Abstract
Progranulin has recently attracted attention due to the discovery of mutations in its encoding gene (GRN) in several cases of frontotemporal lobar degeneration, but also for a possible role in inflammatory processes. In adult central nervous system, GRN mRNA is expressed in forebrain, olfactory bulbs and spinal cord. Progranulin cerebrospinal fluid (CSF) levels were evaluated in 55 patients with multiple sclerosis (MS) as well as in 35 subjects with non-inflammatory neurological diseases (NIND), 7 individuals with other inflammatory neurological disease (OIND) and 8 controls (CON), matched for ethnic background, gender and age. No statistically significant differences were found in patients compared with either NIND, OIND or CON (P > 0.05), even stratifying according to disease subtype or gender. A positive correlation between progranulin CSF levels and age was observed in patients (ρ = 0.29, P = 0.03). According to these data, progranulin does not likely play a major role in the pathogenesis of MS.
Keywords: Multiple sclerosis, Cerebrospinal fluid (CSF), Progranulin
Multiple sclerosis (MS) is considered predominantly an inflammatory autoimmune disease of the central nervous system (CNS), with myelin proteins supposed to act as autoantigens, starting with aberrant activation of specific populations of autoreactive T lymphocytes in the periphery, followed by T cell recruitment into the brain [9]. This process leads to the activation of several inflammatory molecules, which in turn cause demyelination, eventually resulting in axonal damage [5].
Nevertheless, in chronic progressive forms of MS neurodegeneration is prominent, as demonstrated by the development of motor disability, cognitive deficits and brain atrophy. Moreover, several data support the concept that gray matter pathology may precede white matter pathology and occurs in part independently [10]. This makes conceivable that proteins already shown to be involved in more typical neurodegerative diseases, such as frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS) can play a role in the pathogenesis of MS. One of them, the 593 aminoacid secreted glycoprotein progranulin, is a possible candidate. This molecule has recently attracted attention due to the discovery of mutations in its encoding gene (GRN) in several cases of FTLD, but also for a possible role in inflammatory processes. In fact, despite the full length protein displays trophic properties, its proteolitically derived peptides (granulins) act as pro-inflammatory mediators [1].
In adult CNS, GRN mRNA is expressed in forebrain, olfactory bulbs and spinal cord [4]. Other evidence can be found about increased levels of GRN mRNA in several inflammatory neurodegenerative disorders associated with microglial activation such as ALS [7] and virally induced CNS inflammation [6].
Neuronal death is the pathological correlate of disease progression in multiple sclerosis, but the molecular mechanisms underlying neurodegeneration are not completely understood. Pathological events occurring during the development of neurodegenerative disorders could have a role also in MS. For instance, abnormal hyperphosphorylation of tau is implicated in the aetiopathogenesis of FTLD, but there is an emerging evidence of tau-positive inclusions also in chronic progressive MS [2]. Therefore, an implication of progranulin in MS could be conceivable.
Given these premises, we evaluated progranulin levels in CSF from patients with MS as compared with patients with noninflammatory neurological diseases (NIND) and with inflammatory neurological disease (OIND).
Fifty-five patients with MS (15 males and 40 females, age ± SEM: 42.91 ± 1.77) were consecutively recruited at the Multiple Sclerosis Center of Ospedale Maggiore Policlinico, Milan, Italy, and at the Department of Neurology of the Washington University, St Louis, MO, USA. All patients underwent a standard battery of examinations, including medical history, physical and neurological examinations, screening laboratory test, brain magnetic resonance imaging (MRI) and lumbar puncture (LP) at the L4/L5 or L3/L4 inter-space. All patients with MS fulfilled the McDonald's criteria [8]. The course of MS was described as relapsing remitting (RR, n = 35, 6 males and 29 females, age ± SEM: 36.29 ± 1.76), secondary progressive (SP, n = 8, 3 males and 5 females, age ± SEM: 53.75 ± 5.3) or primary progressive (PP, n = 12, 6 males and 6 females, age ± SEM: 50.42. ± 2.58). All patients with RRMS were in an acute phase of the disease.
As control groups, 35 subjects (8 males and 27 females, mean age ± SEM: 47.15 ± 2.84) with NIND, including tension type headache, depression, dizziness, ischemic stroke or small vessel disease, primary epilepsy and normal pressure hydrocephalus, 7 (4 males and 3 females, age ± SEM: 44.92 ± 4.01) with OIND, including viral meningitis, optic neuritis without demyelinating lesions at MRI, and Systemic Lupus Erithematosus, and 8 controls (CON; all of them underwent lumbar puncture for subjective symptoms with no evidence of objective pathological conditions) were recruited. All these subjects were matched for ethnic background, gender and age. All NIND individuals had either a normal brain MRI or evidence of small vessel disease or stroke, and a CSF analysis without evidence of CNS inflammation or autoimmune process such as intrathecal immunoglobulin (Ig) production or presence of oligoclonal Ig. All OIND subjects showed evidence of CNS inflammation with increased leukocytes count and protein.
An informed consent to participate in this study was given by all individuals.
Characteristics of patients and control groups are summarized in Table 1.
Table 1.
Characteristics of MS patients and control groups.
| RRMS | SPMS | PPMS | NIND | OIND | CON | |
|---|---|---|---|---|---|---|
| Number of subjects | 35 | 8 | 12 | 35 | 7 | 8 |
| Gender (M:F) | 6:29 | 3:5 | 6:6 | 8:27 | 4:3 | 4:4 |
| Age at sampling, years (mean ± SEM) | 36.29 ± 1.76 | 53.75 ± 5.3 | 50.42 ± 2.58 | 47.15 ± 2.84 | 44.92 ± 4.01 | 46.39 ± 2.58 |
| Disease duration, years (mean ± SEM) | 4.37 ± 1.30 | 16.21 ± 5.71 | 3.56 ± 1.01 | |||
| GRN CSF levels (ng/ml, mean ± SEM) | 5.72 ± 0.30 | 6.54 ± 0.46 | 6.39 ± 0.69 | 5.95 ± 0.35 | 8.20 ± 1.69 | 6.48 ± 0.65 |
Cerebrospinal fluid samples were centrifuged at 4 °C, aliquoted and stored at −80 °C until analysis. Cell counts, glucose and proteins were determined. Albumin was measured by rate nephelometry. To evaluate the integrity of the brain–blood barrier and the intrathecal IgG production, the albumin quotient (CSF albumin/serum albumin) × 103 and the IgG index (CSF albumin/serum albumin)/(CSF IgG/serum IgG) were calculated.
Progranulin levels were measured with a human specific ELISA kit (AdipoGen, Korea) according to the instruction of the manufacturer. The sensitivity of the assay was set at 32 pg/ml. In order to avoid possible variations due to multiple freezing/thawing cycles, analyses were carried out after the first thawing cycle and no more than after 1-year storage.
One-way ANOVA on ranks was used for comparisons among groups and among patients with different subtypes of the disease. T-test was used for gender-related differences. Pearson's test was used for correlations between age or disease duration and progranulin levels.
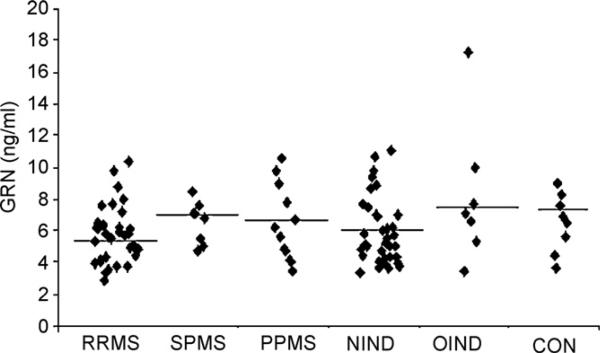
No differences were found in CSF progranulin levels in MS patients compared either with NIND or OIND (5.99 ± 0.25 ng/ml vs 5.95 ± 0.35 ng/ml or 8.20 ± 1.69 ng/ml, respectively, P > 0.05, Fig. 1) as well as with CON (6.48 ± 0.65 ng/ml, P > 0.05, Fig. 1).
Fig. 1.
Scattergram of progranulin CSF levels in different subtypes of MS, NIND, OIND and CON. Black lines represent mean values.
Progranulin CSF levels were not significantly different according to disease subtype (Fig. 1) or gender (P > 0.05, data not shown).
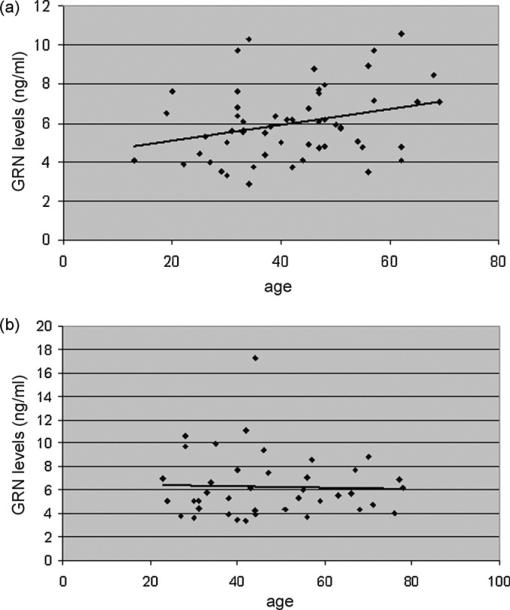
A statistically significant positive correlation was found between progranulin levels and age at sampling in patients (ρ = 0.29, P = 0.03, Fig. 2A), but not in all remaining groups (ρ = 0.07, P > 0.05, Fig. 2B). A trend towards a correlation between disease duration and progranulin levels was observed (ρ = 0.27), although the significance threshold was not reached (P = 0.09, data not shown).
Fig. 2.
Correlation between CSF progranulin levels and age (A) in patients (ρ = 0.29, P = 0.03) (B) in CON, OIND and NIND (ρ = 0.07, P > 0.05).
Stratifying patients according to the presence of active inflammation, expressed by gadolinium-enhancing lesions, a lack of significant correlation was seen.
According to these results, progranulin levels are not altered in CSF from patients with MS as compared with controls, suggesting that this protein does not play a major role in the pathogenesis of MS, either in patients with bout-onset or in PPMS.
Progranulin is an intriguing molecule as it has anti-inflammatory properties, but could be cleaved in granulins, which, conversely, are pro-inflammatory. Therefore, the balance between progranulin and granulins could theoretically have a role in inflammatory diseases, including MS.
Besides inflammation, neurodegeration is the other well-known pathological hallmark of MS and a possible relationship with progranulin levels in biologic fluids has not been clearly investigated so far. It has been demonstrated however that progranulin and granulins act as neurothropic factors that enhance neuronal survival and axonal outgrowth [11], suggesting that the lack of these factors could alter the integrity of neurites, contributing to neuronal death. In this study we demonstrated that there are no differences in progranulin CSF levels between patients and controls, but we did not consider granulins, which could indeed support neuronal survival, as demonstrated in in vitro studies [11], due to the lack of specific granulin antibodies. A bias of this study is the small size of control groups as well as the heterogeneity of controls. Therefore, a confirmation study in a larger and more homogeneous population would be needed to avoid false-negative results.
Notably, progranulin CSF levels likely increase with age in patients, possibly as an attempt to counterbalance damaging processes occurring in such a pathological condition.
In conclusion, our results do not support a major role of progranulin in MS. Nevertheless, it could have a non-specific role in MS, i.e. interacting with additional genetic or environmental factors, progranulin could theoretically contribute to the disease, in line with the recent evidence that progranulin is a “molecular generalist”, contributing to many tasks but acutely essential for few [3]. Therefore, further studies are needed before excluding any involvement of progranulin in the pathogenesis of MS.
Acknowledgements
This work was supported by grants from Bayer, IRCCS Ospedale Maggiore Policlinico Milan, grant Giovani Ricercatori 2008-Italian Ministry of Health (to DG), Monzino Foundation and Ing. Cesare Cusan.
References
- 1.Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J. Neuroinflamm. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Patani R, Reynolds R, Nicholas R, Compston A, Spillantini MG, Chandran S. Evidence for abnormal tau phosphorylation in early aggressive multiple sclerosis. Acta Neuropathol. 2009;117:583–589. doi: 10.1007/s00401-009-0515-2. [DOI] [PubMed] [Google Scholar]
- 3.Bateman A, Bennett HPJ. The granulin gene family: from cancer to dementia. BioEssays. 2009;31:1245–1254. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- 4.Daniel R, Daniels E, He Z, Bateman A. Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Dev. Dyn. 2003;227:593–599. doi: 10.1002/dvdy.10341. [DOI] [PubMed] [Google Scholar]
- 5.Galimberti D, Bresolin N, Scarpini E. Chemokine network in multiple sclerosis: role in pathogenesis and targeting for future treatments. Expert Rev. Neurother. 2004;4:439–453. doi: 10.1586/14737175.4.3.439. [DOI] [PubMed] [Google Scholar]
- 6.Johnston C, Jiang W, Chu T, Levine B. Identification of genes involved in the host response to neurovirulent alphavirus infection. J. Virol. 2001;75:10431–10445. doi: 10.1128/JVI.75.21.10431-10445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malaspina A, Kaushik N, de Belleroche J. Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. J. Neurochem. 2001;77:132–145. doi: 10.1046/j.1471-4159.2001.t01-1-00231.x. [DOI] [PubMed] [Google Scholar]
- 8.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for MS. Ann. Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 9.Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, Issekutz AC, Vestweber D, Butcher EC, Constantin G. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric Gi-linked receptors. J. Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- 10.Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- 11.Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. JBC. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]