Abstract
Context
Visfatin (VF) is a recently described adipokine preferentially secreted by visceral adipose tissue (VAT) with insulin mimetic properties.
Objective
The aim of this study was to examine the association of VF with insulin sensitivity, intramyocellular lipids (IMCL), and inflammation in humans.
Design and Patients
VF mRNA was examined in paired samples of VAT and abdominal sc adipose tissue (SAT) obtained from subjects undergoing surgery. Plasma VF and VF mRNA was also examined in SAT and muscle tissue, obtained by biopsy from well-characterized subjects with normal or impaired glucose tolerance, with a wide range in body mass index (BMI) and insulin sensitivity (SI).
Setting
The study was conducted at a University Hospital and General Clinical Research Center.
Intervention
SI was measured, and fat and muscle biopsies were performed. In impaired glucose tolerance subjects, these procedures were performed before and after treatment with pioglitazone or metformin.
Main Outcome Measures
We measured the relationship between VF and obesity, SI, adipose tissue inflammation, IMCL, and response to insulin sensitizers.
Results
No significant difference in VF mRNA was seen between SAT and VAT depots. VAT VF mRNA associated positively with BMI, whereas SAT VF mRNA decreased with BMI. SAT VF correlated positively with SI, and the association of SAT VF mRNA with SI was independent of BMI. IMCL and markers of inflammation (adipose CD68 and plasma TNFα) were negatively associated with SAT VF. Impaired glucose tolerance subjects treated with pioglitazone showed no change in SAT VF mRNA despite a significant increase in SI. Plasma VF and muscle VF mRNA did not correlate with BMI or SI or IMCL, and there was no change in muscle VF with either pioglitazone or metformin treatments.
Conclusion
SAT VF is highly expressed in lean, more insulin-sensitive subjects and is attenuated in subjects with high IMCL, low SI, and high levels of inflammatory markers. VAT VF and SAT VF are regulated oppositely with BMI.
Obesity is highly associated with insulin resistance and the increased risk to type 2 diabetes and cardiovascular diseases (1). The accumulation of adipose tissue in the abdominal visceral depot is especially correlated with insulin resistance (2). Several recent studies have demonstrated that adipose tissue secretes different proteins, which likely have an important role in the development of obesity-related complications, especially insulin resistance.
Visfatin (VF) is a recently described adipokine that was reported to be highly expressed by visceral adipose tissue (VAT) both in humans and mice (3). Among its actions, VF has been demonstrated to mimic the action of insulin by activating the insulin signal transduction pathway, achieved by binding to the insulin receptor at a site different from that of insulin (3). VF was originally identified as a growth factor for B cell proliferation, pre-B cell colony enhancing factor (PBEF) in lymphocytes. PBEF was subsequently shown to be more ubiquitously expressed by many cells and tissues such as neutrophils, liver, heart, and muscle (4, 5) and to be associated to different functions (6, 7, 8, 9).
Initial studies of VF in humans have obtained results that are somewhat conflicting. Whereas one study showed predominant expression of VF by visceral fat (3), another study found no difference between VF expression in VAT and sc adipose tissue (SAT) (10). A number of studies have examined the relationship between obesity and VF plasma levels or mRNA expression. Although one study found a significant positive association between plasma VF and body mass index (BMI) (10), this has not been confirmed by another study (11). Berndt et al. (10) found only VAT VF mRNA to associate with obesity while SAT VF mRNA did not show any association. Furthermore, on examining the association between VF and insulin sensitivity (SI), they found that neither plasma nor mRNA levels of VF from either VAT or SAT showed any relationship to parameters of SI (10). Circulating VF and SAT VF mRNA levels have been described to be higher in type 2 diabetic subjects in a few studies (11, 12, 13). Further studies are required to obtain a better understanding of the role of VF in obesity induced insulin resistance.
In this study, we examined VF expression in adipose tissue and muscle in addition to the plasma levels of VF in a well-characterized group of subjects with either normal (NGT) or impaired glucose tolerance (IGT) and the association of VF with markers of insulin resistance such as intramyocellular lipids (IMCL) and markers of inflammation such as plasma TNFα and CD68 mRNA. We also examined the effects of two insulin sensitizers with different mechanisms of action, metformin and pioglitazone, on VF expression in IGT subjects.
Subjects and Methods
Human subjects: group 1—adipose tissue from surgery
Paired samples of VAT and SAT were obtained from 15 subjects undergoing elective abdominal surgery. This protocol was approved by the University of Maryland Institutional Review Board, and all patients signed consent for removal of fat during surgery. The surgical procedures included gastric bypass or restriction to treat obesity, cholecystectomy, abdominal hysterectomy, hernia repair, and other routine procedures. Adipose tissue specimens were obtained early in the surgical procedure, samples for RNA extraction were frozen on dry ice immediately after excision. The subjects ranged in age from 24 to 62 yr, and BMI ranged from 29 to 76 kg/m2. These patients were nondiabetic and were free of any neoplastic or major disease by medical history. Three patients had hypertension and were taking medications. In addition, one other patient was taking celecoxib and montelukast.
Group 2—adipose tissue and muscle from biopsies
Additional SAT and muscle tissue were obtained from healthy subjects by biopsy. Nondiabetic subjects were recruited by local advertisement, and all subjects provided written, informed consent under protocols that were approved by the local Institutional Review Board, and studies were conducted at the University of Arkansas for Medical Sciences General Clinical Research Center. None of the subjects had a history of coronary artery disease or were being treated with antiinflammatory medications, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers. Subjects were included if the fasting glucose was less than 126 mg/dl, and the 2-h postchallenge glucose was less than 200 mg/dl, determined by an initial 75-g oral glucose tolerance test. Based on the oral glucose tolerance test, subjects were defined as either NGT (2-h glucose <140 mg/dl) or IGT (2-h glucose 140–199 mg/dl). A total of 75 subjects were recruited (64 women and 11 men, 44.4 ± 1.3 yr of age), including both NGT and IGT subjects who had a wide range of BMI (19–55 kg/m2), percent body fat of (15.5–54.1%), and SI (0.62–26.8 × 10−5 × min−1/pm). All subjects were weight stable and underwent SAT and muscle biopsies, as described previously (14). Body fat was measured by dual x-ray absorbtiometry.
Although the total sample size of this study included 75 subjects, data were unavailable on some subjects for some measurements. Hence, the number of subjects reported for each figure is less than the total sample size.
Group 1 subjects underwent surgery, whereas group 2 subjects, who were volunteers, underwent biopsy. Because of differences between surgical subjects and subject volunteers and the possible effects of anesthesia, data from the two groups cannot be combined and were hence analyzed separately. In this study, mRNA from surgical adipose tissue was only used to examine differential distribution between adipose depots and in examining the association of VAT VF with BMI. For all other associations the larger and better-characterized group II subjects were used.
Adipose tissue and cells
Adipocyte and stromal fractions were isolated from adipose tissue as described previously (15). Briefly, SAT was digested with collagenase, and the adipocytes were separated from the stromal vascular fraction by centrifugation. Cultured human adipocytes were prepared by the induction of differentiation of preadipocytes obtained from de-identified adipose tissue from normal women (BMI < 30 kg/m2) undergoing liposuction, as described previously (15, 16). Differentiation was assessed by Oil Red O staining and the detection of adipocyte-specific mRNA and/or protein expression.
SI measurement
SI was measured by an insulin-modified frequently sampled iv glucose tolerance using 11.4 g/m2 of glucose and 0.04 U/kg of insulin (17). Plasma insulin was determined using a chemoluminescent assay (Molecular Light Technology Research, Ltd., Cardiff, Wales, UK), and plasma glucose was measured by a glucose oxidase assay in duplicate. Insulin sensitivity was calculated according to the insulin and glucose data using the MinMod program (17).
Total RNA isolation and real-time RT-PCR
Total RNA from adipose tissue was isolated using an RNAeasy Lipid Tissue Mini kit from QIAGEN (Valencia, CA), following the manufacturer’s instruction. Total RNA from muscle biopsy samples was isolated using an Ultraspec RNA Isolation System kit from Biotex (Houston, TX), according to the manufacturer’s instruction. The quantity and quality of the isolated RNA was determined by Agilent 2100 Bioanalyser (Palo Alto, CA). One microgram of total RNA was reverse-transcribed using random hexamer primers with TaqMan RT Reagents (Applied Biosystems, Foster City, CA). Reverse-transcribed RNA was amplified with 1X SYBR Green PCR Master Mix (Applied Biosystems) plus 0.3 µm of gene-specific upstream and downstream primers during 55 cycles on a Rotor-Gene 3000 Real-time Thermal Cycler (Sydney, Australia). Each cycle consisted of denaturation at 94 C for 20 sec, annealing at 58 C for 20 sec, and extension at 72 C for 20 sec. Amplified 18S ribosomal RNA expression was used as a standard to normalize the differences in individual samples. All data were expressed in relation to 18S RNA, where the standard curves was generated using pooled RNA from the samples assayed. The primers used were as follows: 18s: 5′-TTCGAACGTCT-GCCCTATCAA-3′ (forward), 5′-ATGGTAGGCACGGCGACTA-3′ (reverse); human VF: 5′-GGCCACAAATTCTAGAGAGCAG-3′ (forward), 5′-CCAAGTGAGCAGATGCTCCTAT-3′ (reverse); human leptin: 5′-CCAAACCGGTGACTTTCTGT-3′ (forward), 5′-TGACACCAAAACCC-TCATCA-3′ (reverse); human CD68: 5′-GCTACATGGCGGTGGAGTA-CAA-3′ (forward), 5′-ATGATGAGAGGCAGCAAGATGG-3′ (reverse).
Plasma cytokine measurements
VF concentration in plasma was quantified using the human VF C-terminal Enzyme Immunoassay (Phoenix, Belamont, CA). Plasma TNFα was quantified by ELISA (R&D Systems, Minneapolis, MN).
Muscle biopsy, immunohistochemistry, and measurement of IMCL
Muscle biopsy samples from the vastus lateralis were performed under local anesthesia and immediately processed for fiber typing and lipid content (IMCL) as described previously (14). Muscle lipid content was determined by Oil Red O staining (18, 14), and images of the muscle sections analyzed using the NIH Image program version 1.60. The total area of lipid droplets in a muscle fiber was divided by the total area of the same muscle fiber, yielding a percentage of lipid content in the muscle fiber. Muscle oxidative capacity was assessed by measuring the activity of succinate dehydrogenase (SDH) as described previously (14, 19).
Statistical analyses
Student’s two-sample t tests were used to compare groups with respect to continuous variables, and paired t tests were used to compare baseline and treatment measurements within a group. Pearson’s correlation coefficients were used to describe the linear association between variables. The distributions of the variables of interest were examined using quantile-quantile plots. Where the data were not normally distributed (VF mRNA, SI, plasma TNFα, and IMCL), natural logarithm transformations were used to attain approximate normality before analysis. All data from samples are expressed as mean ± sem. P ≤ 0.05 was taken to indicate statistical significance.
Results
VF expression profile in adipose tissue depots and cell fractions of adipose tissue
To compare the expression of VF between VAT and SAT, paired samples of VAT and SAT obtained from 15 subjects during elective surgery were analyzed. VF mRNA was measured by real-time PCR as described in Subjects and Methods and normalized to 18S rRNA. The expression of leptin was also measured as a comparison. As shown in Table 1, there was no significant difference in the expression of VF between VAT and SAT depots. However, the expression of leptin was significantly higher in SAT, in agreement with previous studies (20, 21).
TABLE 1.
VF mRNA in different adipose depots, cells, and tissue
| VF | Leptin | |
|---|---|---|
| Surgical adipose tissue depots | ||
| SAT (n = 15) | 0.61 ± 0.18 | 1.74 ± 0.21 |
| VAT (n = 15) | 0.93 ± 0.35 | 0.68 ± 0.12a |
| Cell/biopsy tissue | ||
| SAT (n = 3) | 0.95 ± 0.28 | 1.93 ± 0.62 |
| Adipocytes from SAT (n = 14) | 0.52 ± 0.30 | 2.43 ± 0.70 |
| Stromal fraction from SAT (n = 14) | 1.40 ± 0.13b | 0.25 ± 0.05 |
| Adipocytes from cultured preadipocytes (n = 2) |
1.99 ± 0.46 | 0.26 ± 0.16 |
| Cultured preadipocytes (n = 2) | 0.91 ± 0.17 | ND |
| Muscle tissue (n = 3) | 0.48 ± 0.21 | ND |
The RNA for the surgical samples of SAT and VAT were analyzed separately from the cell/biopsy tissue samples with a different standard curve generated from pooled cDNA in order to compare different tissue or cell fractions better. Data are mean ± se. These numbers represent VF mRNA/18s ratio and have no units. ND, Not detectable.
P < 0.005 vs. SAT;
P < 0.001 vs. adipocytes from SAT.
Adipose tissue is comprised of adipocytes, as well as a number of cell types in the stromal vascular fraction, such as preadipocytes, macrophages, and endothelial cells. To examine the expression of VF in different cell fractions of adipose tissue, SAT obtained by biopsy was digested with collagenase and VF expression was measured in the adipocyte and in the stromal vascular fraction. As shown in Table 1, VF was expressed in adipose tissue and also in muscle. Within adipose tissue, both the adipocyte and stromal vascular fractions expressed VF. However, the stromal vascular fraction of adipose tissue expressed significantly higher VF mRNA than the adipocyte fraction. In adipocytes from cell culture, VF was expressed by both differentiated adipocytes and preadipocytes (Table 1).
Association between VF and obesity
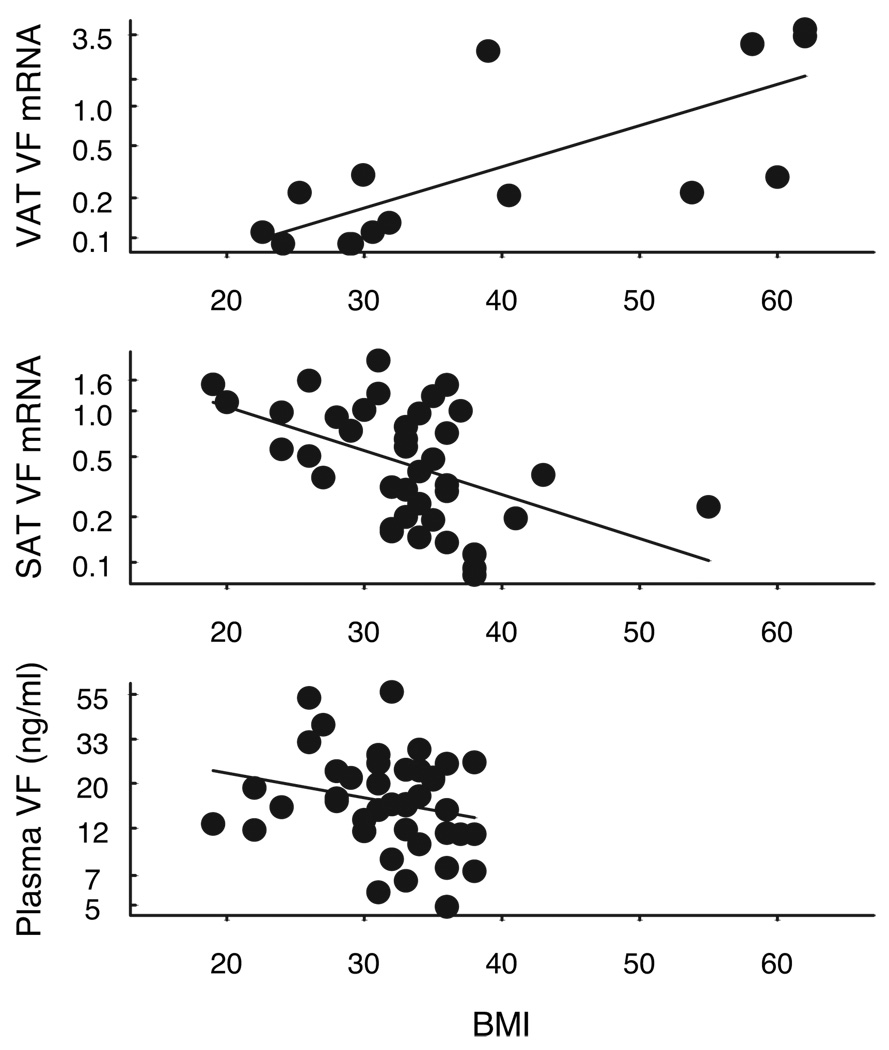
We examined the relationship between obesity (using BMI) and VF in both the surgical cohort of 15 paired samples (group I), as well as in the larger group of subjects in whom only SAT was obtained (group II). The relationship between VAT VF (group I) and BMI is shown in Fig. 1, top. There was a significant linear relationship between BMI and VAT VF mRNA (r = 0.75, P < 0.001, n = 15). To examine the relationship between BMI and VF from SAT, SAT VF mRNA was examined in 39 well-characterized subjects from group II including 36 women, 3 men, age 42 ± 1.7 yr, with either NGT or IGT and having a wide range of BMI (19–55 kg/m2, % body fat 15.5–54.1%) who had SAT biopsies as outpatients. As shown in Fig. 1, middle, BMI correlated negatively with SAT VF expression (r = −0.48, P = 0.002, n = 39). A similar relationship was found using percent body fat as an indicator of obesity (r = −0.32, P < 0.05, n = 39). Thus, whereas SAT VF mRNA was negatively regulated in obesity, VAT VF mRNA showed positive association attributable to some very obese subjects expressing high levels of VF. As shown in Fig. 1, bottom, plasma VF, from group II subjects, showed a negative trend with BMI (r = −0.23, P = 0.14, n = 41), but the relationship was not statistically significant.
FIG. 1.
Relationship between VF mRNA expression and BMI. Top, VAT VF mRNA expression and BMI (n = 15). Middle, SAT VF mRNA expression and BMI (n = 39). VF mRNA was determined by real time RT-PCR analysis and was expressed in relation to endogenous 18S RNA. Bottom, Relationship between plasma VF and BMI (n = 29). Plasma VF was measured by ELISA as described in Subjects and Methods. The natural log of VAT VF mRNA, SAT VF mRNA, and plasma VF, respectively, was plotted against BMI.
SAT VF expression is associated positively with SI
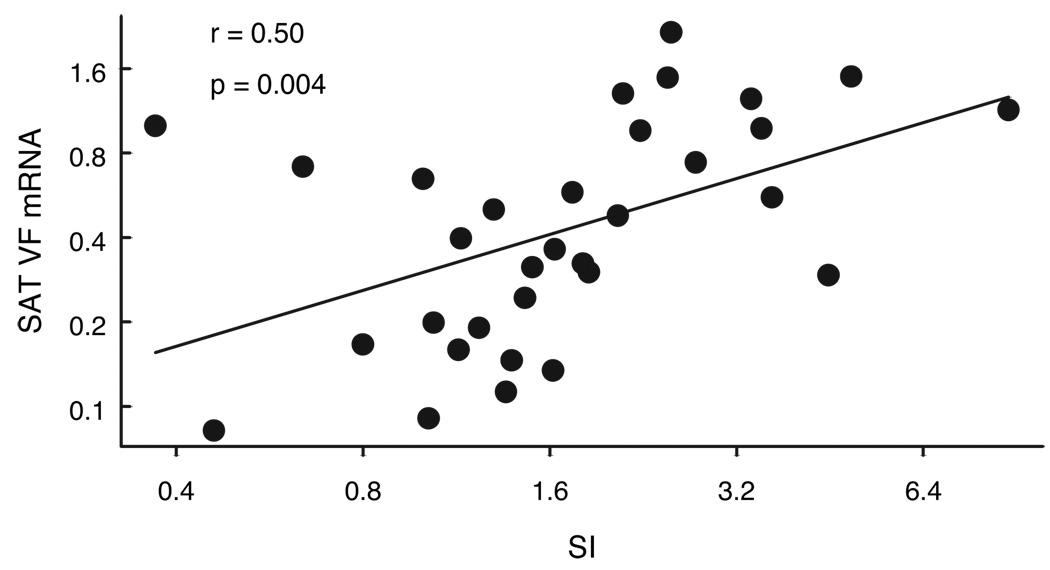
VF was previously demonstrated to have insulin mimetic effects (3). To examine the association of SAT VF gene expression with SI, 39 subjects (36 women, 3 men, age 42 ± 1.7 yr), with either NGT or IGT and having a wide range of BMI (19–55 kg/m2, % body fat 15.5–54.1%) and SI (0.62–14.6 × 10−5 × min−1/pm) were included in the analyses. As seen in Fig. 2, SAT VF expression showed a significant positive association with SI (r = 0.50, P = 0.004, n = 32), such that insulin resistant subjects had lower level of VF expression. There was no association between plasma VF and SI (r = 0.16, P < 0.5, n = 36), and Table 2 lists many of the correlations with SAT VF.
FIG. 2.
Relationship between SAT VF expression and SI (n = 32). VF mRNA level was determined by real-time RT-PCR analysis and was expressed in relation to endogenous 18S RNA. SI value was determined by the method of frequently sampled iv glucose tolerance. The natural log of VF was plotted against the natural log of SI.
TABLE 2.
Correlation coefficients of VF mRNA with associated variables
| Variable | r | P |
|---|---|---|
| % Fat (n = 39) | −0.32 | <0.05 |
| Fat mass (n = 39) | −0.38 | <0.02 |
| SI (n = 32)a | 0.50 | <0.004 |
| Fasting insulin levels (n = 37) | −0.04 | >0.5 |
| Waist (cm) (n = 37) | −0.36 | <0.05 |
| Hip (cm) (n = 37) | −0.31 | <0.1 |
| Waist/hip (n = 37) | −0.30 | <0.1 |
| Plasma TNFα | −0.62 | <0.001 |
| Adipose CD68 mRNA | −0.37 | <0.05 |
| % IMCL type I (n = 31)a | −0.58 | <0.006 |
| % IMCL type II (n = 31)a | −0.66 | <0.0001 |
| % Total IMCL (n = 31)a | −0.59 | <0.001 |
| Muscle fiber area (n = 31) | 0.02 | >0.5 |
| Leptin expression ng/ml (n = 37) | −0.31 | <0.1 |
VF mRNA and the measures were logarithmically transformed.
SAT VF expression is higher in subjects with high SI compared with subjects with low SI, independent of obesity
Insulin resistance tends to increase with obesity. Among the subjects included in Fig. 2, there was a strong negative relationship between BMI and SI (r = −0.65, P < 0.001), as expected (data not shown). Hence, to ascertain the relationship between VF expression and insulin sensitivity, independent of obesity, we separated a subgroup from our study population who were matched for BMI, race, and gender, and divided them into two groups, low SI and high SI based on the median SI value of 1.6. The characteristics of the subjects are shown in Table 3. As shown in Table 3, VF mRNA was significantly higher in the low SI group compared with the BMI-matched more insulin sensitive (P < 0.02). However, plasma VF did not show any significant difference between NGT and IGT subjects.
TABLE 3.
Characteristics of BMI matched low SI and high SI subjects
| Low SI<1.6 (n = 13) |
High SI >1.6 (n = 12) |
|
|---|---|---|
| BMI, kg/m2 | 33.5 ± 0.8 | 32.8 ± 1.0 |
| SI, 10−5 × min−1/pm | 1.7 ± 0.2 | 4.1 ± 0.4b |
| Fasting glucose, mm | 5.0 ± 0.18 | 4.6 ± 0.14 |
| 2-h glucose, mm | 8.8 ± 0.6 | 6.4 ± 0.43a |
| Triglyceride, mm plasma | 1.8 ± 0.2 | 1.4 ± 0.2 |
| SAT VF mRNA | 0.37 ± 0.08 | 0.81 ± 0.2a |
| Plasma VF (ng/ml) | 22.3 ± 4.85 | 17.3 ± 2.18 |
P < 0.02;
P < 0.005 vs. low SI.
VF gene expression in SAT correlates negatively with markers of inflammation
CD68 is a surface marker found predominantly on macrophages (22), and our previous studies demonstrated that CD68 expression in adipose tissue was negatively associated with insulin resistance (15). We examined the relationship between VF expression and markers of adipose tissue inflammation. VF expression tended to be higher in subjects with low CD68 expression (r = −0.37, P = 0.05, n = 30), and a stronger negative association was observed between SAT VF mRNA and plasma TNFα (r = −0.62, P = 0.001, n = 37) (Table 2). Because of the relationship between VF mRNA and SI, we examined the partial correlation coefficients between VF mRNA, adipose tissue CD68, and plasma TNFα after adjusting for BMI and SI. Adjusting for BMI and SI weakened the relationship between VF mRNA and both TNFα and CD68. After this adjustment there was only a tendency of a negative relationship between VF mRNA and plasma TNFα (r = −0.38, P = 0.08), and there was no significant relationship between VF and CD68.
VF expression in SAT correlates negatively with IMCL
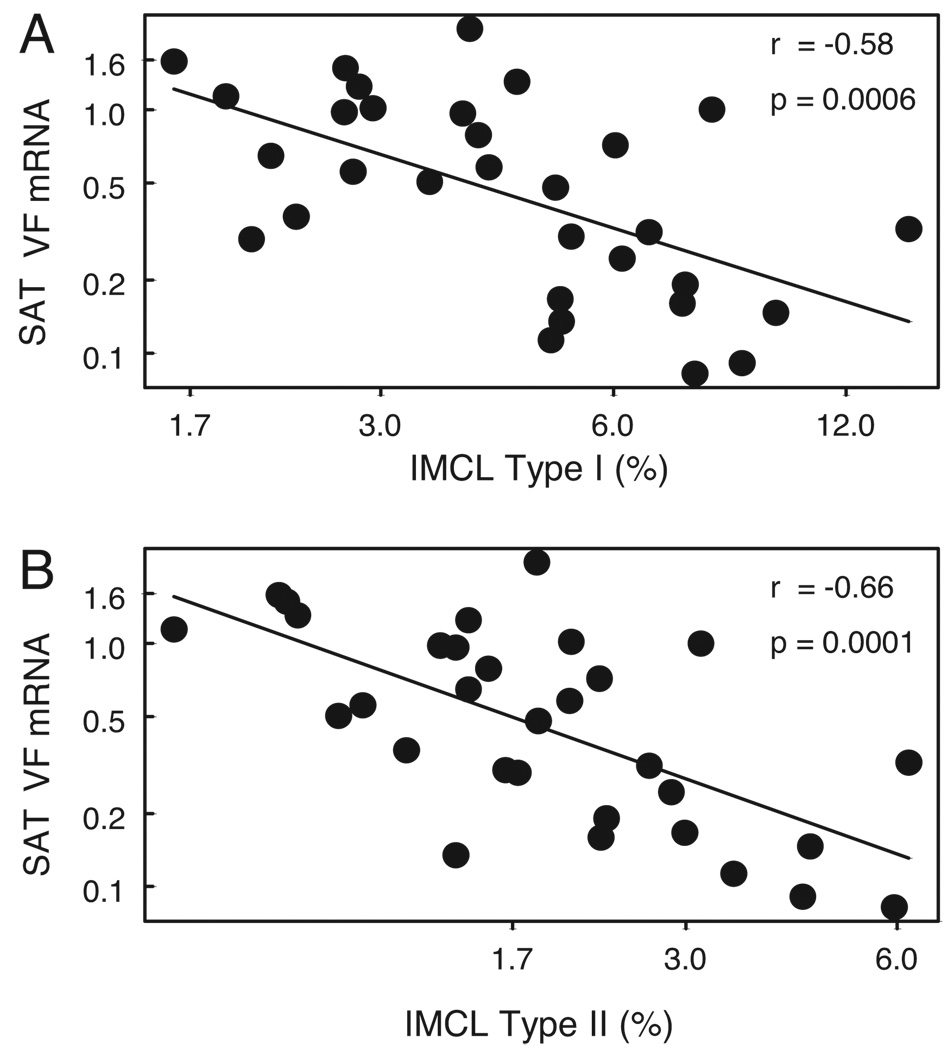
Earlier studies have demonstrated increased IMCL accumulation in obese, insulin-resistant subjects (23, 24). Hence, the relationship between SAT VF expression and IMCL, a marker of SI, was examined in 31 IGT and NGT subjects whose IMCL data were available. IMCL was measured in these subjects, as described in Subjects and Methods. As depicted in Fig. 3, SAT VF expression showed a significant negative correlation with IMCL in both type 1 and type 2 fibers (r = −0.58, P = 0.0006, n = 31 and r = −0.66, P = 0.0001, n = 31, respectively). Both obesity and insulin resistance are also associated with IMCL. Hence, to determine whether the relationship between VF and IMCL was attributable to the association between VF and BMI and SI, the association between VF and IMCL was adjusted for BMI and SI. After adjustment for BMI and SI, the correlation coefficient between SAT VF mRNA and IMCL was lower (r = −0.34, P = 0.08 and r = −0.50, P < 0.01 for type 1 and type 2 fibers, respectively), and still remained statistically significant for IMCL in type 2 fibers (Table 4). A significant negative association was also observed between plasma VF and IMCL in both type 1 (r = −0.43, P = 0.01, n = 32) and type 2 fibers (r = −0.42, P = 0.02 n = 32). However, this association was less robust and was no longer significant after adjusting for BMI and SI (r = −0.25 P = 0.19 and r = −0.14, P = 0.48 for plasma VF vs. type 1 and type 2 fibers, respectively). Thus, adipose tissue VF mRNA is lower in subjects who accumulated more IMCL.
FIG. 3.
Relationship between SATVF expression and IMCL (n = 31). A, Type 1 muscle fiber IMCL. B, Type 2 muscle fiber IMCL. The natural log of SAT VF mRNA was plotted against the natural log of IMCL type I and type II, respectively.
TABLE 4.
Correlation coefficients of VF mRNA and plasma VF with IMCL before and after adjusting for BMI and SI
| IMCL type 1a |
IMCL type 2a |
|||||
|---|---|---|---|---|---|---|
| n | r | P | n | r | P | |
| VF mRNAa | ||||||
| Unadjusted | 31 | −0.58 | <0.001 | 31 | −0.66 | <0.001 |
| Adjustedb | 29 | −0.34 | 0.074 | 29 | −0.50 | 0.005 |
| Plasma VFa | ||||||
| Unadjusted | 32 | −0.43 | 0.013 | 32 | −0.42 | 0.017 |
| Adjustedb | 29 | −0.25 | 0.20 | 29 | −0.14 | 0.48 |
VF and IMCL measures are logarithmically transformed.
Adjusted for BMI and SI.
Because of the association between VF mRNA and IMCL, we wondered whether VF could be responsible for changes in muscle lipid oxidation. Therefore, the relationship between SAT adipose VF expression and muscle SDH activity was examined. No significant correlation was seen between SAT VF and SDH activity of either type 1 or type 2 fibers (r = 0.28, P = 0.09 and r = 0.08, P = 0.63, respectively).
Thiazolidinedione (TZD) treatment of IGT subjects does not alter VF expression in SAT
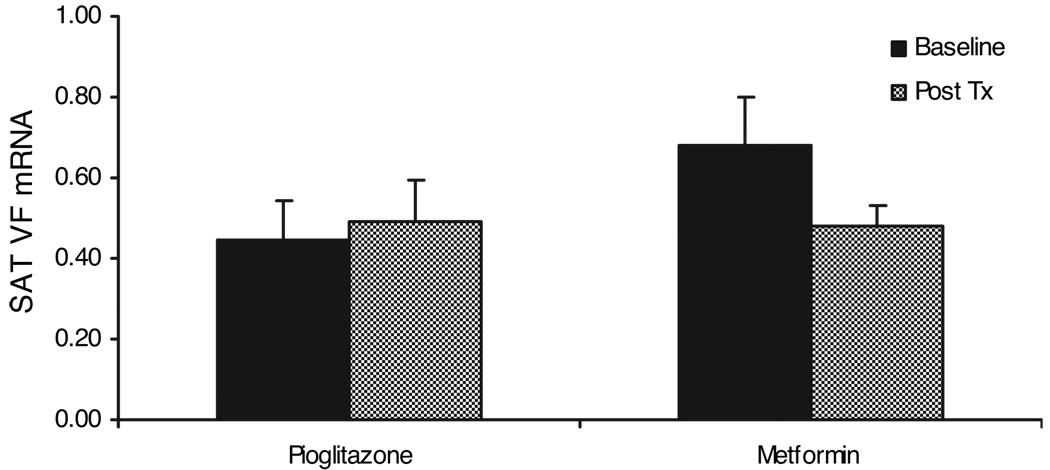
Because SAT VF expression was higher in insulin-sensitive subjects, IGT subjects were treated with two different insulin sensitizers, pioglitazone and metformin, to determine whether these treatments would result in an increase in VF expression. Thirty-six IGT subjects were randomized to receive either pioglitazone or metformin for 10 weeks. In this group, mean BMI was 32.75 ± 0.57 kg/m2, and mean SI at baseline was 2.40 × 10−5 ± 0.22 min−1/pm. As described previously (14), treatment with pioglitazone resulted in a significant increase in SI (from 2.35 to 3.52 × 10−5 min−1/pm (P < 0.005), whereas there was no significant change in SI from metformin treatment. VF mRNA levels were measured in SAT, obtained before and after treatment with either pioglitazone or metformin. As shown in the Fig. 4, neither pioglitazone nor metformin treatment resulted in any change in VF mRNA.
FIG. 4.
Effects of pioglitazone (n = 17) and metformin (n = 19) treatment on VF expression in adipose tissue. VF mRNA levels were determined as described in Subjects and Methods.
Muscle VF expression was measured in the same subjects, but showed no significant association with BMI, SI, IMCL or SDH activity, and there was no difference in muscle VF expression between IGT and NGT subjects or before and after pioglitazone or metformin treatments (data not shown). These data suggest that VF expression is not regulated in muscle.
Discussion
VF was originally called PBEF, and was noted to be involved in the maturation of B cell precursors (5). More recently, VF was characterized as an adipokine which was highly expressed in visceral fat and exhibited insulin-like functions (3). These insulin mimetic functions were mediated through stimulation of the insulin signal transduction pathway through induction of phosphorylation of signal transduction proteins in the insulin signaling pathway, and also through binding to the insulin receptor at a site distinct from that of insulin (25). In addition, VF lowered blood glucose in mice and mice with a loss of function mutation of VF demonstrated higher blood glucose levels (3). Because VF was originally identified as a gene expressed predominantly by VAT, there was speculation that VF may be either a marker of VAT volume, or in some way associated with the abnormal condition known as metabolic syndrome.
In these studies, we examined VF expression in adipose tissue and muscle from nondiabetic subjects. From the paired surgical samples, there was no difference in VF mRNA levels between SAT and VAT. This lack of difference between VF in VAT and SAT is in agreement with a recent study involving 189 subjects (10). The expression of VF in adipose tissue, its fractions and in muscle, suggests that VF is widely expressed, and in adipose tissue it is expressed by both adipocytes and other cells in adipose tissue. When different adipose tissue fractions from whole adipose tissue were examined, the stromal vascular fraction expressed significantly more VF than the adipocyte fraction, suggesting that adipocytes are not the major source of VF in the adipose tissue. Results from cultured adipose cells demonstrate that VF is expressed by both the differentiated adipocytes and preadipocytes, the latter of which is a component of the stromal vascular fraction. A recent study suggested that VF is highly expressed in the macrophage enriched stromal fraction in VAT (26). Our study was not able to distinguish between macrophages and other cells of the stromal vascular fraction. However, the negative association between VF and CD68 and TNFα suggests that VF is not highly expressed by macrophages and indeed plays an anti inflammatory role in adipose tissue.
On examining the relationship to obesity, VF expression in VAT varied considerably among the 15 subjects we examined. Overall there was a significant positive association with BMI similar to the positive relationship described by one study (3), but in contrast to another study (26), in which no correlation between VAT VF mRNA and BMI was noted. In contrast, SAT VF expression was significantly negatively associated with BMI, suggesting that VF may be differentially regulated in the two adipose depots. These data would also suggest that the relative abundance of VF mRNA in the visceral vs. sc depots would depend heavily on the degree of obesity of the subjects. Plasma VF, however, showed no correlation with BMI, perhaps because of the differential regulation of VF expression in the different adipose depots. Hence, the increase in VAT VF with obesity may be balanced by the decrease in SAT VF, such that plasma VF is not affected by increasing BMI. Further studies are needed to understand the basis to the variation in VF expression among individual subjects.
Previous studies have not examined the relationship between VF and insulin resistance. In our subjects, SAT VF mRNA correlated significantly with SI, such as insulin-resistant subjects had lower expression of VF. Insulin sensitivity is dependent on a number of factors, including BMI. To assess the relationship between VF and SI independent from obesity, and to control for other factors such as gender, race, and age, we compared VF expression in a more homogeneous group of BMI-matched NGT and IGT white female subjects. IGT subjects demonstrated significantly lower VF expression, suggesting that SAT VF expression was associated with insulin sensitivity. Again, however, there was no relationship between plasma VF and SI.
The precise role, if any, of VF in insulin resistance is not clear. A previous study (3) demonstrated that both VF and insulin had similar affinity for the insulin receptor. However, under physiological conditions, the circulating levels of VF are only approximately 10% of insulin. Thus, despite the insulin sensitizing action of VF in vitro, VF may not play a role as an insulin sensitizer in vivo, although VF may be a biomarker of the insulin resistant state. VF could be exerting local autocrine/paracrine effects on VAT by its insulin-like adipogenic and lipogenic effects. If this is the case, however, it is unclear why VF mRNA would increase with obesity in VAT, yet decrease in SAT.
The precise etiology of insulin resistance is unknown, but many studies have suggested that lipotoxicity, or ectopic fat accumulation, may be responsible for insulin resistance (23, 27). We assessed the relationship between IMCL and VF and found that SAT VF mRNA was negatively associated with IMCL in both the oxidative types I and nonoxidative type 2 muscle fibers. IMCL is related to obesity and insulin resistance, but we found that the association between VF and IMCL remained significant on adjusting for BMI and SI with IMCL type 2 fibers, and was of borderline significance in type 1 fibers. Although the precise regulation of muscle lipid accumulation is not clear, a number of studies have suggested that insulin-resistant subjects demonstrate defects in mitochondrial lipid oxidation (16, 28). To determine whether VF expression was related to muscle oxidative capacity, SDH activity was assessed. However, SDH activity in either type 1 or type 2 fibers was not associated with adipose VF. Thus, these studies would suggest that SAT VF expression is associated with muscle lipid accumulation, perhaps not in a direct fashion, but as a marker for some other adipose tissue factor that results in less muscle lipid accrual.
TZDs are agonists for PPARγ, which is predominantly located in adipose tissue, and improve peripheral insulin sensitivity (29). Recent studies have suggested that the improvement in SI from TZDs occurs through a mobilization of lipid to SAT, thus protecting insulin sensitive tissues and visceral adipose tissue from ectopic fat accumulation (14, 14, 30). Because VF may be an insulin mimetic, it is reasonable that an insulin sensitizer would increase VF expression. However, when IGT subjects were treated with pioglitazone and metformin, there was no change in SAT VF expression, despite an improvement in SI after pioglitazone treatment. Thus, the association between VF and SI does not extend to the improvement in SI that occurs through TZD treatment.
In summary, VF expression was not significantly different between the SAT and VAT adipose tissue depots. VAT and SAT VF correlated in opposite manners with obesity, and SAT VF correlated positively with SI and negatively with IMCL, CD68, and plasma TNFα. These findings are consistent with the possible role of VF as an adipokine that will augment insulin action. SAT VF did not increase after treatment with pioglitazone or metformin, suggesting complex regulation that does not accompany TZD mediated changes in SI. VF is expressed by many cells and tissues, and hence plasma levels are not reflective of obesity or insulin resistance.
Acknowledgments
This work was supported by a Merit Review Grant from the Veterans Administration (to P.A.K.); Grant M01RR14288 of the General Clinical Research Center (to P.A.K.); Grants DK 39176 and DK 71277 (to P.A.K.), DK 71346 (to R.E.M.), and DK 52398 (to S.K.F.) from the National Institutes of Health; and funding from the American Diabetes Foundation and Geriatrics Research Education and Clinical Center of the Baltimore VA (to S.K.F.).
Abbreviations
- BMI
Body mass index
- IGT
impaired glucose tolerance
- IMCL
intramyocellular lipid(s)
- NGT
normal glucose tolerance
- PBEF
pre-B cell colony enhancing factor
- SAT
sc adipose tissue
- SDH
succinate dehydrogenase
- SI
insulin sensitivity
- VAT
visceral adipose tissue
- VF
visfatin
Footnotes
Disclosure Statement: V.V., A.Y.-B., A.M.B., B.P., M.-J.L., T.S., L.M.K., H.J.S., R.E.M. and S.K.F. have nothing to disclose. P.A.K. has received lecture fees from Takeda Pharmaceuticals and Merck. N.R. has received investigator initiated grant support from Takeda Pharmaceuticals and Abbott.
References
- 1.Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Annu Rev Med. 2003;54:453–471. doi: 10.1146/annurev.med.54.101601.152403. [DOI] [PubMed] [Google Scholar]
- 2.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 3.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 4.Kitani T, Okuno S, Fujisawa H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003;544:74–78. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 5.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colonyenhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye SQ, Zhang LQ, Adyshev D, Usatyuk PV, Garcia AN, Lavoie TL, Verin AD, Natarajan V, Garcia JG. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc Res. 2005;70:142–151. doi: 10.1016/j.mvr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 9.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 11.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 12.Hammarstedt A, Pihlajamaki J, Rotter SV, Gogg S, Jansson PA, Laakso M, Smith U. Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab. 2006;91:1181–1184. doi: 10.1210/jc.2005-1395. [DOI] [PubMed] [Google Scholar]
- 13.Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–1581. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 14.Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930–E934. doi: 10.1152/ajpendo.00522.2004. [DOI] [PubMed] [Google Scholar]
- 15.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 16.Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metabolism. 2001;50:407–413. doi: 10.1053/meta.2001.21690. [DOI] [PubMed] [Google Scholar]
- 17.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 18.Malenfant P, Joanisse DR, Theriault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord. 2001;25:1316–1321. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- 19.Kern PA, Simsolo RB, Fournier M. Effect of weight loss on muscle fiber type, fiber size, capillarity, and succinate dehydrogenase activity in humans. J Clin Endocrinol Metab. 1999;84:4185–4190. doi: 10.1210/jcem.84.11.6090. [DOI] [PubMed] [Google Scholar]
- 20.Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, Arner P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 21.Van Gaal LF, Wauters MA, Mertens IL, Considine RV, De Leeuw IH. Clinical endocrinology of human leptin. Int J Obes Relat Metab Disord. 1999;23 Suppl 1:29–36. doi: 10.1038/sj.ijo.0800792. [DOI] [PubMed] [Google Scholar]
- 22.Holness CL, da Silva RP, Fawcett J, Gordon S, Simmons DL. Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem. 1993;268:9661–9666. [PubMed] [Google Scholar]
- 23.Krssak M, Falk PK, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 24.Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA. Effect of weight loss on muscle lipid content in morbidly obese subjects. Am J Physiol Endocrinol Metab. 2003;284:E726–E732. doi: 10.1152/ajpendo.00371.2002. [DOI] [PubMed] [Google Scholar]
- 25.Hug C, Lodish HF. Medicine. Visfatin: a new adipokine. Science. 2005;307:366–367. doi: 10.1126/science.1106933. [DOI] [PubMed] [Google Scholar]
- 26.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 27.Friedman J. Fat in all the wrong places. Nature. 2002;415:268–269. doi: 10.1038/415268a. [DOI] [PubMed] [Google Scholar]
- 28.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry RR. Insulin resistance: from predisposing factor to therapeutic target in type 2 diabetes. Clin Ther. 2003;25 Suppl B:B47–B63. doi: 10.1016/s0149-2918(03)80242-4. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]