Abstract
Phosphorylation of cardiac troponin is a key mechanism involved in regulation of contractile function. In vitro kinase assays revealed that lysates prepared from resting cardiomyocytes contain cardiac troponin I (cTnI) and cTnT kinase activity. cTnI phosphorylation is inhibited by pharmacologic inhibitors of PKA, PKC, Rho kinase and PKC effectors such as RSK and PKD; these kinase inhibitors do not inhibit phosphorylation of cTnT. Rather, cTnT phosphorylation is decreased by the Raf inhibitor GW5074. In vitro kinase assays show that recombinant Raf phosphorylates cTnT, and that Raf-dependent cTnT phosphorylation is abrogated by a T206E substitution; Raf does not phosphorylate cTnI. These studies identify Raf-dependent cTnT-Thr206 phosphorylation as a novel mechanism that would link growth factor-dependent signaling pathways to dynamic changes in cardiac contractile function.
Keywords: Cardiac troponin T, Raf-1, GW5074, cardiomyocytes, phosphorylation
Introduction
Myofilament contraction and relaxation is regulated, in a Ca2+-dependent manner, by the cooperative interaction of cardiac troponin (cTn) with tropomyosin (Tm). The troponin complex is comprised of three distinct proteins with specific functions. Troponin C (TnC) binds Ca2+, troponin I (TnI) inhibits the myosin-actin cross-bridge formation, and troponin T (TnT) binds Tm and tethers the cTn complex to the thin filament. TnT functions as a lever to transmit signals generated by Ca2+-dependent conformational changes in TnC-TnI to Tm, regulating actin-myosin interactions (Kobayashi & Solaro 2005). The functional importance of the cTn complex is underlined by the numerous charge mutations in these proteins that lead to familial hypertrophic cardiomyopathies (Morimoto 2008). Charged residues or phosphorylations control the intermolecular interactions between these sarcomeric proteins and critically regulate cardiac contractile function.
The focus of much of the literature has been on cardiac TnI, which is phosphorylated by several serine/threonine kinases (PKA, PKC, PKD, ROCK), leading to important changes in contractile function (Haworth et al 2004, Layland et al 2005, Vahebi et al 2005). While TnT is not phosphorylated by PKA, its phosphorylation by PKC (at Thr197, Ser201, Thr206, and Thr287 sites in the functionally important C-terminal half of the protein that interacts with TnI, TnC, and possibly Tm) has been linked to impaired myofilament function (Sumandea et al 2003). TnT phosphorylation by ASK1 (a stress-activated kinase that has been implicated specifically in TNF and oxidant stress responses) also has been associated with a decrease in cardiomyocyte contractility (He et al 2003). The functional importance of TnT phosphorylation has been exposed in transgenic models where replacement of cardiac TnT with skeletal TnT (that lacks regulatory phosphorylation sites) blunts PKC-dependent depression of cardiac contraction (Montgomery et al 2001). Mutagenesis studies (with PKC phosphorylation sites substituted by either alanine or phosphomimetic glutamate residues) implicate Thr206 as a functionally important phosphorylation site (Sumandea et al 2003).
In the course of recent studies that examined the role of PKCδ as a TnI kinase (Sumandea et al 2008), we identified a TnT kinase activity that could not be attributed to PKC in cardiomyocytes lysates. Studies reported herein identify Raf as a novel cardiomyocyte TnT kinase.
Material and Methods
Materials
Recombinant PKCδ was from Calbiochem. Recombinant MEK, PKA, PKCα, PKCβII, PKCε and PKD1 were from Upstate. Recombinant truncated/activated Raf-1 (amino acids 306-648 in the catalytic domain, with Y340D/Y341D substitutions) and full-length Pak-1 were obtained from Invitrogen. Other chemicals were reagent grade.
Cardiomyocyte culture
Cardiomyocytes were isolated from hearts of 2 day-old Wistar rats by a trypsin dispersion procedure that uses a differential attachment procedure followed by irradiation to enrich for cardiomyocytes. Cells were plated on protamine sulfate-coated culture dishes at a density of 5 × 106 cells/100-mm dish and grown in MEM (Gibco, BRL) supplemented with 10% fetal calf serum for 4 days. Cells were lysed in homogenization buffer (20 mM Tris-Cl, pH7.5, 2 mM EDTA, 2 mM EGTA, 0.5 mM DTT, 0.2% Triton X-100, 50 μg/ml aprotinin, 50 μg/ml leupeptin, 50 μg/ml benzamidine, 1 mM PMSF, 5 μM pepstatin A, 1 mM sodium orthovanadate, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 0.1 μM calyculin). Cell extracts were used for in vitro kinase assays.
Troponin Expression and Purification
Recombinant heterotrimeric troponin complexes were prepared according to very stringent conditions designed to avoid the presence of any contaminants, as previously described (Sumandea et al 2003). Briefly, each subunit (cardiac TnT-WT and -T206E, TnI and TnC) was expressed independently in E. Coli, denatured in a 6M urea solution and purified to >90% using a combination of ammonium sulfate fractionation, anionic and cationic exchange chromatography (on an AKTA-FPLC system), and extensive dialysis. Recombinant heterotrimeric cTn complexes were reconstituted by mixing equimolar amounts of cTnT (either wt or T206E), cTnI and cTnC in a solubilization buffer containing 6 M Urea, 50 mM Tris pH 8.0, 1 M NaCl, 5 mM MgCl2, 1 mM CaCl2, 1 mM DTT. The solution was subjected to sequential dialysis using 0.7 M NaCl, 0.4 M NaCl, and finally 0.05 M NaCl in the above buffer minus urea (CaCl2 was omitted from the final dialysis step). cTn complex was then further purified from any contaminants on a high performance Resource Q column (GE Healthcare). The troponin complex elutes as a sharp peak well separated from any unincorporated monomeric TnI or TnC subunits. Troponin complexes were then tested using in vitro reconstituted myofilament assays for their ability to regulate myosin ATPase activity in a [Ca2+] dependent manner.
In vitro kinase assays with cell extracts
In vitro kinase assays were performed with 11 μg of cardiomyocyte extract. Assays were performed in 110 μl of a reaction buffer containing 20 mM Tris-Cl, pH 7.5, 3 mM MgCl2, 0.7 mM EDTA, 0.7 mM EGTA, 0.24 mM DTT, 0.9 mM sodium vanadate, 120 mM NaCl, 0.073% Triton X-100, 0.36 mM phenylmethylsulfonyl fluoride, 0.36 mM sodium orthovanadate, 36 mM sodium fluoride, 3.6 mM sodium pyrophosphate, 0.06 μM calyculin, 15 μg of recombinant cardiac troponin complex (cTn), and [γ-32P]ATP (2 μCi, 66 μM). Incubations were for 16 min at 30°C.
In vitro kinase assays with recombinant PKCs, PKD1 and PKA
In vitro kinase assays were performed in 110 μl of a reaction buffer containing 38 mM Tris-Cl, pH 7.5, 6 mM MgCl2, 0.6 mM EDTA, 0.6 mM EGTA, 1.6 mM DTT, 136 mM NaCl, 4.2% glycerol, 83 μg/ml phosphatidylserine, 160 nM PMA, 4 μg of cTn, and [γ-32P]ATP (2 μCi, 66 μM). Incubations were for 16 min at 30°C in the presence of PKCδ (0.032 units), PKCα (0.063 units), PKCβII (0.063 units), PKCε (0.184 units), PKD1 (0.026 units), or PKA (3.84 units).
In vitro kinase assays with recombinant c-Raf-1 and Pak
In vitro kinase assays were performed in 40 μl of a reaction buffer containing 30 mM HEPES, pH 7.4, 10 mM MgCl2, 5 mM EGTA, 1 mM DTT, 115 mM NaCl, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate, 7.5 μg of cTn, and [γ-32P]ATP (15 μCi, 15 μM). Incubations were for 30 min at 25°C in the absence or presence of c-Raf-1 (15 ng) or Pak (20 ng).
Results
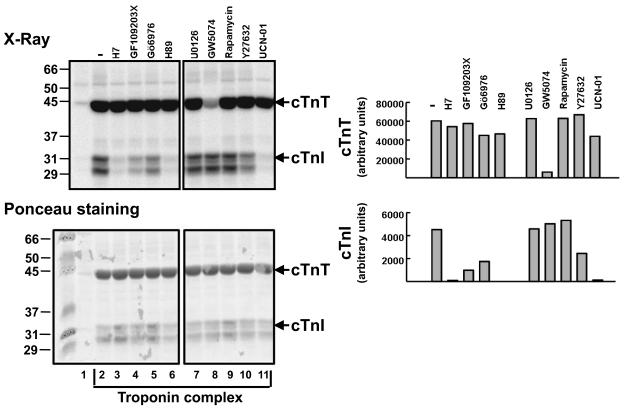
In vitro kinase assays were performed with lysates from resting cardiomyocytes in buffers containing [32P]ATP and cTn complex as substrate (containing equimolar concentrations of cTnT, cTnC, and cTnI). These studies show that both cTnI and cTnT kinase activities are recovered in cardiomyocyte lysates (Fig. 1A and 1B). cTnI phosphorylation is effectively blocked by H7 (a relatively non-specific serine/threonine kinase inhibitor), GF109203X (which inhibits PKC isoforms and RSK), H89 (a PKA inhibitor), and UCN-01. UCN-01 is a 7-hydroxystaurosporine derivative that is relatively selective for PDK-1 and checkpoint kinases at low concentrations, but inhibits PKC isoforms at the concentrations used in this study (Guo et al 2006, Komander et al 2003). Cardiomyocytes co-express multiple PKC isoforms, including conventional PKCs (cPKCs) such as PKCα and arguably PKCβ and novel PKCs (nPKCs) such as PKCδ and PKCε. While the general consensus is that cPKCs are 5-8-fold more abundant than nPKC in most cardiomyocyte preparations (Komander et al 2003), cTnI phosphorylation is attenuated (but not completely inhibited) by Gö6976, which selectively inhibits cPKC isoforms (and not nPKCs) and also blocks the catalytic activity of PKD (a different enzyme that also has been implicated as a cTnI kinase (Haworth et al 2004)). cTnI phosphorylation also is attenuated by Y27632, a Rho kinase (ROCK) inhibitor. Ponceau staining of membranes show that cTnI appear as either a single band or as a doublet in different preparations of the troponin complex. This represents an unfortunate technical feature of this set of experiments; it is presumed to reflect some cTnI degradation during the assays (and has no biological significance). The different cTnI mobilities do not result from differences in phosphorylation pattern.
Figure 1. Cardiac lystaes contain cTnT kinase activity that is blocked by GW5074 (a Raf inhibitor) and not by inhibitors of various other serine/threonine kinases.
Lysates from resting cardiomyocytes were subjected to in vitro kinase assays without (lane 1) and with troponin complex (lanes 2-11). The following kinase inhibitors were used in the assays: 50 μM H7, 5 μM GF109203X, 5 μM Go6976, 20 μM H89, 5 μM U0126, 10 μM GW5074, 10 nM Rapamycin, 10 μM Y27632, and 10 μM UCN-01. It is worth noting that UCN-01 specifically blocks PDK-1 and checkpoint kinases at low concentrations, but UCN-01 also inhibits PKC isoforms at the concentrations used in these studies. Autoradiogram and Ponceau staining are depicted in the left panels, with a quantification of the data in the right panel. These results were replicated in two similar experiments. Similar results also were obtained in experiments that used lysates from H2O2-treated cardiomyocytes cultures.
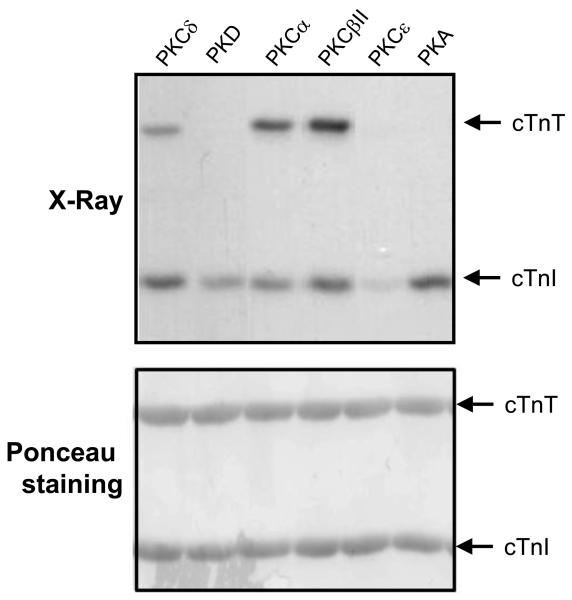
These results are consistent with recent studies implicating PKA, PKCs, PKD, RSK, and ROCK as cTnI kinases (Haworth et al 2004, Itoh et al 2005, Noland et al 1996, Vahebi et al 2005). Of note, none of these kinase inhibitors blocked cTnT phosphorylation. While this is consistent with the additional observation that recombinant PKCε, PKD and PKA phosphorylate cTnI but they do not act as cTnT kinases, it is at odds with results of kinase assays with PKCα (an abundant enzyme in cardiomyocyte lysates (Rohde et al 2000) and other PKC isoforms (such as PKCδ and PKCβII) that act as dual in vitro cTnI/cTnT kinases (Figure 2). An explanation for the failure to detect GF109203X-sensitive cTnT kinase activity (attributable to PKCα) in cardiomyocytes lysates is not obvious and will require further analysis. Nevertheless, figure 1 shows that cTnT phosphorylation is effectively blocked by GW5074, an inhibitor of Raf and PIM kinases, that also exerts a lesser inhibitory effect on RIP2, HIPK2, and MST2 (Bain et al 2007). GW5074 does not block cTnI kinase activity. These results suggest that a GW5074-sensitive kinase is the predominant cTnT kinase recovered in cardiomyocyte lysates. Of note, studies performed in parallel with lysates from H2O2-treated cardiomyocytes provided qualitatively identical results, indicating that oxidative stress does not result in the activation of additional/alternative cardiomyocyte troponin kinases (data not shown).
Figure 2. Differential phosphorylation of cTnT and cTnI by PKC-α, βII, δ, ε, PKD and PKA.
Recombinant troponin complexes were subjected to in vitro kinase assays (top) in buffers containing [γ-32P]ATP and either PKCα, PKCβII, PKCδ, PKCε, PKD or PKA. Phosphorylated forms of cTnT and cTnI were separated by SDS-PAGE and visualized by autoradiography. Ponceau staining (bottom) shows equal protein loading in each lane. Results were replicated in three separate experiments.
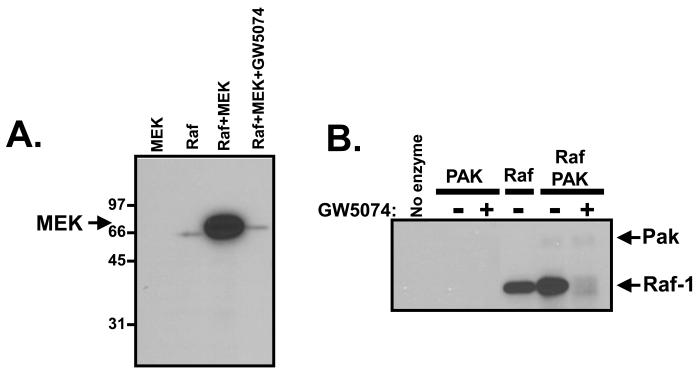
We used in vitro kinase assays to directly explore the potential role of Raf as a cTnT kinase. Initial studies used MEK as a conventional Raf substrate. These studies show that: 1) Raf phosphorylates the conventional Raf substrate MEK (Fig 3A) 2) Raf undergoes an autophosphorylation reaction during in vitro kinase assays (Fig 3A and 3B). A single radioactive band corresponding to the autophosphorylated Raf-1 enzyme is detected when this Raf preparation is subjected to in vitro kinase assays with 32P-ATP; no other contaminating enzymes/substrates are detected in this preparation (even with long exposures of the gel). 3) Raf autophosphorylation is increased by the Raf activator Pak (Fig 3B), and 4) Raf activity (measured as either autophosphorylation or phosphorylation of MEK) is inhibited by GW5074 (Figures 3A and 3B). We then applied this information to studies of troponin phosphorylation. Fig 3C shows Raf and Pak phosphorylate different components of the troponin complex; Pak phosphorylates cTnI and Raf phosphorylates cTnT. Raf-dependent cTnT phosphorylation is increased by Pak, which activates Raf. Raf does not exert a reciprocal effect to influence Pak-dependent cTnI phosphorylation. Pak and Raf kinase activities are further resolved with GW5074, which abrogates Raf-dependent cTnT without blocking Pak-dependent cTnI phosphorylation. Of note, cTnI phosphorylation by Pak was consistently increased by GW5074 (in assays both with Pak alone and with Pak + Raf). These results suggest that GW5074 might act as a direct Pak activator and deserves further study.
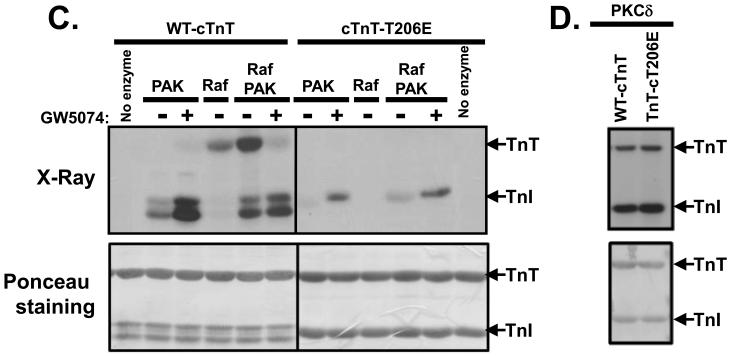
Figure 3. c-Raf-1 is a cTnT-Thr206 kinase.
In vitro kinase assays were performed with c-Raf-1 using MEK as substrate (Panel A), Pak and/or c-Raf-1 in the absence (−) or presence (+) of 10 μM GW5074 (Panel B), with WT-cTnT or cTnT-T206E as substrate (Panel C) or PKCδ with WT-cTnT or cTnT-T206E as substrate (Panel D). Of note, the data in individual panels in this figure are from a single experiment, and permit a side-by-side comparison of c-Raf-1 activity towards MEK (which contains at least two Raf-1 phosphorylation sites) and TnT (which is phosphorylated by Raf-1 specifically at Thr206). Results were replicated in three separate experiments.
Troponin T phosphorylation sites have been mapped to Thr197, Ser201, Thr206, and Thr287. To determine whether Raf phosphorylate cTnT at Thr206 (the functionally critical phosphorylation site) we used recombinant troponin complexes containing equimolar amounts of cTnI, cTnC, and cTnT (either WT or harboring T206E substitution). Figure 3C shows that cTnT-T206E is not a substrate for Raf, including in assays that contain Pak to increase Raf activity. These results indicate that Raf acts as a selective cTnT-Thr206 kinase. Interestingly, Pak-dependent cTnI phosphorylation is decreased in assays with cTnT-T206E as substrate. While this finding was surprising, nonphosphorylatable TnT or TnI mutants that exert long-range effect to decrease the phosphorylation of the other subunit have been described in previous studies (Montgomery et al 2001, Montgomery et al 2002). Finally, Fig 3D shows that PKCδ (a different serine/threonine kinase) phosphorylates WT- and T206E-substituted cTnT in a similar manner; PKCδ also phosphorylates cTnI. These results validate the integrity of the cTnI-T206E protein and indicate that PKCδ phosphorylates cTnT at sites other than Thr206.
DISCUSSION
Raf proteins have recently emerged as key mediators of cellular growth, differentiation, and survival mechanisms, in the heart and other tissues. The focus of most studies has been on the role of Raf-1 to activate the ERK-MAPK pathway. Using molecular approaches involving overexpressing dominant-negative forms of Raf, Raf-1 has been implicated in endothelin-1- or phenylephrine-dependent cardiomyocyte growth, sarcomeric reorganization, and atrial natriuretic factor gene expression in cardiomyocytes cultures (Harris et al 2004) and Raf proteins have been implicated in ERK activation, ventricular hypertrophy, and survival in mice subjected to aortic banding (TAC). Studies in genetically modified mice suggest anti-apoptotic mechanisms for Raf-1 in the heart. Cardiac-specific disruption of the Raf-1 gene (Raf CKO) leads to increased cardiomyocytes apoptosis, left ventricular dilatation, and decreased systolic function at 10 weeks of age that is associated with an increase in ASK1, JNK, and p38 MAPK activities (without a detectable defect in agonist-dependent ERK activation)(Harris et al 2004). Additional studies implicate ASK1 as a particularly important Raf-1 effector that contributes to in vivo cardiac remodeling, showing that the effect of Raf-1 knockout to induce apoptosis and cardiac dilatation and to decrease cardiac contractile function is prevented in the Raf1-ASK1 double knockout (Yamaguchi et al 2004).
Studies reported herein identify an additional role for Raf, as a cTnT-Thr206 kinase. Since cTnT-Thr206 phosphorylation has been implicated as a mechanism that attenuates force, actomyosin Mg-ATPase rate, Ca2+ sensitivity, and tension cost of mouse cardiac fiber bundles (Sumandea et al 2003), dynamic increases in cTnT-Thr206 phosphorylation may be beneficial in instances of high-energy demand and protect the heart from over-stimulation. Finally, it is interesting to note that the Pak-Raf-Ask1 pathway may be uniquely poised to regulate contractility since Pak phosphorylates cTnI, Raf phosphorylates cTnT-Thr206 and ASK1 phosphorylates cTnT (at T197ERKS201, in the vicinity of the KRQT206EREK sequence). Future studies that examine whether Raf-1- and ASK1-dependent cTnT phosphorylations are independently regulated or whether these phosphorylation reactions are controlled in an interdependent fashion will be important, as this would function to add further complexity to the process.
Acknowledgments
This work was supported by United States Public Health Service - National Heart, Lung and Blood Institute grants HL-77860, HL-74161, and AHA-SDG 0335199N.
Abbreviations
- PKA
Protein kinase A
- PKC
Protein kinase C
- PKD
Protein kinase D
- PDK
Phosphoinositide-dependent protein kinase
- ASK-1
Apoptosis signal-regulating kinase -1
- RSK
Ribosomal S6 kinase
- ROCK
Rho-A-dependent kinase
REFERENCES
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Sabri A, Elouardighi H, Rybin V, Steinberg SF. Alpha1-adrenergic receptors activate AKT via a Pyk2/PDK-1 pathway that is tonically inhibited by novel protein kinase C isoforms in cardiomyocytes. Circ Res. 2006;99:1367–1375. doi: 10.1161/01.RES.0000252830.01581.fd. [DOI] [PubMed] [Google Scholar]
- Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, et al. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- He X, Liu Y, Sharma V, Dirksen RT, Waugh R, Sheu SS, et al. ASK1 associates with troponin T and induces troponin T phosphorylation and contractile dysfunction in cardiomyocytes. Am J Pathol. 2003;163:243–251. doi: 10.1016/S0002-9440(10)63647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Ding B, Bains CP, Wang N, Takeishi Y, Jalili T, et al. Role of p90 ribosomal S6 kinase (p90RSK) in reactive oxygen species and protein kinase C beta (PKC-beta)-mediated cardiac troponin I phosphorylation. J Biol Chem. 2005;280:24135–24142. doi: 10.1074/jbc.M413015200. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- Komander D, Kular GS, Bain J, Elliott M, Alessi DR, Van Aalten DM. Structural basis for UCN-01 (7-hydroxystaurosporine) specificity and PDK1 (3-phosphoinositide-dependent protein kinase-1) inhibition. Biochem J. 2003;375:255–262. doi: 10.1042/BJ20031119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Montgomery DE, Chandra M, Huang Q, Jin J, Solaro RJ. Transgenic incorporation of skeletal TnT into cardiac myofilaments blunts PKC-mediated depression of force. Am J Physiol Heart Circ Physiol. 2001;280:H1011–1018. doi: 10.1152/ajpheart.2001.280.3.H1011. [DOI] [PubMed] [Google Scholar]
- Montgomery DE, Wolska BM, Pyle WG, Roman BB, Dowell JC, Buttrick PM, et al. alpha-Adrenergic response and myofilament activity in mouse hearts lacking PKC phosphorylation sites on cardiac TnI. American Journal of Physiology - Heart & Circulatory Physiology. 2002;282:H2397–2405. doi: 10.1152/ajpheart.00714.2001. [DOI] [PubMed] [Google Scholar]
- Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77:659–666. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- Noland TA, Jr., Raynor RL, Jideama NM, Guo X, Kazanietz MG, Blumberg PM, et al. Differential regulation of cardiac actomyosin S-1 MgATPase by protein kinase C isozyme-specific phosphorylation of specific sites in cardiac troponin I and its phosphorylation site mutants. Biochemistry. 1996;35:14923–14931. doi: 10.1021/bi9616357. [DOI] [PubMed] [Google Scholar]
- Rohde S, Sabri A, Kamasamudran R, Steinberg SF. The alpha(1)-adrenoceptor subtype- and protein kinase C isoform-dependence of Norepinephrine's actions in cardiomyocytes. J Mol Cell Cardiol. 2000;32:1193–1209. doi: 10.1006/jmcc.2000.1153. [DOI] [PubMed] [Google Scholar]
- Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003;278:35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- Sumandea MP, Rybin VO, Hinken AC, Wang C, Kobayashi T, Harleton E, et al. Tyrosine Phosphorylation Modifies Protein Kinase C {delta}-dependent Phosphorylation of Cardiac Troponin I. J Biol Chem. 2008;283:22680–22689. doi: 10.1074/jbc.M802396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahebi S, Kobayashi T, Warren CM, de Tombe PP, Solaro RJ. Functional effects of rho-kinase-dependent phosphorylation of specific sites on cardiac troponin. Circ Res. 2005;96:740–747. doi: 10.1161/01.RES.0000162457.56568.7d. [DOI] [PubMed] [Google Scholar]
- Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]