Abstract
Context
Retinol binding protein 4 (RBP4) was recently found to be expressed and secreted by adipose tissue, and was strongly associated with insulin resistance.
Objective
The aim was to determine the relationship between RBP4 and obesity, insulin resistance, and other markers of insulin resistance in humans.
Design and Patients
RBP4 mRNA levels in adipose tissue and muscle of nondiabetic human subjects with either normal or impaired glucose tolerance (IGT) were studied, along with plasma RBP4. RBP4 gene expression was also measured in adipose tissue fractions, and from visceral and sc adipose tissue (SAT) from surgical patients.
Setting
The study was conducted at University Hospital and General Clinical Research Center.
Intervention
Insulin sensitivity (SI) was measured, and fat and muscle biopsies were performed. In IGT subjects, these procedures were performed before and after treatment with metformin or pioglitazone.
Main Outcome Measures
The relationship between RBP4 expression and obesity, SI, adipose tissue inflammation, and intramyocellular lipid level, and response to insulin sensitizers was measured.
Results
RBP4 was expressed predominantly from the adipocyte fraction of SAT. Although SAT RBP4 expression and the plasma RBP4 level demonstrated no significant relationship with body mass index or SI, there was a strong positive correlation between RBP4 mRNA and adipose inflammation (monocyte chemoattractant protein-1 and CD68), and glucose transporter 4 mRNA. Treatment of IGT subjects with pioglitazone resulted in an increase in SI and an increaseinRBP4gene expression in both adipose tissue and muscle, but not in plasma RBP4 level, and the in vitro treatment of cultured adipocytes with pioglitazone yielded a similar increase in RBP4 mRNA.
Conclusions
RBP4 gene expression in humans is associated with inflammatory markers, but not with insulin resistance. The increase in RBP4 mRNA after pioglitazone treatment is unusual, suggesting a complex regulation of this novel adipokine.
Obesity is associated with the global increase in type 2 diabetes (1, 2) and metabolic syndrome, features of which include insulin resistance, impaired insulin secretion, hepatic steatosis, dyslipidemia, and atherosclerosis (3, 4). Insulin resistance is the central feature of this syndrome (5), and many studies have demonstrated a strong link between visceral adipose tissue (VAT) and insulin resistance (6, 7). More recent studies have shown that adipose secretory products are important in the development of insulin resistance, through either a hormonal effect or local effects in adipose tissue. The accumulation of adipose tissue resident macrophages and the elaboration of inflammatory cytokines have also been implicated in the development of obesity related insulin resistance (8–11).
Adipose tissue is an active endocrine organ, secreting a number of molecules that either enhance or impair insulin action. In recent studies, retinol binding protein 4 (RBP4) has been identified as a circulating adipokine that was highly expressed in the adipose tissue of the adipocyte-specific glucose transporter 4 (Glut4) knockout mouse (12). Furthermore, insulin resistance was induced in mice that were either over-expressing RBP4 or were injected with recombinant RBP4, whereas RBP4 knockout mice showed increased insulin sensitivity (SI). In addition, RBP4 was significantly increased not only in mice with obesity and insulin resistance, but also in humans with diabetes. Treatment of Glut4 knockout mice with a thiazolidinedione (TZD) resulted in a decrease in RBP4 expression, although there was no change in RBP4 after TZD treatment of control mice, and no change in liver RBP4 expression (12). In humans, older studies found that plasma RBP4 was either elevated or unchanged in diabetic subjects (12–15). In recent studies plasma RBP4 levels were highly correlated with obesity and insulin resistance in two studies (16, 17), whereas another study showed no relationship between plasma RBP4 and insulin resistance (18). Adipose tissue RBP4 mRNA levels were measured in one study (18), but no study has determined the response of RBP4 to insulin sensitizers. To understand better the role of RBP4 in regulating SI, we studied RBP4 gene expression in adipose tissue and muscle of human subjects with either normal or impaired glucose tolerance (NGT and IGT, respectively). The relationships between RBP4 expression in adipose tissue with SI and adipose tissue inflammation markers [monocyte chemoattractant protein-1 (MCP1) and CD68] were also explored, along with the effects of the insulin sensitizers pioglitazone and metformin on RBP4 gene expression in IGT subjects.
Subjects and Methods
Human subjects
Surgical adipose tissue
Paired VAT and SAT samples were obtained from 16 subjects undergoing elective abdominal surgery. All patients signed consent under a protocol approved by the University of Maryland Institutional review board. The surgical procedures included cholecystectomy, abdominal hysterectomy, hernia repair, and other routine procedures, and ranged in age from 24–62, and body mass index (BMI) ranged from 29–76 kg/m2. These patients were generally healthy, nondiabetic, and free of inflammatory disease by medical history. Subjects taking β-blockers, steroids, or other medications likely to affect adipocyte or lipid metabolism were excluded, but one subject was taking an angiotensin-converting enzyme inhibitor to treat hypertension.
Adipose tissue and muscle biopsies
Additional SAT and muscle tissues were obtained by biopsies from healthy subjects recruited by a local advertisement. All subjects provided written, informed consent under protocols that were approved by the University of Arkansas for Medical Sciences institutional review board, and studies were conducted at the University of Arkansas for Medical Sciences/Central Arkansas Veterans Health Care System General Clinical Research Center. None of the subjects had a history of coronary artery disease, or were being treated with fibrates, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers. Subjects were included if the fasting glucose was less than 110 mg/dl, and the 2 h post-challenge glucose was less than 200 mg/dl, determined by an initial 75-g oral glucose tolerance test. Based on the oral glucose tolerance test, subjects were defined as either NGT (2-h glucose <140 mg/dl) or IGT (2-h glucose 140–199 mg/dl). A total of 86 subjects were recruited (70 women and 16 men, 21–66 yr old), of which 54 were NGT, and 32 were IGT. This study group had a wide range of BMI (19–55 kg/m2) and SI (1.02–26.77 × 10−5 × min−1/pm). All subjects were weight stable, and underwent SAT and muscle biopsies and SI testing using a frequently sampled iv glucose tolerance test, as described previously (19). IGT subjects were then randomized to receive either metformin or pioglitazone for a 2-wk dose escalation followed by 8 wk at a maximum dose (1000-mg metformin twice a day, or 45 mg of pioglitazone daily). After 10 wk of treatment, the oral and iv glucose tolerance tests and biopsies were repeated. Many of the samples used in this study were also included in previous studies (19–21).
Adipose tissue fractions and cells
Adipose cell fractions and stromal fractions were isolated from adipose tissues using a collagenase digestion, as described previously (9). Briefly, SAT was digested with collagenase, and the adipocytes were separated from the stromal vascular fractions by centrifugation. Cultured human adipocytes were also prepared by the induction of differentiation of preadipocytes. For these samples, discarded SAT was obtained from normal women undergoing liposuction (9,22). An additional source of human adipocytes was studied using a cell line, which was derived from the stromal fraction of SAT of an infant with Simpson-Golabi-Behmel syndrome (SGBS), as described previously (23). These cells were kindly provided by Dr. Martin Wabitsch (Pediatric Endocrinology, Department of Pediatrics, University of Ulm, 89075 Ulm, Germany) and cultured according to the published methods. Briefly, SGBS cells were plated and allowed to reach confluence before adding: differentiation medium DMEM:F12 with dexamethasone 25 nm (Sigma-Aldrich, St. Louis, MO); IBMX 500 mm (Sigma-Aldrich); rosiglitazone 2 mm; human transferrin 0.01 mg/ml (Sigma-Aldrich); insulin 2 × 108 m (Novo Nordisk, Princeton, NJ); cortisol 10−7 m (Sigma-Aldrich); T3 0.2 nm (Sigma-Aldrich); biotin 33 mm (Sigma-Aldrich); and pantothenate 17 mm (Sigma-Aldrich) for 4 d. Cell medium was then changed to medium without any TZD containing DMEM with human transferrin 0.01 mg/ml, insulin 2 × 108 m, cortisol 10−7 m, T3 0.2 nm, biotin 33 mm (Sigma-Aldrich), and pantothenate 17 mm (Sigma-Aldrich) for a further 4 d. These cells were then treated with pioglitazone (1.5 µm) for 24 and 48 h. Differentiation in both cell lines was assessed by Oil Red O staining, and the detection of adipocyte-specific mRNA and/or protein expression.
SI measurement
SI was measured by an insulin-modified frequently sampled iv glucose tolerance test using 11.4 g/m2-glucose and 0.04 U/kg-insulin (24). Plasma insulin was determined using a chemiluminescent assay (Molecular Light Technology Research, Ltd., Cardiff, Wales, UK), and plasma glucose was measured by a glucose oxidase assay in duplicate. SI was calculated according to the insulin and glucose data using the MINMOD Millennium program (25).
RNA isolation and real-time RT-PCR
Total RNA from human adipose tissue and cultured cells was isolated using an RNAeasy Lipid Tissue Mini kit from QIAGEN, Inc. (Valencia, CA), and total RNA from muscle biopsies was isolated using an Ultraspec RNA Isolation System kit (Biotex, Houston, TX). The quantity and quality of the isolated RNA were determined by the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA). Realtime RT-PCR was performed as described previously (9). The primer sequences of 18S, CD68, and MCP-1 were as published previously (9). The primer sequences of RBP4 were as follows: forward-GCCTCTTTCTGCAG-GACAAC and reverse-GCACACGTCCCAGTTATTCA.
Plasma RBP4 measurement
Plasma RBP4 was measured either using an ELISA kit (ALPCO Diagnostics, Salem, NH) or by Western blot with a monoclonal antibody to human RBP4 (ALPCO Diagnostics). Plasma samples were run in duplicate on a 4–20% Tris-HCL Citerion Precast Gel (Bio-Rad Laboratories, Hercules, CA,) and transferred onto a nitrocellulose membrane at 100 mV for 1 h at 4 C. Membranes were blocked for 1 h at room temperature with 5% skim milk powder in Tris-buffered saline containing 0.1% Tween 20. Anti-RBP4 (1:1000) was applied overnight at 4 C. After washes, the blot was incubated for 1 h at room temperature with horseradish peroxidase antirabbit IgG. The blot was analyzed using Lumi-Light Western blotting substrate (Roche, Indianapolis, IN) and the chemiluminescence was recorded using the Chemi-Doc XRS imager system with Quantity One 1-D analysis software (Bio-Rad Laboratories). Densitometric analyses were carried out with Scion Image software (Scion Corp., Frederick, MD).
Muscle biopsy, immunohistochemistry, and measurement of intramyocellular lipid (IMCL)
Muscle biopsies from the vastus lateralis were performed under local anesthesia, and immediately processed for fiber typing and lipid content, using Oil Red O staining and imaging as described previously (9, 19, 26). IMCL data were expressed as a percentage of lipid content in the muscle fiber by dividing the total area of lipid droplets in a muscle fiber by the total area of the same muscle fiber. To assess the oxidative capacity of the muscle, we measured the activity of succinate dehydrogenase, as described previously (19, 27).
Statistical analysis
The distributions of the variables of interest were examined for normality using quantile-quartile plots, normal probability plots, and Shapiro-Wilks’ W. Student’s two-sample t tests were used to compare groups with respect to continuous variables. Paired t tests were used to compare baseline and treatment measurements within a group. For those cases in which the data were nonnormally distributed, transformations were used to attain approximate normality before t test analysis. Specifically, natural logarithms were used to transform RBP4 (mRNA and protein), IMCL, CD68, MCP1, GLUT4, and SI data. Spearman’s rank order correlation coefficients (rS) were used to describe the linear association between variables. All data from samples were expressed as mean ± sem. P value < 0.05 was considered significant. Statistical analyses were performed using SAS (v8.02; SAS Institute Inc., Cary, NC).
Results
Detection of RBP4 in adipose tissue depots and cell fractions of adipose tissue
To compare the expression of RBP4 in different cell types, mRNA levels of RBP4 were determined using real-time RT-PCR in a variety of different human adipose tissue samples and adipose cell fractions. The expression of leptin, which is known to be differentially expressed in the adipose depot, was also examined (28, 29). To examine the relative expression of RBP4 in SAT vs. VAT, we examined 16 paired surgical samples and found that RBP4 expression was 3-fold higher in SAT vs. VAT (n = 16; P < 0.005) (Table 1). RBP4 expression was also examined in adipocytes vs. stromal cells using SAT from biopsies, as described in Subjects and Methods. As shown in Table 1, RBP4 from whole SAT or adipocyte fractions showed at least a 7-fold higher expression than that of stromal fraction (P < 0.005). To assess the expression of RBP4 during adipogenic differentiation, human preadipocytes from adult-derived human adipose stem (ADHAS) cells were induced to differentiate into adipocytes, as described in Subjects and Methods. Differentiated adipocytes expressed RBP4, although the level was not as high as SAT or adipocytes from SAT. RBP4 expression was not detectable in preadipocytes, and RBP4 expression was very low in muscle, compared with adipose tissue.
TABLE 1.
RBP4 mRNA in different adipose depots, cells, and tissue
| RBP4/18S | Leptin/18S | |
|---|---|---|
| Surgical adipose tissue depot (n = 16) | ||
| SAT | 1.76 ± 0.19 | 1.74 ± 0.21 |
| VAT | 0.58 ± 0.08a | 0.68 ± 0.12a |
| Cell (n = 2)/tissue (n = 3) | ||
| SAT | 1.76 ± 0.72 | 1.93 ± 0.62 |
| Adipocytes from SAT | 2.46 ± 0.62 | 2.43 ± 0.70 |
| Stromal fraction from SAT | 0.25 ± 0.02 | 0.25 ± 0.05 |
| Adipocytes from human preadipocytes | 0.31 ± 0.10 | 0.26 ± 0.16 |
| Cultured human preadipocytes | ND | ND |
The RNA for the surgical samples of SAT and VAT were analyzed separately from the cell/tissue samples, with a different standard curve generated by pooled cDNA. Data are mean ± SE. ND, Not detectable.
P < 0.005 vs. SAT.
Adipose tissue RBP4 expression and plasma RBP4 levels do not correlate with BMI or SI
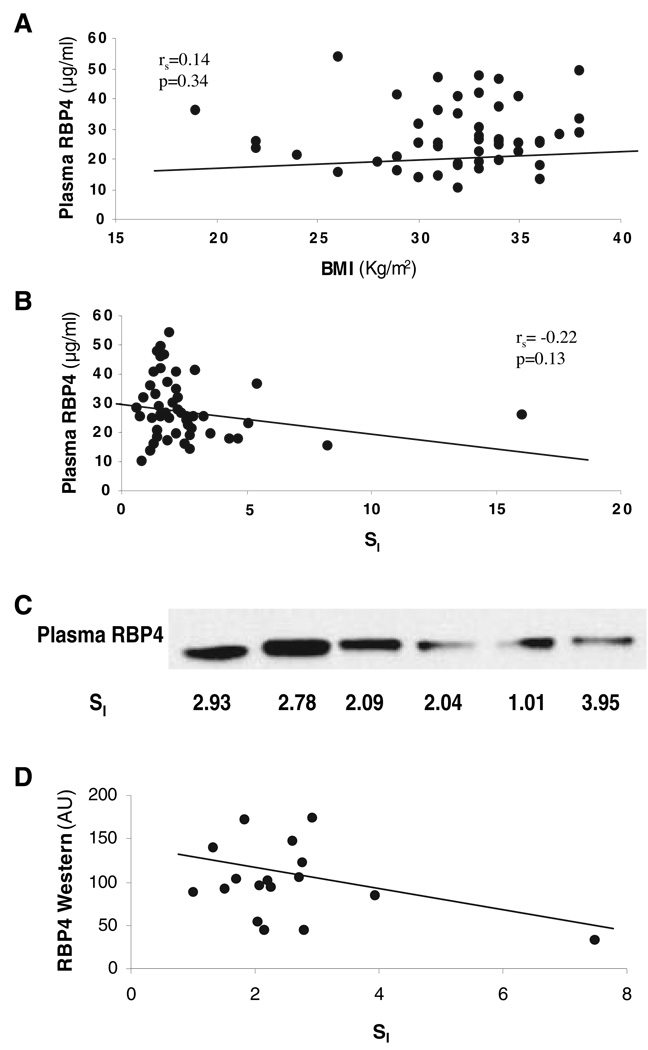
In a previous study, elevated blood RBP4 caused insulin resistance in RBP4 transgenic mice (12). Therefore, we examined the relationship between RBP4 mRNA levels from SAT biopsies of 86 subjects, covering a wide range of BMI (19–55 kg/m2) and SI (1.02–22.67 × 10−5 × min−1/pm). There was no significant relationship between SAT RBP4 expression and BMI [rs = 0.13; P = 0.25 (n = 86)]. In addition, there was no correlation between RBP4 expression and SI [rs = −0.01; P = 0.97 (n = 75)]. We also measured plasma RBP4 levels of 52 samples among these 86 subjects with ELISA assay. Similar to RBP4 gene expression, plasma RBP4 levels did not correlate with either BMI [rs = 0.14; P = 0.34 (n = 52)] or SI [rs = −0.22; P = 0.13 (n = 50)], as shown in Fig. 1, A and B. To make sure that the aforementioned results were not due to the use of plasma (rather than serum) or to other assay artifacts, we obtained fresh serum and plasma samples from 18 subjects, and these samples were analyzed using both ELISA and Western blotting. In these serum and plasma samples, there was no significant relationship between RBP4 by ELISA and either BMI or SI. Similar results were obtained using an EIA assay (ALPCO Diagnostics). Finally, plasma RBP4 levels were measured by Western blotting using monoclonal antibodies. As shown in Fig. 1C, a single band at approximately 25 kDa was identified. When the data were analyzed and quantitated by densitometry, there was no significant relationship with blood RBP4 levels and either BMI or SI (Fig. 1D).
FIG. 1.
Relationships between plasma RBP4 and BMI (A) and SI (B–D). Plasma RBP4 was measured with either ELISA assay (A and B) or Western blotting assay (C and D), as described in Subjects and Methods. The data in D panel were generated from the mean of four repeated Western blots. AU, Arbitrary units.
Although there was no statistical relationship between RBP4 and SI in a large population, we wondered whether RBP4 would be related to insulin resistance in a more homogeneous population. Therefore, we examined RBP4 expression and the RBP4 plasma level in a population that was well matched for gender, race, age, and BMI, but differed in SI. Among the 86 subjects described previously, RBP4 expression was compared among Caucasian women between the ages of 21 and 59, with a BMI between 28 and 38 kg/m2, who were either insulin resistant (SI <3 × 10−5 × min−1 /pm) or insulin sensitive (SI >3 × 10−5 × min−1/pm). As shown in Table 2, there were no differences between insulin sensitive and insulin resistance groups in BMI or age, yet there were significant differences in SI and 2-h glucose, as expected. There was no significant difference in RBP4 expression in SAT between the insulin sensitive and insulin resistance groups (Table 2). Although the average plasma RBP4 level of the insulin sensitive group (24.57 µg/ml) was lower than that in the insulin resistance group (30.81 µg/ml), it did not reach statistical significance (P = 0.07).
TABLE 2.
Characteristics of insulin-resistant and insulin-sensitive subjects
| Insulin-resistant (n = 17) |
Insulin-sensitive (n = 19) |
|
|---|---|---|
| Age (yr) | 43.4 ± 1.9 | 47.0 ± 2.0 |
| BMI (kg/m2) | 34.2 ± 0.7 | 32.5 ± 0.5 |
| SI (10−5 × min−1/pm) | 2.24 ± 0.14 | 4.65 ± 0.30a |
| Fasting insulin (pm) | 96.5 ± 22.0 | 44.3 ± 7.22b |
| Fasting glucose (mm) | 4.81 ± 0.12 | 4.77 ± 0.15 |
| 2-h glucose (mm) | 8.39 ± 0.51 | 6.91 ± 0.52b |
| Triglyceride (mm) | 1.87 ± 0.15 | 1.38 ± 0.10 |
| LDL-cholesterol (mm) | 2.75 ± 0.18 | 3.05 ± 0.26b |
| HDL-cholesterol (mm) | 1.16 ± 0.06 | 1.54 ± 0.06a |
| RBP4 mRNA/18S | 1.67 ± 0.15 | 1.99 ± 0.19 |
| Plasma RBP4 (µg/ml) | 30.8 ± 2.84 (n = 15) | 24.5 ± 1.89 (n = 16) |
HDL, High-density lipoprotein; LDL, low-density lipoprotein.
P < 0.0001 vs. insulin resistance.
P < 0.05 vs. insulin resistance.
Adipose tissue RBP4 expression levels correlate with adipose tissue GLUT4, CD68, and MCP1 expression
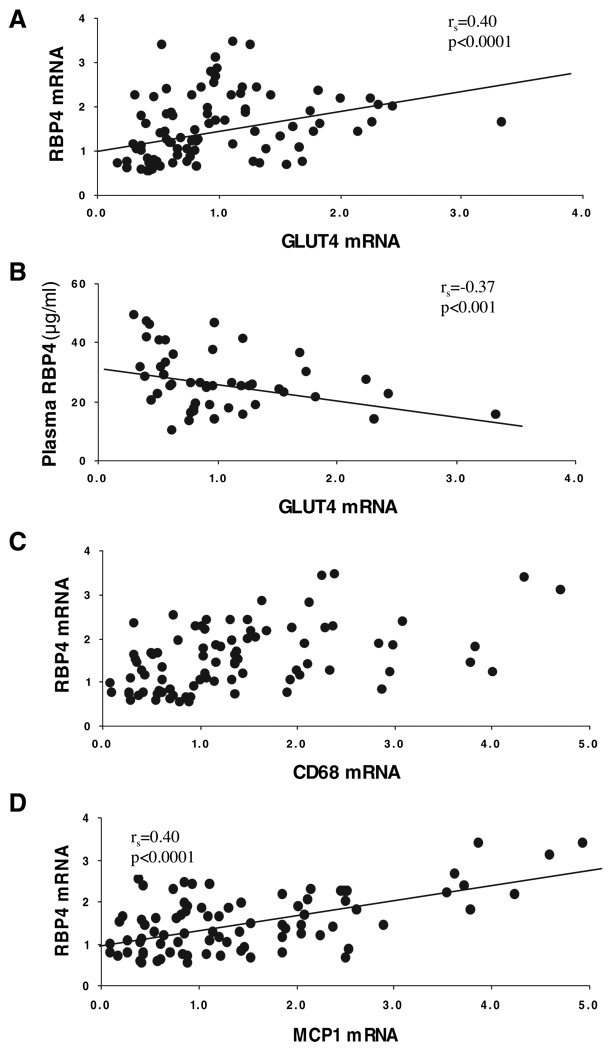
Adipose GLUT4 mRNA and protein are known to be decreased with obesity and insulin resistance (30, 31), and the subjects in this study also demonstrated a strong positive correlation between adipose GLUT4 mRNA and SI [rs =0.56; P < 0.0001 (n = 74)], and a negative correlation between adipose GLUT4 mRNA and BMI [rs = −0.34; P < 0.01 (n = 85)], supporting previous studies. Animal and human studies showed a strong negative relationship between GLUT4 expression in adipose tissue and RBP4 expression (12). However, in our studies of SAT from 85 subjects, GLUT4 mRNA showed a positive correlation with adipose RBP4 mRNA (Fig. 2A), and even after adjustment for BMI and SI, this relationship remained significant and became stronger [from rs = 0.40, P < 0.0001 (n = 85) to rs = 0.52, P < 0.0001(n = 74)]. In contrast, there was a negative relationship between adipose GLUT4 mRNA and plasma RBP4 level (Fig. 2B), and this relationship was no longer significant after BMI and SI adjustment (Table 3).
FIG. 2.
Relationship between adipose tissue GLUT4 mRNA and RBP4 expression (A) and plasma RBP4 (B). Relationship between adipose tissue RBP4 and CD68 (C) and MCP1 (D). Levels of RBP4 mRNA, GLUT4 mRNA, MCP1 mRNA, and CD68 mRNA were determined by real-time RT-PCR as described in Subjects and Methods. Levels of plasma RBP4 were determined by ELISA assay.
TABLE 3.
Spearman’s correlation coefficients of RBP4 with BMI, SI, IMCL, GLUT4, CD68, and MCP1
| Adipose tissue RBP4 Mrna (n) |
Adipose tissue RBP4 mRNA after adjustment with BMI and SI (n) |
Plasma RBP4 (n) |
Plasma RBP4 after adjustment with BMI and SI (n) |
|
|---|---|---|---|---|
| BMI | 0.13 (86) | N/A | 0.14 (52) | N/A |
| SI | −0.01 (75) | N/A | −0.22 (50) | N/A |
| IMCL type 1 fiber | 0.28 (59)a | 0.28 (55)a | 0.18 (42) | 0.05 (40) |
| IMCL type 2 fiber | 0.21 (58) | 0.26 (54) | 0.30 (42) | 0.19 (40) |
| GLUT4 Mrna | 0.40 (85)b | 0.52 (74)b | −0.37 (48)c | −0.21 (46) |
| CD68 mRNA | 0.48 (85)b | 0.50 (74)b | 0.04 (47) | 0.004 (45) |
| MCP1 mRNA | 0.40 (85)b | 0.39 (75)d | 0.005 (48) | −0.05 (46) |
P < 0.05.
P < 0.0001.
P < 0.01.
P < 0.001.
In previous studies, IMCL was associated with insulin resistance. However, there was only a weak association between RBP4 mRNA and IMCL in type 1 fibers, and no relationship between IMCL and blood RBP4 (Table 3).
Earlier studies showed that macrophage infiltration of adipose tissue was associated with obesity and insulin resistance (8–10, 27). To explore the possible relationship between RBP4 and macrophage infiltration in adipose tissue, we measured the expression of CD68, which is a macrophage marker, as well as the expression of MCP1, which is expressed by macrophages, as well as other cells (9). In the 85 adipose tissue samples examined, there was a strong correlation between the expression of RBP4 and both CD68 (rs = 0.48; P < 0.0001) and MCP1 (rs = 0.40; P < 0.0001), as shown in Fig. 2, C and D. The relationships between RBP4 and CD68, and between RBP4 and MCP1 remained significant after adjustment for BMI and SI (Table 3). Therefore, RBP4 expression was strongly linked to inflammation in adipose tissue. However, circulating RBP4 level was not correlated with either CD68 or MCP1 mRNA.
Pioglitazone increased RBP4 expression in adipose and muscle tissue, but not plasma RBP4
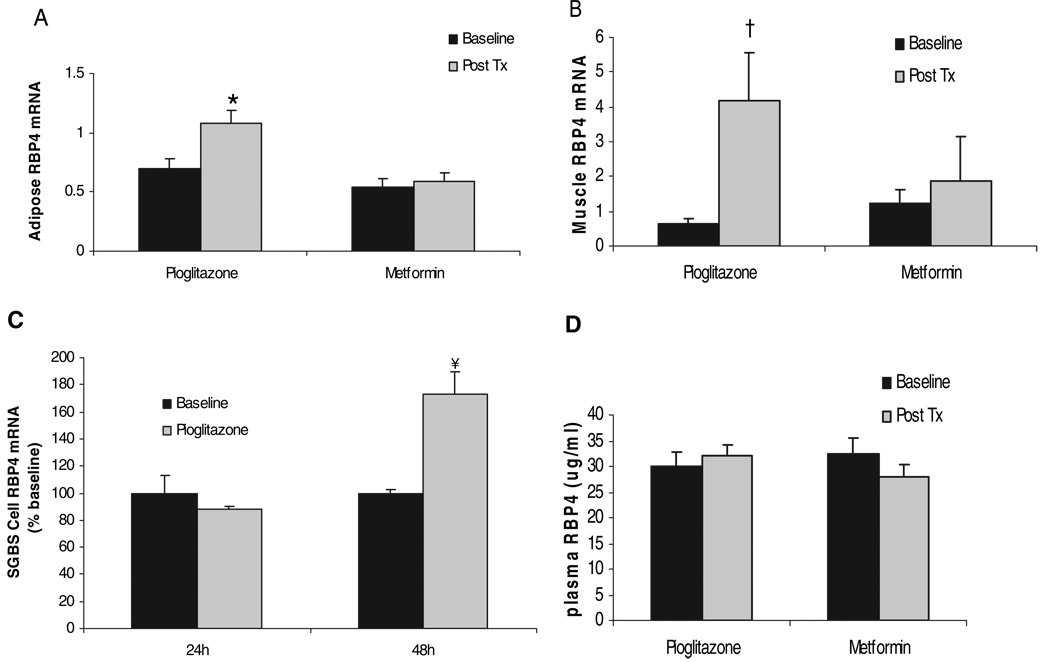
When adipose-Glut4−/− transgenic mice were treated with rosiglitazone, the elevated RBP4 mRNA levels were reduced in adipose tissue, in parallel with increased SI and glucose intolerance (12, 32). To determine whether pioglitazone had similar effects on RBP4 expression in humans, 39 IGT subjects were randomized to receive treatment for 10 wk with either pioglitazone or metformin. These subject characteristics have been described previously (19). Mean BMI was 33 kg/m2, and age was 48 yr, and there were no significant differences in baseline characteristics between pioglitazone and metformin groups. Treatment with pioglitazone, but not metformin, increased SI from 2.93 to 4.04 × 10−5 min−1/pm (P = 0.005).
RBP4 mRNA levels were determined using real-time RT-PCR in adipose tissue and muscle obtained from subjects before and after treatment. As shown in Fig. 3A, pioglitazone treatment resulted in a 56% (n = 18; P = 0.001) increase of RBP4 mRNA level, whereas metformin treatment had no effect on RBP4 expression. In a similar fashion, treatment with pioglitazone, but not metformin, resulted in an increase in RBP4 expression in muscle tissue of more than 6-fold (P = 0.02) (Fig. 3B). To examine further the effects of pioglitazone on RBP4, we studied the effect of pioglitazone on RBP4 expression in vitro in cultured human SGBS adipocytes. As shown in Fig. 3C, pioglitazone yielded an increase of RBP4 expression after 48-h treatment. Thus, these data in differentiated SGBS adipocytes reflected those demonstrated in vivo and suggested a direct effect of pioglitazone on RBP4 mRNA expression. However, plasma RBP4 levels, measured with the ELISA method from the same subjects, did not show a significant increase after pioglitazone treatment (Fig. 3D).
FIG. 3.
Effects of pioglitazone and metformin treatment on RBP4 expression in adipose tissue (A), muscle tissue (B), differentiated SGBS adipocytes (C), and on plasma RBP4 level (D). *, P = 0.001 vs. baseline of adipose tissue. †, P = 0.02 vs. baseline of muscle tissue. ¥, P < 0.05 vs. baseline of SGBS cells. Post Tx, After treatment.
Discussion
Recent studies in mice revealed the novel hypothesis that RBP4 could be an important new adipokine, and would explain much of the adipocyte-muscle connection that links obesity and insulin resistance (33). Although RBP4 was thought to function only to deliver retinol to peripheral tissues (34, 35), microarray studies found high levels of RBP4 expression in the adipose tissue of insulin-resistant GLUT4 knockout mice (12). Overexpression of RBP4 led to insulin resistance, and the deletion or reduction of RBP4 levels led to improved SI. In addition, blood levels of RBP4 were elevated in several mouse models of insulin resistance, and were elevated in human subjects with obesity and diabetes. These data suggested that adipose tissue RBP4 had systemic effects on peripheral SI, either directly through the delivery of retinol or other lipids to peripheral tissues, or through the production of other adipokines. Others have suggested that adipose tissue, through RBP4, acts as a fuel sensor, such that the decrease in glucose uptake, as would occur in the GLUT4 knockout mouse, results in the secretion of RBP4 to subsequently decrease muscle glucose uptake (12).
To understand better the role of RBP4 in humans with varying degrees of insulin resistance and obesity, we measured RBP4 mRNA levels in nondiabetic humans covering a wide range of BMI and insulin resistance. RBP4 expression was highest in SAT, and was present only at low levels in the stromal fraction of SAT, and was not detectable in cultured preadipocytes. There was no significant relationship between either RBP4 mRNA level or plasma RBP4 level with either BMI or SI, and RBP4 expression and protein level in plasma in insulin sensitive subjects was not significantly different from insulin resistance subjects. These findings were confirmed with both plasma and serum samples using both Western and ELISA assays to measure the RBP4 protein.
As described previously, blood RBP4 is highly associated with insulin resistance in rodents (12), and in some (16, 17), but not all (18), human studies. It is not clear why such differences are present among similarly conducted human studies. The inclusion of diabetic subjects could alter the regulation of RBP4. However, both our study and others (16–18) included nondiabetics. Different study populations and ethnic groups could explain some of the differences. Our subjects were mostly Caucasian women, whereas the subjects reported by Cho et al. (17) were drawn from an Asian population, and most of the subjects in the study by Graham et al. (16) were men. To be sure that the absence of a correlation between blood RBP4 and SI was not due to sample preparation or the ELISA, we reanalyzed fresh serum and plasma samples with both ELISA and Western blots, and still found no significant correlation between RBP4 and BMI or SI. As noted by Janke et al. (18), adipose RBP4 mRNA and blood RBP4 levels correlated poorly, suggesting that blood RBP4 is secreted by another tissue, or is subject to modification in plasma, or is regulated posttranscriptionally. Neither this study nor others have measured cellular RBP4 protein in humans, and, therefore, the extent of posttranscriptional regulation is not known.
RBP4 was originally identified as an insulin resistance gene by its elevated levels in Glut4 knockout mice. Therefore, it follows that there was a strong correlation noted between adipose tissue expression of GLUT4 and RBP4. Together, these data suggest an important link between RBP4 and GLUT4, although not necessarily with insulin resistance.
Insulin resistance is part of a larger syndrome, which includes the infiltration of macrophages into adipose tissue, the expression of inflammatory cytokines, and the accumulation of lipid in ectopic sites (9–11, 36, 37). Although there were only weak associations between RBP4 and IMCL, there were significant associations with inflammation. Inflammatory pathways are activated in adipose tissue in many insulin-resistant states, and the adipose tissue of obesity contains increased number of resident macrophages, which is the potential source of circulating cytokines and/or chemokines (10, 11). Macrophage infiltration into adipose tissue is likely increased by the secretion of chemotactic molecules, such as MCP1, which is expressed by macrophages, as well as by adipocytes and other cells, especially in obese, insulin-resistant subjects (9, 38, 39). As described previously, there was a significant positive relationship between adipose tissue CD68 and RBP4 expression, and also a significant relationship between adipose MCP1 and RBP4 expression. Adipose tissue RBP4 protein was not measured, and, therefore, it is not known whether inflammatory markers are associated with tissue RBP4 protein. CD68 is a macrophage surface marker, and the expression of CD68 correlates with SI, and with the production of TNFα and IL-6 (9). Thus, adipose tissue RBP4 expression was not associated with SI but was associated with important physiological features of insulin resistance, including adipose tissue inflammation.
TZDs are peroxisome proliferator-activated receptor γ agonists that improve peripheral SI through a reduction of inflammation, a reduction in ectopic lipid accumulation, and other effects (36). We examined the effects of pioglitazone treatment on adipose tissue and muscle RBP4 expression and plasma RBP4 in subjects with IGT. Based on the known improvement in features of the insulin resistance syndrome that occur with TZD treatment, one may have expected that pioglitazone treatment would decrease adipose RBP4 expression. Indeed, when Glut4−/− mice were treated with rosiglitazone, the mice demonstrated a reduced Rbp4 mRNA level, and improved SI and glucose tolerance (12). However, we observed the opposite effects: a significant increase in both adipose and muscle RBP4 expression after the pioglitazone treatment of IGT humans. When pioglitazone was added to cultured adipocytes, an increase in RBP4 mRNA occurred, suggesting that this is a direct effect on the adipocyte, and not secondary to systemic effects of the drug. Within adipose tissue, RBP4 was expressed mainly in adipocytes in a differentiation-dependent manner, and this differentiation-dependent expression of RBP4 has also been shown previously in adipocytes (40). A steady increase in RBP4 secretion from adipose during adipogenesis had been reported (18). Therefore, peroxisome proliferator-activated receptor γ activated by pioglitazone may trigger adipocyte differentiation, thereby increasing RBP4 expression.
These data in human subjects support an association between RBP4 and some feature of the metabolic syndrome but demonstrated no correlation between RBP4 and BMI or SI. However, the response to pioglitazone was more difficult to reconcile with a putative role of RBP4 as an insulin resistance factor. It is possible that RBP4 is not directly involved in the development of insulin resistance in humans but is expressed in response to other phenomena associated with the insulin resistance syndrome. These data also suggest that the regulation of SI in a cross section of a human population is very different from the response to an insulin sensitizer drug.
In summary, we examined RBP4 expression in a broad spectrum of nondiabetic human subjects, and found strong positive correlation between RBP4 and adipose inflammation, but no significant relationship with BMI or SI. Treatment of IGT subjects with pioglitazone resulted in a somewhat paradoxical increase in RBP4 expression in both adipose tissue and muscle, despite an increase of SI. Together, these studies do not support a definitive role for RBP4 in the regulation of insulin resistance but suggest that the role of this adipocyte protein in humans is complex and may be associated with adipose tissue inflammation.
Acknowledgments
This work was supported by a merit review grant from the Veterans Administration (to P.A.K. and N.R.); Grant M01RR14288 of the General Clinical Research Center; Grants DK 39176 and DK 71277 (to P.A.K.), DK 71346 (to R.E.M.), and DK 52398 (to S.K.F.) from the National Institutes of Health, and the American Diabetes Association (to S.K.F).
Abbreviations
- BMI
Body mass index
- GLUT
glucose transporter
- IGT
impaired glucose tolerance/tolerant
- IMCL
intramyocellular lipid
- MCP1
monocyte chemoattractant protein-1
- NGT
normal glucose tolerance/tolerant
- RBP4
retinol binding protein 4
- rs
Spearman’s rank order correlation coefficients
- SAT
sc adipose tissue
- SGBS
Simpson-Golabi-Behmel syndrome
- SI
insulin sensitivity
- TZD
thiazolidinedi-one
- VAT
visceral adipose tissue
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad A, Serdula MK, Dietz WH, Bowman B, Marks J, Koplan J. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 3.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 4.Karelis AD, St. Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 6.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87:5044–5051. doi: 10.1210/jc.2002-020570. [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27:S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 9.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 13.Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997;16:39–45. doi: 10.1080/07315724.1997.10718647. [DOI] [PubMed] [Google Scholar]
- 14.Abahusain MA, Wright J, Dickerson JW, de Vol EB. Retinol, α-tocopherol and carotenoids in diabetes. Eur J Clin Nutr. 1999;53:630–635. doi: 10.1038/sj.ejcn.1600825. [DOI] [PubMed] [Google Scholar]
- 15.Mohan V, Snehalatha C, Bhattacharyya PK, Ramachandran A, Viswanathan M. Nutritional profile of fibrocalculous pancreatic diabetes and primary forms of diabetes seen in southern India. Diabetes Res Clin Pract. 1991;11:203–207. doi: 10.1016/s0168-8227(05)80034-8. [DOI] [PubMed] [Google Scholar]
- 16.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 17.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 18.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 19.Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930–E934. doi: 10.1152/ajpendo.00522.2004. [DOI] [PubMed] [Google Scholar]
- 20.Varma V, Yao-Borengasser A, Rasouli N, Bodles AM, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ, III, McGehee RE, Jr, Fried SK, Kern PA. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab. 2007;92:666–672. doi: 10.1210/jc.2006-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao-Borengasser A, Rasouli N, Varma V, Miles LM, Phanavanh B, Starks TN, Phan J, Spencer HJ, III, McGehee RE, Jr, Reue K, Kern PA. Lipin expression is attenuated in adipose tissue of insulin-resistant human subjects and increases with peroxisome proliferator-activated receptor γ activation. Diabetes. 2006;55:2811–2818. doi: 10.2337/db05-1688. [DOI] [PubMed] [Google Scholar]
- 22.Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metab Clin Exp. 2001;50:407–413. doi: 10.1053/meta.2001.21690. [DOI] [PubMed] [Google Scholar]
- 23.Wabitsch M, Brenner RE, Melzner I, Braun M, Moller P, Heinze E, Debatin KM, Hauner H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord. 2001;25:8–15. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- 24.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 26.Malenfant P, Joanisse DR, Theriault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord. 2001;25:1316–1321. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- 27.Kern PA, Simsolo RB, Fournier M. Effect of weight loss on muscle fiber type, fiber size, capillarity, and succinate dehydrogenase activity in humans. J Clin Endocrinol Metab. 1999;84:4185–4190. doi: 10.1210/jcem.84.11.6090. [DOI] [PubMed] [Google Scholar]
- 28.Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, Arner P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 29.Van Gaal LF, Wauters MA, Mertens IL, Considine RV, De Leeuw IH. Clinical endocrinology of human leptin. Int J Obes Relat Metab Disord. 1999;23 Suppl 1:29–36. doi: 10.1038/sj.ijo.0800792. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001;15:1101–1103. [PubMed] [Google Scholar]
- 32.Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest. 2004;114:1666–1675. doi: 10.1172/JCI21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamori Y, Sakaue H, Kasuga M. RBP4, an unexpected adipokine. Nat Med. 2006;12:30–31. doi: 10.1038/nm0106-30. [DOI] [PubMed] [Google Scholar]
- 34.Lenhard JM, Weiel JE, Paulik MA, Furfine ES. Stimulation of vitamin A(1) acid signaling by the HIV protease inhibitor indinavir. Biochem Pharmacol. 2000;59:1063–1068. doi: 10.1016/s0006-2952(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 35.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasouli N, Molavi B, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab. 2007;9:1–10. doi: 10.1111/j.1463-1326.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 37.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 38.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes Relat Metab Disord. 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 39.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zovich DC, Orologa A, Okuno M, Kong LW, Talmage DA, Piantedosi R, Goodman DS, Blaner WS. Differentiation-dependent expression of retinoid-binding proteins in BFC-1 β adipocytes. J Biol Chem. 1992;267:13884–13889. [PubMed] [Google Scholar]