Abstract
The circulation of Aichi virus in a major urban area was demonstrated using molecular detection with samples recovered from a major river polluted with sewage discharges in Caracas, Venezuela. Five out of 11 water samples studied were positive, being classified by phylogenetic analysis as genotype B. Analysis of sewage waters appears to be a useful methodology to uncover the presence of a hitherto undetected fecal pathogen in a given geographical area.
Sewage pollution plays a major role in the transmission of multiple viral pathogens associated with gastrointestinal diseases in human populations (10a). Understanding the distribution and persistence of sewage-borne viral pathogens in highly contaminated waters from different geographical areas may provide relevant information on the epidemiology of enteric viral disease worldwide (20). Aichi viruses have emerged as viral agents associated with food-borne nonbacterial acute gastroenteritis in humans. Seroprevalence studies conducted in Japan and Europe suggest that infections with this agent are quite frequent (16, 26). In the Western hemisphere, Aichi virus has been described only in Brazil, in clinical samples (7, 14, 16, 17, 19, 22, 24, 25, 27). In Venezuela, it is unknown whether Aichi viruses circulate in the population, and their eventual associations with diarrheal cases or sporadic outbreaks have not been documented.
Aichi viruses are small round viruses that are about 30 nm in diameter and have an RNA genome of positive polarity, with a length of approximately 8.3 kb. These viruses, together with the bovine and porcine kobuviruses, are classified into the genus Kobuvirus of the Picornaviridae family (11, 13, 18). Three genotypes of the Aichi virus (A to C) have been proposed based on sequence analysis of 519 nucleotides at the 3CD junction region (1, 24, 27).
The molecular detection and characterization of Aichi virus have been conducted so far in isolates from clinical samples (16, 17, 19, 24) and sea food (8), but to our knowledge, there are no reports of detection in water samples.
The present study was conducted in order to determine the occurrence and circulation of Aichi virus in a major urban area of Venezuela, using molecular detection and sequence analysis of viral particles recovered from surface waters heavily polluted with sewage discharges. A total of 11 samples were collected in an urban stream known as Guaire River, with samplings made twice a month from October 2007 to February 2008, with three samplings in February. The description of the sampling location and predominant point pollution source has been documented in previous publications (2, 21). The recovery of viral particles and RNA extraction followed procedures previously described (21). The molecular detection and characterization of Aichi viruses involved reverse transcription and nested PCR, using primers and cycling conditions that amplify a 519-bp fragment located between the 3CD region and the N terminus, 3D, of the Aichi virus genome (24). A new set of primers identified as AiF2 (62835′-CAA GGA CTT GCG GCG CTT CAT CG-3′6305) and AiR2b (66725′-GCA CCC YTC YCC CGC CTA CGG TG-3′6694) was designed for a second round of amplification, with an expected product of 411 bp. These primers anneal to an inner region of the first amplicon, and their sequence was based on the consensus sequence generated after multiple alignments of four complete genome sequences of Aichi virus available in the GenBank database (accession numbers DQ028632, FJ890523, NC001918, and AB010145). The nucleotide position reported for each new primer was based on the genomic sequence of the reference strain AB010145. For reverse transcription, 10 μl of the extracted RNA was mixed with 15 μl of molecular grade water, incubated at 95°C for 5 min, and placed on ice for RNA denaturation. The denatured RNA was reverse transcribed with random primers (0,02 μg) in the presence of avian myeloblastosis virus (AMV) reverse transcriptase (10 U), deoxynucleoside triphosphate mix (0.2 mM), and RNasin (40 U) in reverse transcription buffer to a final volume of 50 μl. The mixture was incubated at 42°C for 1 h followed by incubation at 95°C for 15 min. The first round of PCR amplification included 10 μl of cDNA, 2.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate [dNTP]), 20 pmol of each primer (6261/6779), and 2.5 U of Platinum Taq polymerase in PCR buffer to a final volume of 50 μl. Cycling conditions included 40 cycles of 95°C for 30 s, 55°C for 30 s, and 68°C for 1 min. The second round of amplification was conducted under the same cycling conditions with the new primer set and 1 μl of the first round amplification product. All reagents were purchased from Invitrogen (Carlsbad, CA), except AMV, which was supplied by Promega (Madison, WI).
PCR products were purified with the PCR purification kit (Qiagen GmbH, Germany), by following the manufacturer's instructions, and sequenced commercially by Macrogen Sequencing Service (Macrogen, South Korea). Both strands were sequenced, and the resultant nucleotide sequences were compared with reference sequences of strains available in the GenBank database. Sequence assembly and alignment were conducted with the DNAMan software 5.2.2 (Lynnon Biosoft, Quebec, Canada) and the phylogenetic analysis with the MEGA 4.1 software (23).
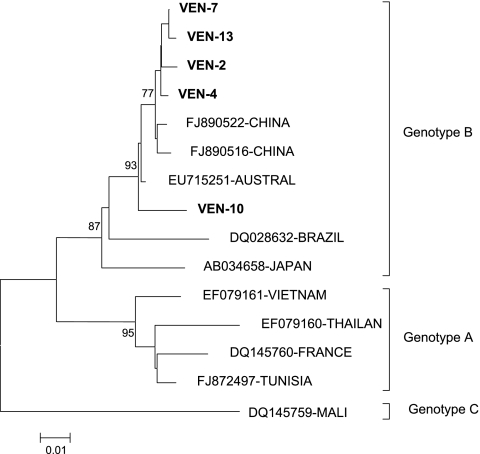
Aichi virus was detected by the reverse transcription-PCR (RT-PCR) screening method in 5 of the 11 water samples tested. Nucleotide sequences of the partial 3CD region of five Aichi virus-positive samples (Ven7, Ven13, Ven2, Ven4, Ven10) were highly conserved (99.2% to 97%), with amino acid identities of 99 to 100%. Phylogenetic analysis of partial 3CD nucleotide sequences of Aichi virus strains from Venezuela and reference sequences from GenBank is shown in Fig. 1. The phylogenetic tree indicated that all five virus strains belonged to group B of Aichi virus, according to the classification proposed by Yamashita et al. (24). All five Venezuelan isolates are located in the same cluster, with one sample (Ven10), with a 2.6 to 2.8% nucleotide divergence from the other samples, located on a different minor branch, indicating some degree of variability among Venezuelan isolates. In addition, the nucleotide sequences of Aichi viruses from the sewage-polluted river in Venezuela shared a close similarity with Aichi virus strains from different geographical areas, thereby evidencing the circulation of closely related strains worldwide (12).
FIG. 1.
Phylogenetic analysis of the Aichi viruses recovered from urban sewage, based on a 391-bp fragment of the genomic 3CD region junction, corresponding to positions 6322 to 6713 of the genomic sequence of the reference strain AB010145. The tree was constructed using the minimum evolution method as implemented in the package MEGA 4. The bar is in units of base substitutions per site. Bootstrap values (>75) are indicated for the corresponding nodes, based on a resampling analysis of 1,000 replicates. Venezuelan isolates are indicated in boldface type.
Although more sampling is required in order to make inferences about the prevalence of Aichi virus infection in the study area, the high proportion of positive samples may indicate a widespread circulation of Aichi virus among the Venezuelan population. Viral RNA of Aichi virus has been detected in variable proportions (0.3 to 20.5%) in human fecal samples (1, 12, 22, 26), and with a high seroprevalence in different countries (7, 16, 26). Further studies are warranted in order to elucidate the significance of these findings and to establish the clinical importance of Aichi virus and other enterically transmitted viruses, such as hepatitis E virus, in Venezuela.
Recently, various agents putatively associated with human or animal diarrhea have been described: klassevirus, bocavirus, salivirus, parechovirus, and torque teno virus (3-5, 10, 15). Klassevirus and bocavirus have been detected in sewage (3, 7) and torque teno virus in river waters (9). Sewage represents a natural pool of thousands of individual specimens, so the analysis of sewage waters appears to be a very useful methodology to uncover the presence of a hitherto undetected fecal pathogen in a given geographical area.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study were deposited in the GenBank database under the following accession numbers: GU807430 (Ven2), GU807429 (Ven4), GU807431 (Ven7), GU807432 (Ven10), and GU807433 (Ven13).
Acknowledgments
This work was partially supported through Projects IVIC-98 and IVIC-883.
Footnotes
Published ahead of print on 23 April 2010.
REFERENCES
- 1.Ambert-Balay, K., M. Lorrot, F. Bon, M. Giraudon, J. Kaplon, M. Wolfer, P. Lebon, D. Gendrel, and P. Pothier. 2008. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J. Clin. Microbiol. 46:1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betancourt, W. Q., L. Querales, Y. F. Sulbaran, J. Rodriguez-Diaz, L. Caraballo, and F. H. Pujol. 2010. Molecular characterization of sewage-borne pathogens and detection of sewage markers in an urban stream in Caracas, Venezuela. Appl. Environ. Microbiol. 76:2023-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blinkova, O., K. Rosario, L. Li, A. Kapoor, B. Slikas, F. Bernardin, M. Breitbart, and E. Delwart. 2009. Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J. Clin. Microbiol. 47:3507-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braham, S., M. Iturriza-Gómara, and J. Gray. 2009. Detection of TT virus by single-primer sequence-independent amplification in multiple samples collected from an outbreak of gastroenteritis. Arch. Virol. 154:981-985. [DOI] [PubMed] [Google Scholar]
- 5.Drexler, J. F., K. Grywna, A. Stöcker, P. S. Almeida, T. C. Medrado-Ribeiro, M. Eschbach-Bludau, N. Petersen, H. da Costa-Ribeiro, Jr., and C. Drosten. 2009. Novel human parechovirus from Brazil. Emerg. Infect. Dis. 15:310-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Goyer, M., L. S. Aho, J. B. Bour, K. Ambert-Balay, and P. Pothier. 2008. Seroprevalence distribution of Aichi virus among a French population in 2006-2007. Arch. Virol. 153:1171-1174. [DOI] [PubMed] [Google Scholar]
- 8.Hansman, G. S., T. Oka, T. C. Li, O. Nishio, M. Noda, and N. Takeda. 2008. Detection of human enteric viruses in Japanese clams. J. Food Prot. 71:1689-1695. [DOI] [PubMed] [Google Scholar]
- 9.Haramoto, E., M. Kitajima, H. Katayama, and S. Ohgaki. 2010. Real-time PCR detection of adenoviruses, polyomaviruses, and torque teno viruses in river water in Japan. Water Res. 44:1747-1752. [DOI] [PubMed] [Google Scholar]
- 10.Holtz, L., S. Finkbeiner, G. Zhao, C. Kirkwood, R. Girones, J. Pipas, and D. Wang. 2009. Klassevirus 1, a previously undescribed member of the family Picornaviridae, is globally widespread. Virol. J. 6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Hurst, C., and P. Murphy. 1997. The transmission and prevention of infectious disease, p. 3-54. In C. J. Hurst (ed.), Modeling disease transmission and its prevention by disinfection, 1st ed. Cambridge University Press, New York, NY.
- 11.ICTVdB Management. 2006. 00.052.0.08. Kobuvirus. In C. Büchen-Osmond (ed.), ICTVdB—The Universal Virus Database, version 4. Columbia University, New York, NY. http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/.
- 12.Kaikkonen, S., S. Rasanen, M. Ramet, and T. Vesikari. 2009. Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol. Infect. 7:1-6. [DOI] [PubMed] [Google Scholar]
- 13.Khamrin, P., N. Maneekarn, A. Kongkaew, S. Kongkaew, S. Okitsu, and H. Ushijima. 2009. Porcine kobuvirus in piglets, Thailand. Emerg. Infect. Dis. 15:2075-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Guyader, F. S., J. C. Le Saux, K. Ambert-Balay, J. Krol, O. Serais, S. Parnaudeau, H. Giraudon, G. Delmas, M. Pommepuy, P. Pothier, and R. L. Atmar. 2008. Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 46:4011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, L., J. Victoria, A. Kapoor, O. Blinkova, C. Wang, F. Babrzadeh, C. J. Mason, P. Pandey, H. Triki, O. Bahri, B. S. Oderinde, M. M. Baba, D. N. Bukbuk, J. M. Besser, J. M. Bartkus, and E. L. Delwart. 2009. A novel picornavirus associated with gastroenteritis. J. Virol. 83:12002-12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh, D. Y., P. A. Silva, B. Hauroeder, S. Diedrich, D. D. Cardoso, and E. Schreier. 2006. Molecular characterization of the first Aichi viruses isolated in Europe and in South America. Arch. Virol. 151:1199-1206. [DOI] [PubMed] [Google Scholar]
- 17.Pham, N. T., Q. D. Trinh, P. Khamrin, T. A. Nguyen, S. K. Dey, T. G. Phan, P. Hoang le, N. Maneekarn, S. Okitsu, M. Mizuguchi, and H. Ushijima. 2008. Sequence analysis of the capsid gene of Aichi viruses detected from Japan, Bangladesh, Thailand, and Vietnam. J. Med. Virol. 80:1222-1227. [DOI] [PubMed] [Google Scholar]
- 18.Reuter, G., A. Boldizsár, and P. Pankovics. 2009. Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae. Arch. Virol. 154:101-108. [DOI] [PubMed] [Google Scholar]
- 19.Reuter, G., A. Boldizsár, G. Papp, and P. Pankovics. 2009. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch. Virol. 154:1529-1532. [DOI] [PubMed] [Google Scholar]
- 20.Roda-Husman, A. M., and J. Bartram. 2007. Global supply of virus-safe drinking water, p. 127-162. In A. Bosh (ed.), Human viruses in water, 1st ed. Elsevier, Amsterdam, Netherlands. [DOI] [PMC free article] [PubMed]
- 21.Rodríguez-Díaz, J., L. Caraballo, E. Vizzi, L. Querales, F. Liprandi, H. Takiff, and W. Betancourt. 2009. Detection and characterization of waterborne gastroenteritis viruses in urban sewage and sewage-polluted river waters in Caracas, Venezuela. Appl. Environ. Microbiol. 75:387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sdiri-Loulizi, K., M. Hassine, H. Gharbi-Khelifi, N. Sakly, S. Chouchane, M. N. Guericke, P. Pothier, M. Aouni, and K. Ambert-Balay. 2009. Detection and genomic characterization of Aichi viruses in stool samples from children in Monastir, Tunisia. J. Clin. Microbiol. 47:2275-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita, T., M. Sugiyama, H. Tsuzuki, K. Sakae, Y. Suzuki, and Y. Miyazaki. 2000. Application of a reverse transcription-PCR for identification and differentiation of Aichi virus, a new member of the Picornavirus family associated with gastroenteritis in humans. J. Clin. Microbiol. 38:2955-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita, T., K. Sakae, S. Kobayashi, Y. Ishihara, T. Miyake, A. Mubina, and S. Isomura. 1995. Isolation of cytopathic small round virus (Aichi virus) from Pakistani children and Japanese travelers from Southeast Asia. Microbiol. Immunol. 39:433-435. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita, T., K. Sakae, Y. Ishihara, S. Isomura, and E. Utagawa. 1993. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J. Clin. Microbiol. 31:2938-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita, T., S. Kobayashi, K. Sakae, S. Nakata, S. Chiba, Y. Ishihara, and S. Isomura. 1991. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J. Infect. Dis. 164:954-957. [DOI] [PubMed] [Google Scholar]