similar patterns are exhibited in other modern nations and should identify population-wide avoidable risks that could account for unexplained increases in these residual cancers.
Keywords: Generational risk, Cancer trends, SEER, Age-Period-Cohort model, Cancer incidence, Cancer mortality, Tobacco, Screening, Occupational cancer, Environmental cancer
Background
Analyses of cancer trends can be used to gauge progress against cancer,1, 2 estimate the proportion of cancers associated with workplace or environmental exposures,3 and predict future demand for health care.4 In 1981, Doll and Peto estimated that smoking accounted for most of the cancer increases in whites aged 35-69 and occupational factors accounted for less than 4% of all cancers as of 1977.5, 6 That widely used estimate of more than three decades ago did not include incidence data, nor did it include data on blacks, who proportionally sustained greater relative exposures to industrial workplace risk factors.
Some thirty years later, the decline in overall cancer mortality in the United States (U.S.) chiefly reflects successful efforts to discourage smoking and advances in screening and treatment for breast, cervical, prostate, and colorectal cancers. In light of these welcome declines in cancer deaths, it is important to examine trends associated with other factors, in order to project future demands for health care and explore novel risk factors that may underlie these trends.
An analysis of trends in cancer rates must take into account the fact that rates generally increase with age. Age-adjusted rates are weighted averages of age-specific rates, with weights proportional to a standard age distribution. In contrast, age-specific rates can reveal patterns within narrow age groups that are not apparent from age-adjusted rates. For example, about 80% of all cancers occur in persons over age 60, who constituted less than 15% of the population in 2000. Thus, age-adjusted cancer rates generally reflect changes in the younger segment of the population and can obscure changes among the elderly. Cancer trends can be evaluated in terms of age-specific (or age-adjusted) rates relative to the year of cancer diagnosis (incidence) or death (mortality), or relative to birth year. These time effects are called period and cohort effects, respectively.
Changes in diagnostic technology, screening policies, medical practice, and underlying risk factors such as smoking can alter cancer trends. In an effort to differentiate among these factors, we created three groupings: cancers principally associated with tobacco use, cancers detectable by screening, and all other cancers. Examining cancer trends in this context should indicate overall progress against cancer after adjusting for welcome benefits of tobacco control and effective screening policies.
Our analysis adjusts for age, period, and cohort effects on cancer rates and it expands on our earlier work on cancer trends7 in three ways. We examine cancer mortality in addition to cancer incidence; we correct incidence data for reporting delays using National Cancer Institute (NCI) formulae; and we evaluate 30 years rather than 20 years of incidence data.
Materials and Methods
Focusing solely on long-term linear trends in log-transformed rates provides a simple framework for examining changes in cancer incidence and mortality that can highlight both successes and challenges for cancer prevention and care. We summarize these trends in terms of average annual percentage change and generational risk. The latter concept assesses relative cancer rates, comparing rates between one point in time and another 25 years (i.e., one “generation”) earlier.
Cancer Data
We analyzed data from the U.S. Surveillance, Epidemiology, and End Results (SEER) Program, using SEER*Stat software (version 6.2.4) to obtain race-, sex-, age-, year-, and site-specific cancer incidence8 and mortality9 counts, as well as race-, sex-, age-, and year-specific population sizes. We used NCI correction factors10, 11 and the methods of Clegg et al.12 to account for incidence reporting delays. Incidence data came from the nine earliest SEER populations. Primary cancer sites were identified using the “SEER Incidence Site Recode ICD-O-3, 1/27/2003” (http://seer.cancer.gov/siterecode), a coding scheme that maps ICD-O-3 primary site and histology data (converted from ICD-O-2, where necessary) into conventional primary site groupings that are consistent over time.
Parallel to earlier work,7 we created three broad categories of cancer: tobacco-related, screen-detectable, and all other. In our current analysis, the tobacco-related category includes 100% of primary cancers of the lung and bronchus, esophagus, larynx, oral cavity and pharynx, urinary bladder, pancreas, and kidney and renal pelvis. These cancers correspond to those identified by the International Agency for Research on Cancer (IARC) as being caused by cigarette smoking.13 The only difference is that we include kidney in addition to renal pelvis. Table 1 shows the estimated percentage of deaths attributable to smoking for each of these cancers, most of which are below 75%. Some cancers in this category may be attributable to second-hand smoke. This category does not include leukemia or stomach cancer, as reports indicate that proportionally few deaths from these cancers are attributable to smoking.14, 15 The screen-detectable category includes 100% of primary cancers of the prostate, female breast, cervix, colon, and rectum. Table 2 shows the estimated percentage of deaths prevented by screening for each of these cancers. Some recent changes in cancer patterns for these sites can be attributed, at least in part, to variation in the use of screening for early detection and treatment.
Table 1.
Annual numbers of deaths and estimated percentages of smoking-attributable mortality (SAM) by cause and gender in the United States, 2000-2004.54
| Male |
Female |
|||
|---|---|---|---|---|
| Cause of death | Deaths | SAM (%) | Deaths | SAM (%) |
| Trachea, lung, bronchus | 90,025 | 87.4 | 66,874 | 70.0 |
| Larynx | 2,984 | 82.0 | 778 | 72.4 |
| Lip, oral cavity, pharynx | 5,126 | 73.1 | 2,494 | 45.9 |
| Esophagus | 9,707 | 71.7 | 2,926 | 55.7 |
| Urinary bladder | 8,508 | 45.9 | 3,951 | 27.2 |
| Kidney and renal pelvis | 7,469 | 37.8 | 4,527 | 4.8 |
| Pancreas | 14,845 | 21.2 | 15,481 | 22.8 |
| Total | 138,664 | 73.4 | 97,031 | 56.7 |
Table 2.
Estimated percent reduction in mortality attributed to screening for selected cancer sites.
| Cancer Site | Percent Reduction in Mortality (95% CI)1 |
Population | Reference |
|---|---|---|---|
| Female Breast | 28-65 2 | USA | Berry et al., 200523 |
| Cervical | 53 (23-72) | Sweden | Mählck et al., 199455 |
| 35 | Australia | Taylor et al., 200156 | |
| 36-75 3 | USA | Austin, 200557 | |
| Prostate | 27 (10-44) | 7 European countries | Schröder et al., 200958 |
| 0 | USA | Andriole et al., 200959 | |
| Colorectal | 33 (27-49) | USA (annual screening) | Mandel et al., 199960 |
| 21 (3-38) | USA (biennial screening) | Mandel et al., 199960 | |
| 18 (1-32) | Denmark | Kronborg et al., 199661 |
CI = confidence interval
Ranges from 7 different models, median value is 46
Estimated ranges
The goal of our classification scheme was to produce a residual category of cancer sites minimally impacted by temporal changes in smoking and screening. Not all cancers in our tobacco-related category are caused by smoking, and thus the associated trends are not entirely due to changes in tobacco use. Similarly, trends in the screen-detectable category are not entirely due to changes in screening practices. In fact, a small fraction of cancers in our residual category are affected by smoking and screening. However, trends in our residual category are mainly due to changes in factors other than smoking or screening, and may therefore yield a general indicator of workplace and other environmental influences.
We analyzed cancer mortality data from the entire U.S., identifying cause of death using the “SEER Cause of Death Recode 1969+, 9/17/2004” (http://seer.cancer.gov/codrecode), a scheme that maps ICD-9 (converted from ICD-8, where necessary) and ICD-10 cause of death codes into conventional cause of death categories that are consistent over time. We grouped causes of death into the same cancer categories used in our incidence analysis.
We restricted incidence and mortality analyses to blacks and whites aged 20-84 years. We did not model cancer endpoints occurring after age 84 for two reasons: to match our previous analysis7 and because recognition and classification of cancer in the elderly are particularly vulnerable to variability in health care access and diagnostic practices. To avoid instability due to counts near zero, we excluded black men aged 20-24 from our incidence analysis of screen-detectable cancers. For each combination of cancer category, race, sex, and 5-year age group between 20-24 and 80-84 years, we calculated incidence for each 5-year period between 1975-79 and 2000-04 and mortality for each 5-year period between 1970-74 and 2000-04.
Statistical Analysis
We fitted separate age-period-cohort (APC) models7, 16 to incidence and mortality data for each combination of race, sex, and cancer category. Our incidence and mortality analyses involved 6 and 7 time periods, respectively, which together with the 13 age groups produced 18 and 19 birth cohorts. We used Proc Genmod (SAS, Version 9.1, Cary, NC) to perform Poisson regression, where the cancer rate was modeled as a log-linear function of age, period, and cohort. If the residuals were over-dispersed, as determined by the Pearson goodness-of-fit test (p<0.05), we fitted an APC model that allowed extra-Poisson variation.17
The APC model can be expressed in terms of trend lines associated with age, period, and cohort effects, as well as deviations from linearity. The sum of the period and cohort trend line slopes can be estimated, though the individual slopes cannot. From the estimate of this sum (D, for net drift), we calculate the average annual percentage change, AAPC = 100(eD/5 – 1), a standard summary reflecting the yearly increment in the long-term trend in cancer rates over time. We also calculate the 25-year generational risk, GR25 = (eD/5)25 = e5D, an equivalent though less standard trend summary based on the ratio of cancer rates across a 25-year time span (or one “generation”). Both formulas divide D by 5 to rescale from 5-year to 1-year time units. Values of AAPC greater (less) than 0 indicate that cancer risk in one year is higher (lower) than in the previous year; values of GR25 greater (less) than 1 indicate that cancer risk increased (decreased) over 25 years. In our linear trend analysis, period and cohort effects cannot be separated and are interpreted in a combined fashion as general time effects; see Dinse et al.7 for details. Unadjusted p-values for assessing trends are given in Tables 3 and 4. Footnotes describe a conservative adjustment for multiple testing, which only affects our conclusion about the mortality trend of tobacco-related cancer among black women.
Table 3.
Summary Measures of Long-term Trends in Cancer Incidence (1975-2004) in the United States by Race, Sex, and Cancer Category.
| Cancer Category |
Race-sex Group | AAPC (95% C.I.) | GR25 (95% C.I.) | P-value |
|---|---|---|---|---|
| Tobacco- related |
White Men | −0.72 (−0.87,−0.57) | 0.84 (0.80, 0.87) | < 0.001 |
| Black Men | −0.98 (−1.28,−0.68) | 0.78 (0.72, 0.84) | < 0.001 | |
| White Women | 0.99 ( 0.82, 1.15) | 1.28 (1.23, 1.33) | < 0.001 | |
| Black Women | 0.32 (−0.07, 0.70) | 1.08 (0.98, 1.19) | 0.054 | |
| Screen- detectable |
White Men | 2.24 ( 1.73, 2.75) | 1.74 (1.54, 1.97) | < 0.001 |
| Black Men | 2.27 ( 1.32, 3.24) | 1.75 (1.39, 2.22) | < 0.001 | |
| White Women | 0.38 ( 0.25, 0.50) | 1.10 (1.06, 1.13) | < 0.001 | |
| Black Women | 0.12 (−0.11, 0.35) | 1.03 (0.97, 1.09) | 0.151 | |
| Other | White Men | 1.17 ( 0.96, 1.39) | 1.34 (1.27, 1.41) | < 0.001 |
| Black Men | 0.94 ( 0.63, 1.26) | 1.26 (1.17, 1.37) | < 0.001 | |
| White Women | 0.82 ( 0.71, 0.93) | 1.23 (1.19, 1.26) | < 0.001 | |
| Black Women | 0.83 ( 0.66, 0.99) | 1.23 (1.18, 1.28) | < 0.001 |
AAPC = Average Annual Percent Change; values above (below) 0 indicate increases (decreases) GR25 = 25-year Generational Risk; values above (below) 1 indicate increases (decreases) C.I. = Confidence Interval
P-value = significance level for testing the null hypothesis of no linear trend (AAPC=0; GR25=1). Without adjusting for multiple testing, typically the null hypothesis is rejected if p < 0.05. A simple Bonferroni correction adjusts for the fact that 12 tests were performed by comparing the p-value to 0.05/12 = 0.0042, which in this case would not change any conclusions.
Table 4.
Summary Measures of Long-term Trends in Cancer Mortality (1970-2004) in the United States by Race, Sex, and Cancer Category.
| Cancer Category |
Race-sex Group | AAPC (95% C.I.) | GR25 (95% C.I.) | P-value |
|---|---|---|---|---|
| Tobacco- related |
White Men | −0.93 (−1.02,−0.84) | 0.79 (0.77, 0.81) | < 0.001 |
| Black Men | −1.04 (−1.19,−0.89) | 0.77 (0.74, 0.80) | < 0.001 | |
| White Women | 0.71 ( 0.59, 0.84) | 1.19 (1.16, 1.23) | < 0.001 | |
| Black Women | 0.26 ( 0.07, 0.46) | 1.07 (1.02, 1.12) | 0.0044 | |
| Screen- detectable |
White Men | −1.15 (−1.31,−0.98) | 0.75 (0.72, 0.78) | < 0.001 |
| Black Men | −0.43 (−0.65,−0.21) | 0.90 (0.85, 0.95) | < 0.001 | |
| White Women | −1.49 (−1.56,−1.41) | 0.69 (0.68, 0.70) | < 0.001 | |
| Black Women | −0.85 (−0.98,−0.72) | 0.81 (0.78, 0.83) | < 0.001 | |
| Other | White Men | −0.58 (−0.63,−0.53) | 0.86 (0.85, 0.88) | < 0.001 |
| Black Men | −0.43 (−0.51,−0.34) | 0.90 (0.88, 0.92) | < 0.001 | |
| White Women | −0.81 (−0.84,−0.77) | 0.82 (0.81, 0.82) | < 0.001 | |
| Black Women | −0.67 (−0.75,−0.59) | 0.85 (0.83, 0.86) | < 0.001 |
AAPC = Average Annual Percent Change; values above (below) 0 indicate increases (decreases) GR25 = 25-year Generational Risk; values above (below) 1 indicate increases (decreases) C.I. = Confidence Interval
P-value = significance level for testing the null hypothesis of no linear trend (AAPC=0; GR25=1). Without adjusting for multiple testing, typically the null hypothesis is rejected if p < 0.05. A simple Bonferroni correction adjusts for the fact that 12 tests were performed by comparing the p-value to 0.05/12 = 0.0042, which in this case would only (barely) affect black women in the tobacco-related category.
Results
In all three cancer groupings, incidence generally increased over time, whereas mortality decreased. The only exceptions to this rule were for tobacco-related cancers, where both incidence and mortality decreased in men but increased in women. For each sex and cancer category, overall trends for blacks and whites were very similar. We provide estimates of both AAPC and GR25 to summarize long-term trends for each combination of race, sex, cancer endpoint, and cancer category (Tables 3 and 4). All trends were statistically significant except for incidence trends of tobacco-related and screen-detectable cancers in black women.
Tobacco-related Cancer
The incidence of cancer related to tobacco use decreased in men (−0.72% annually for whites, −0.98% annually for blacks) and increased in women (0.99% for whites, 0.32% for blacks), though the increase in black women was not statistically significant (Table 3). Likewise, mortality rates decreased in men (−0.93% for whites, −1.04% for blacks) and increased in women (0.71% for whites, 0.26% for blacks), though the statistical significance was marginal in black women after adjusting for multiple testing (Table 4). Over a 25-year time span, incidence and mortality rates decreased 16-23% in men and increased 7-28% in women. Qualitatively, the trends were similar for both races, especially among men, but the increasing trends in incidence and mortality were steeper for white women than black women.
Screen-detectable Cancer
The incidence of cancer detectable by screening increased in both men (2.24% annually for whites, 2.27% annually for blacks) and women (0.38% for whites, 0.12% for blacks), though the increase in black women was not statistically significant (Table 3). Conversely, mortality due to screen-detectable cancer decreased in both men (−1.15% for whites, −0.43% for blacks) and women (−1.49% for whites, −0.85% for blacks) (Table 4). Over 25 years, incidence was 74-75% higher in men but only 3-10% higher in women, whereas mortality was 10-25% lower in men and 19-31% lower in women. Changes in both incidence and mortality were more favorable in women than men. The races were similar with respect to incidence, but mortality decreased more in whites than blacks.
Cancer Unrelated to Tobacco or Screening
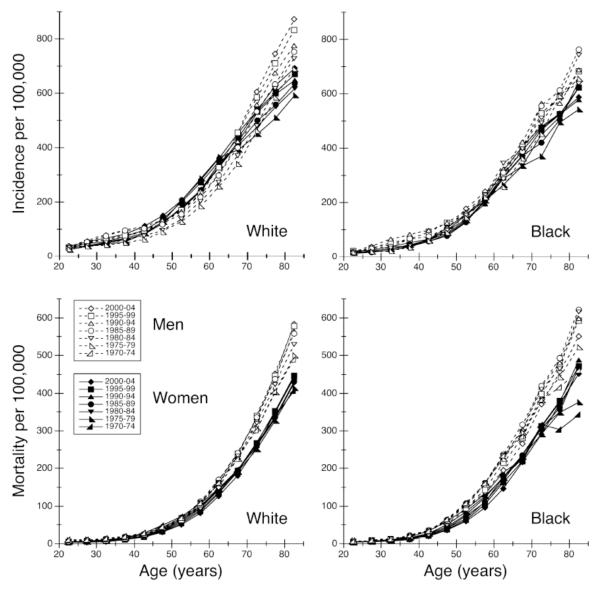
The data for cancers in the residual category are displayed in Figure 1 with age-specific plots of incidence and mortality rates by race, sex, and time period. Time trends estimated under the APC model show that cancer incidence increased in both men (1.17% annually for whites, 0.94% annually for blacks) and women (0.82% for whites, 0.83% for blacks) (Table 3). Conversely, mortality decreased from one year to the next in both men (−0.58% for whites, −0.43% for blacks) and women (−0.81% for whites, −0.67% for blacks) (Table 4). Compared with rates 25 years earlier, incidence rose 23-34% and mortality declined 10-18% in all race-sex groups. Among whites, incidence rose faster in men than women; whereas among blacks, men and women showed similar increases in incidence. Mortality declined more in women than men and more in whites than blacks.
Figure 1.
Age-specific incidence (1975-2004) and mortality (1970-2004) of cancers unrelated to tobacco or screening in the United States by race, sex and time period.
Discussion
Tobacco-related Cancer
Our analysis confirms that incidence (1975-2004) and mortality (1970-2004) of tobacco-related cancer in the U.S. have been decreasing in men, but increasing in women, with consistent results for blacks and whites. Declines in tobacco-related cancer incidence and mortality rates were similar in white and black men, even though until recently more white than black men smoked, and black smokers tended to smoke fewer cigarettes.18 Factors other than tobacco have been found in epidemiologic studies to contribute to patterns of cancer at these sites, including: ionizing radiation, diet, obesity, air and water pollution, urbanization, radon, pharmaceuticals and occupational exposures.19 In contrast, incidence and mortality rates for cancers associated with tobacco use have been rising in women, a trend that may reflect higher proportions of women with a history of smoking among older cohorts currently entering age groups most at risk. With the renewed popularity of smoking in the recent generation of young women,18 this unfortunate trend may continue, though its impact will not be evident for many years.
Screen-detectable Cancer
Temporally circumscribed increases in incidence should result from increased mammographic screening for breast cancer, prostate-specific antigen (PSA) blood testing for prostate cancer, and endoscopic screening or fecal occult blood testing for colorectal cancer. If early detection improves survival, we anticipate concurrent decreases in mortality from these cancers. Reduced colorectal and cervical cancer incidence and mortality are expected consequences, respectively, of increased fecal screening and Papanicolaou and human papilloma virus (HPV) testing and treatment for pre-malignant cervical dysplasia.20
For screen-detectable cancers, we found increasing incidence and decreasing mortality. Declining funding for screening, and cultural differences in attitude toward cervical and breast cancer screening among certain subgroups,21, 22 may explain recent downturns in screen-detectable cancer incidence.1, 20 Our previous analysis7 found an increase of 41-54% in female breast cancer incidence over 25 years. Incorporating data for another 10 years, we estimated an increase of 3-10% in screen-detectable cancer in women, consistent with a recent downturn in breast cancer, though our new category also included cervical and colorectal cancer. Based on mathematical models, Berry et al.23 concluded that advances in screening and treatment lowered U.S. breast cancer mortality. Other factors possibly affecting breast cancer trends include reduced use of hormone replacement therapy24,25, 26 and changes in reproductive practices.27, 28 Several analysts offer conflicting opinions regarding the contribution of increased PSA testing to recent downward trends in prostate cancer incidence and mortality.29, 30 Trends in mortality differ across sexes and races, and trends in incidence differ across sexes, suggesting possible gender and racial disparities in access to medical care and cancer screening 31.
Cancer Unrelated to Tobacco or Screening
Over the past three decades, black and white men and women have experienced increasing incidence of, and decreasing mortality from, cancer unrelated to tobacco or screening. Incidence trends are remarkably similar, with white men exhibiting relatively higher incidence of non-Hodgkin’s lymphoma, melanoma of the skin, leukemia, and brain and other nervous system cancer,32 compared to women and black men. Because declining mortality is consistent across both sexes and races, this may signal general advances in cancer treatment and health care.33 This similarity of trends across sexes and races may reflect the fact that there are few methods for screening for cancers in the residual category. Some cancers in our tobacco-related category are elevated in non-smokers occupationally exposed to solvents,34 pesticides,35 and other workplace toxins.19, 36, 37 Likewise, some cancers in our screen-detectable category have been linked with smoking or workplace exposures. By assuming all these cancers are associated with tobacco or screening, we underestimate the role of occupational factors on cancer incidence and mortality trends in our residual category.
For women, cohort factors that may account for increasing incidence of cancers unrelated to tobacco or screening should be explored, including changes in occupational exposures, expanded use of toxic cleaning and personal care products,38 increased obesity,39, 40 and increased use of pharmaceutical or recreational drugs.41, 42 The percentage of adult women working outside the home rose from 34% in 1950 to 60% in 2000,43 and women comprised 44% of the U.S. labor force by 2004.44 Exposures to benzene, solvents, radiation, and pesticides, as well as employment in food processing, textile, health care, and garment industries, have been linked to cancer risks in women in numerous studies.45
For both men and women, the impact on cancer risk of industrial, agricultural, and other occupational exposures, as well as pharmaceutical and recreational drugs, remains a matter of intense interest. Epidemiologic studies have implicated workplace exposures to heavy metals, pesticides, solvents, cutting oils, and engine exhausts,46 as well as working night shifts,47 as important avoidable causes of cancer. Among other suggested risk factors for cancer are obesity48 and inappropriate use of computerized tomographic scans.49, 50 We suggest that disproportionate exposures to workplace carcinogens have occurred in blue-collar jobs, which represent a decreasing percentage of all jobs in the U.S. today.51 Although our analysis could not test the hypothesis, the decline in blue-collar employment and technological improvements in U.S workforce productivity should reduce cancer risks associated with industrial jobs.
Strengths and Limitations
Changes in cancer rates can be summarized in various ways. One common approach calculates the annual percent change in age-adjusted rates over a given time period, relying on combined weighted averages of age-specific rates over all age groups. This approach cannot identify trends that occur only within specific age groups. A strength of the APC analysis that we conduct here is that it simultaneously incorporates effects for each age group, time period, and birth cohort, providing a more robust estimate of the AAPC. Generational risk provides an innovative summary of linear time trends in terms of rate ratios over 25 years for the GR25, in contrast with yearly relative differences in rates for the AAPC. Whether viewed in terms of AAPC or GR25, temporal changes for a given age group involve simultaneous transitions through adjacent time periods and birth cohorts.
One potential limitation of our approach is that long-term linear trends may only provide part of the picture, as temporal patterns of cancer often exhibit curvature. Although an APC model allows time effects to deviate from linearity, we focus here on the linear aspects of these trends to produce a simple, single-number summary of how cancer rates are changing over time.
By design, our cancer classification scheme underestimates incidence and mortality rates in the residual category as a result of overestimating rates of cancer related to tobacco. About 84% and 77% of all lung cancer deaths have been attributed to smoking in men and women, respectively, but only half or less of all bladder, kidney, esophageal, and pancreatic cancer deaths are thought to be smoking-related.15 In contrast, our tobacco-related category excludes some sites recently linked to smoking, including leukemia, stomach and liver, because the proportions of these cancers attributable to tobacco are much lower. Evidence of a modest association between liver cancer and smoking comes chiefly from Asian countries, where exposures to co-promoting hepatocellular risk factors such as hepatitis and aflatoxin are much more common than in the U.S.52 The net results of our analysis is to over-attribute cancer cases and deaths to tobacco, and thus underestimate rates of cancer unrelated to tobacco or screening..The impact on long-term trends is unclear, however, as underestimated rates do not necessarily imply underestimated trends. Changes in trends depend on whether changes in rates are larger or smaller at early times relative to later times.
Conclusions
The decline in incidence and mortality of tobacco-related cancers in men is a welcome reflection of successful U.S. policies to discourage smoking. The absence of such declines in women reflects their more recent and persisting use of tobacco. Screening and early-treatment programs may partially explain decreasing incidences of colorectal, prostate, breast and cervical cancer. Despite these successes and declines in blue-collar employment, adults today have significantly higher risks of cancer not linked to tobacco or screening than their parents did. Future work should examine whether such increases are evident in other industrial nations. If the increasing trends in cancer not related to tobacco or screening we report here persist, health care systems will be unable to meet the rising needs for care that they will generate.53 Specific studies are required to evaluate whether modifiable social or environmental factors may underlie these unexplained trends. This knowledge could lay the groundwork for preventive public health policies that will ultimately reduce the demand for cancer care. The increased generational risk of cancers not associated with tobacco or screening that we find here merits the most serious concern.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. Support to the Center for Environmental Oncology came from the Heinz Endowments, the DSF Charitable Foundation, the University of Pittsburgh Cancer Institute, the Pennsylvania Tobacco Settlement Funds, the Devra Lee Davis Charitable Foundation, the Milton Fine Fund, and the Collegium Ramazzini. Ethics committee approval is not required for the study. The authors appreciate the valuable counsel and critiques provided by David Umbach and constructive comments provided by Grace Kissling, Lisa DeRoo, Maryann Donovan, Evelyn Talbott, David Hoel, and Annie Sasco.
Footnotes
There are no financial disclosures from any authors.
References
- 1.Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, et al. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107(8):1711–42. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 2.Bailar JC, 3rd, Smith EM. Progress against cancer? N Engl J Med. 1986;314(19):1226–32. doi: 10.1056/NEJM198605083141905. [DOI] [PubMed] [Google Scholar]
- 3.Morrell S, Kerr C, Driscoll T, Taylor R, Salkeld G, Corbett S. Best estimate of the magnitude of mortality due to occupational exposure to hazardous substances. Occup Environ Med. 1998;55(9):634–41. doi: 10.1136/oem.55.9.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute; Bethesda, MD: 2007. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site. Available from http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 5.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191–308. [PubMed] [Google Scholar]
- 6.Peto R. Smoking and death: the past 40 years and the next 40. Bmj. 1994;309(6959):937–9. doi: 10.1136/bmj.309.6959.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinse GE, Umbach DM, Sasco AJ, Hoel DG, Davis DL. Unexplained increases in cancer incidence in the United States from 1975 to 1994: possible sentinel health indicators? Annu Rev Public Health. 1999;20:173–209. doi: 10.1146/annurev.publhealth.20.1.173. [DOI] [PubMed] [Google Scholar]
- 8.Surveillance Epidemiology and End Results (SEER) Program . SEER*Stat Database: Incidence-SEER 9 Regs, Nov 2006 sub (1973-2004) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2007. SEER 1973-2004 public use data. www.seer.cancer.gov. released April 2007, based on the November 2006 submission. [Google Scholar]
- 9.Surveillance Epidemiology and End Results (SEER) Program . SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969-2004) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2007. SEER 1973-2004 public use data. www.seer.cancer.gov. released April 2007. Under lying mortality data provided by NCHS ( www.cdc.gov/nchs) [Google Scholar]
- 10.Midthune DN, Fay MP, Clegg LX, Feuer EJ. Modeling reporting delays and reporting corrections in cancer registry data. Journal of the American Statistical Association. 2005;100(469):61. [Google Scholar]
- 11.Surveillance Epidemiology and End Results (SEER) Program Cancer Query Systems: Delay-Adjusted SEER Incidence Rates. 2006.
- 12.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–45. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2004. [PMC free article] [PubMed]
- 14.English DR, Holman CDJ, Milne E, Winter MG, Hulse GK, Codde JP. The quantification of drug caused morbidity and mortality in Australia. Commonwealth Department of Human Services and Health; AGPS: 1995. [Google Scholar]
- 15.Centers for Disease Control and Prevention Annual smoking-attributable mortality, years of potential life lost, and productivity loses-United States, 1997-2001. MMWR Morbitity Mortality Weekly Report. 2005;54(25):625–28. [PubMed] [Google Scholar]
- 16.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39(2):311–24. [PubMed] [Google Scholar]
- 17.Breslow NE. Extra-Poisson Variation in Log-linear Models. Applied Statistics. 1984;33(1):38. [Google Scholar]
- 18.Centers for Disease Control and Prevention Cigarette smoking among adults--United States, 2004. MMWR - Morbidity & Mortality Weekly Report. 2005;54(44):1121–4. [PubMed] [Google Scholar]
- 19.Axelson O, Davis DL, Forestiere F, Schneiderman M, Wagener D. Lung cancer not attributable to smoking. Ann N Y Acad Sci. 1990;609:165–76. doi: 10.1111/j.1749-6632.1990.tb32065.x. discussion 76-8. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute . Cancer Trend Progress Report - 2005 update. vol. 2007. National Cancer Institute; Bethesda, Maryland: 2005. [Google Scholar]
- 21.Breen N, K AC, Meissner HI, Taplin SH, Tangka FK, Tiro JA, et al. Reported drop in mammography : is this cause for concern? Cancer. 2007;109(12):2405–9. doi: 10.1002/cncr.22723. [DOI] [PubMed] [Google Scholar]
- 22.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97(6):1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 23.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 24.Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49–50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 25.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 26.Davis DL. The Secret History of the War on Cancer. Basic Books; New York, NY: 2007. [Google Scholar]
- 27.Sweeney C, Baumgartner KB, Byers T, Giuliano AR, Herrick JS, Murtaugh MA, et al. Reproductive history in relation to breast cancer risk among Hispanic and non-Hispanic white women. Cancer Causes Control. 2007 doi: 10.1007/s10552-007-9098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler LM, Gold EB, Greendale GA, Crandall CJ, Modugno F, Oestreicher N, et al. Menstrual and reproductive factors in relation to mammographic density: the Study of Women’s Health Across the Nation (SWAN) Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer. 2003;97(6):1507–16. doi: 10.1002/cncr.11212. [DOI] [PubMed] [Google Scholar]
- 30.Etzioni R, Legler JM, Feuer EJ, Merrill RM, Cronin KA, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer--part III: Quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst. 1999;91(12):1033–9. doi: 10.1093/jnci/91.12.1033. [DOI] [PubMed] [Google Scholar]
- 31.Jones BA, Liu WL, Araujo AB, Kasl SV, Silvera SN, Soler-Vila H, et al. Explaining the race difference in prostate cancer stage at diagnosis. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2825–34. doi: 10.1158/1055-9965.EPI-08-0203. [DOI] [PubMed] [Google Scholar]
- 32.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 33.Wong MD, Ettner SL, Boscardin WJ, Shapiro MF. The contribution of cancer incidence, stage at diagnosis and survival to racial differences in years of life expectancy. J Gen Intern Med. 2009;24(4):475–81. doi: 10.1007/s11606-009-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynge E, Anttila A, Hemminki K. Organic solvents and cancer. Cancer Causes Control. 1997;8(3):406–19. doi: 10.1023/a:1018461406120. [DOI] [PubMed] [Google Scholar]
- 35.Merhi M, Raynal H, Cahuzac E, Vinson F, Cravedi JP, Gamet-Payrastre L. Occupational exposure to pesticides and risk of hematopoietic cancers: meta-analysis of case-control studies. Cancer Causes & Control. 2007;18(10):1209–26. doi: 10.1007/s10552-007-9061-1. [DOI] [PubMed] [Google Scholar]
- 36.McGregor D. Industrial chemicals and human cancer. Biotherapy. 1998;11(2-3):181–8. doi: 10.1023/a:1007911002652. [DOI] [PubMed] [Google Scholar]
- 37.Blair A, Steenland K, Shy C, O’Berg M, Halperin W, Thomas T. Control of smoking in occupational epidemiologic studies: methods and needs. Am J Ind Med. 1988;13(1):3–4. doi: 10.1002/ajim.4700130102. [DOI] [PubMed] [Google Scholar]
- 38.Donovan M, Tiwary CM, Axelrod D, Sasco AJ, Jones L, Hajek R, et al. Personal care products that contain estrogens or xenoestrogens may increase breast cancer risk. Med Hypotheses. 2007;68(4):756–66. doi: 10.1016/j.mehy.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 39.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Bmj. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93(9):1062–7. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandes LJ. Hormetic effects of hormones, antihormones, and antidepressants on cancer cell growth in culture: in vivo correlates. Crit Rev Toxicol. 2005;35(6):587–92. doi: 10.1080/10408440500246801. [DOI] [PubMed] [Google Scholar]
- 42.Nelson RA, Levine AM, Marks G, Bernstein L. Alcohol, tobacco and recreational drug use and the risk of non-Hodgkin’s lymphoma. Br J Cancer. 1997;76(11):1532–7. doi: 10.1038/bjc.1997.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toossi M. A century of change: the U.S. labor force from 1950 to 2050. Monthly Labor Review. 2002;125:15–28. [Google Scholar]
- 44.United States Office of Personnel Management Central Personnel Data File (CPDF) Executive Branch (non-Postal) Employment by gender, Rave/National Origin, Disability Status, Veterans Status, Disabled Veterans, 1994-2004. 2006;vol. 2007
- 45.Zahm SH, Blair A. Occupational cancer among women: where have we been and where are we going? Am J Ind Med. 2003;44(6):565–75. doi: 10.1002/ajim.10270. [DOI] [PubMed] [Google Scholar]
- 46.Ward E. Overview of preventable industrial causes of occupational cancer. Environ Health Perspect. 1995;103(Suppl 8):197–203. doi: 10.1289/ehp.95103s8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet Oncology. 2007;8(12):1065–66. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 48.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 49.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 50.Amis ES, Jr., Butler PF, Applegate KE, Birnbaum SB, Brateman LF, Hevezi JM, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272–84. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Hall NE, Rosenman KD. Cancer by industry: analysis of a population-based cancer registry with an emphasis on blue-collar workers. Am J Ind Med. 1991;19(2):145–59. doi: 10.1002/ajim.4700190203. [DOI] [PubMed] [Google Scholar]
- 52.Montesano R, Hainaut P, Wild CP. Hepatocellular carcinoma: from gene to public health. J Natl Cancer Inst. 1997;89(24):1844–51. doi: 10.1093/jnci/89.24.1844. [DOI] [PubMed] [Google Scholar]
- 53.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future Supply and Demand for Oncologists : Challenges to Assuring Access to Oncology Services. Journal of Oncology Practice. 2007;3(2):79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Center for Disease Control and Prevention Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses, United States, 2000-2004. MMWR Morbidity and Mortality Weekly Report. 2008;57(45):1226–28. [PubMed] [Google Scholar]
- 55.Mahlck CG, Jonsson H, Lenner P. Pap smear screening and changes in cervical cancer mortality in Sweden. Int J Gynaecol Obstet. 1994;44(3):267–72. doi: 10.1016/0020-7292(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 56.Taylor RJ, Morrell SL, Mamoon HA, Wain GV. Effects of screening on cervical cancer incidence and mortality in New South Wales implied by influences of period of diagnosis and birth cohort. J Epidemiol Community Health. 2001;55(11):782–8. doi: 10.1136/jech.55.11.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Austin H. An epidemic averted through medical screening. J Med Screen. 2005;12(1):1–2. doi: 10.1258/0969141053279068. [DOI] [PubMed] [Google Scholar]
- 58.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 59.Andriole GL, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 61.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]