Abstract
To investigate the fine-scale diversity of the polyphosphate-accumulating organisms (PAO) “Candidatus Accumulibacter phosphatis” (henceforth referred to as “Ca. Accumulibacter”), two laboratory-scale sequencing batch reactors (SBRs) for enhanced biological phosphorus removal (EBPR) were operated with sodium acetate as the sole carbon source. During SBR operations, activated sludge always contained morphologically different “Ca. Accumulibacter” strains showing typical EBPR performances, as confirmed by the combined technique of fluorescence in situ hybridization (FISH) and microautoradiography (MAR). Fragments of “Ca. Accumulibacter” 16S rRNA genes were retrieved from the sludge. Phylogenetic analyses together with sequences from the GenBank database showed that “Ca. Accumulibacter” 16S rRNA genes of the EBPR sludge were clearly differentiated into four “Ca. Accumulibacter” clades, Acc-SG1, Acc-SG2, Acc-SG3, and Acc-SG4. The specific FISH probes Acc444, Acc184, Acc72, and Acc119 targeting these clades and some helpers and competitors were designed by using the ARB program. Microbial characterization by FISH analysis using specific FISH probes also clearly indicated the presence of different “Ca. Accumulibacter” cell morphotypes. Especially, members of Acc-SG3, targeted by probe Acc72, were coccobacillus-shaped cells with a size of approximately 2 to 3 μm, while members of Acc-SG1, Acc-SG2, and Acc-SG4, targeted by Acc444, Acc184, and Acc119, respectively, were coccus-shaped cells approximately 1 μm in size. Subsequently, cells targeted by each FISH probe were sorted by use of a flow cytometer, and their polyphosphate kinase 1 (ppk1) gene homologs were amplified by using a ppk1-specific PCR primer set for “Ca. Accumulibacter.” The phylogenetic tree based on sequences of the ppk1 gene homologs was basically congruent with that of the 16S rRNA genes, but members of Acc-SG3 with a distinct morphology comprised two different ppk1 genes. These results suggest that “Ca. Accumulibacter” strains may be diverse physiologically and ecologically and represent distinct populations with genetically determined adaptations in EBPR systems.
Enhanced biological phosphorus removal (EBPR) has been applied in many wastewater treatment plants to reduce the phosphorus that causes eutrophication in surface waters. EBPR employs polyphosphate-accumulating organisms (PAOs), which are enriched through alternating aerobic-anaerobic cycles (34). Since PAOs are essential for an understanding of EBPR, many candidates have been proposed as potential PAOs, such as Acinetobacter spp. (11), Tetrasphaera spp. (31), Microlunatus phosphovorus (36), Lampropedia spp. (40), and Gram-positive Actinobacteria (24). However, those organisms do not exhibit all of the characteristics of the EBPR biochemistry model. Recently developed culture-independent approaches such as PCR-clone libraries, fluorescence in situ hybridization (FISH), and microautoradiography (MAR) have highlighted an uncultured Rhodocyclus-related bacterium, “Candidatus Accumulibacter phosphatis” (henceforth referred to as “Ca. Accumulibacter”), as one of the most important PAO candidates (2, 5, 16, 22, 23, 27, 28, 47).
Numerous studies have sought to investigate uncultured “Ca. Accumulibacter” and have shown the presence of genetically and physiologically different members with a global geographic distribution (3, 9, 22, 27, 39). For example, Kong et al. (22) identified two morphologically different “Ca. Accumulibacter” cells of small cocci and large coccobacilli labeled with PAOmix (PAO462, PAO651, and PAO846) in laboratory-scale EBPR reactors. Additional results showing phenotypic and morphological diversities of “Ca. Accumulibacter” cells also existed with respect to the different roles of denitrifying PAO (DPAO) in the EBPR process (3, 9, 23). Carvalho et al. (3) detected two different morphotypes of “Ca. Accumulibacter” with different nitrate reduction capabilities. The presence of other “Ca. Accumulibacter” strains with 15% genome sequence divergence from the dominant strains in metagenomic analyses is likely to explain these morphological and phenotypic differences (12). McMahon et al. (33) suggested the use of the polyphosphate kinase (ppk) gene, which is involved in the production of polyphosphate, for a finer elucidation of “Ca. Accumulibacter” diversity. He et al. (15) grouped “Ca. Accumulibacter” strains into five distinct clades, designated clades I, IIA, IIB, IIC, and IID, using ppk gene sequence information. Flowers and colleagues (9) previously reported that “Ca. Accumulibacter” cells of clade IA had nitrate reduction activity with phosphorus uptake but that “Ca. Accumulibacter” cells of clade IIA did not.
FISH-fluorescence activated cell sorting (FACS) techniques have been used for the separation of specific microbial cells from complex microbial consortia and their metabolic gene analysis (14, 46). For example, Miyauchi et al. (35) sorted PAOmix probe-labeled “Ca. Accumulibacter” cells from EBPR sludge and analyzed their nitrite reductase gene (nirS) diversity. In the current study, we found that four different “Ca. Accumulibacter” clades (Acc-SG1, Acc-SG2, Acc-SG3, and Acc-SG4) were present in the EBPR sludge of laboratory-scale reactors supplied with acetate as the sole carbon source. We analyzed their morphological characteristics and ppk gene sequence information using a suite of FISH and FACS approaches and linked fine-scale phylogenetic diversities of “Ca. Accumulibacter” strains with their morphological characteristics and metabolic genes. This study will be useful for further genetic and physiological studies of different “Ca. Accumulibacter” clades.
MATERIALS AND METHODS
Operation of sequencing batch reactors.
Two laboratory-scale sequencing batch reactors (SBRs) were originally inoculated with activated sludge obtained from a wastewater treatment plant at Pohang University of Science and Technology in South Korea. Both 4-liter reactors were operated under identical conditions with aerobic-anaerobic 8-h cycles with a 10-day sludge retention time at 20°C. More details were given previously (20). In brief, each cycle consisted of 20 min of anaerobic filling, 1 h 40 min of an anaerobic reaction, 4 h of an aerobic reaction, 90 min of settling, and 30 min of decanting. The feed contained 770 mg/liter sodium acetate, 40 mg/liter NH4+-N, 15 mg/liter PO43−-P, and trace-element mixtures (18). pH was monitored by using a pH controller (Kobiotech, South Korea). Soluble orthophosphate was analyzed by using an ICS-1000 ion chromatograph (Dionex).
16S rRNA gene retrieval and phylogenetic analysis.
Total genomic DNA from sludge samples was extracted by using a FastDNA Spin kit (Qbiogene) according to the manufacturer's instructions, and bacterial 16S rRNA gene clone libraries were constructed. Primers 27f and 1492r (25) were used for a modified two-step PCR amplification to reduce chimeric products (29). The resulting PCR amplicons were purified and ligated into the pCR2.1 vector by using a TOPO cloning kit (Invitrogen) according to the manufacturer's instructions. After blue-white screening of the colonies, inserted 16S rRNA genes of more than 250 clones were analyzed on the basis of restriction fragment length polymorphism (RFLP) after their PCR amplifications and HaeIII and HhaI double digestions, as described previously (19, 21). All representative clones with unique RFLP patterns were partially sequenced with the M13 reverse primer from the TOPO cloning kit. The resulting sequences were submitted to GenBank for BlastN searches. If the representative sequences were related to “Ca. Accumulibacter,” the “Ca. Accumulibacter”-related clones were then more completely sequenced to determine the potential diversity among “Ca. Accumulibacter” clades. The resulting sequences were compared with available “Ca. Accumulibacter” 16S rRNA gene sequences from the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST/), and a phylogenetic tree was constructed by using the neighbor-joining (NJ) algorithm available in the PHYLIP software package, version 3.6 (8).
FISH probe design and evaluation.
Specific FISH probes that targeted four clades and some helpers and competitors were designed by using the probe design tool in the ARB software package (30). Based on a comparative analysis of reliable sequences with >1,200 bp in the ARB software package and our in-house clone sequences, the program selected specific regions within the highly similar four “Candidatus Accumulibacter” clades, which allowed their discrimination from each other. Probes were subsequently confirmed for specificity by using Probe Match at the website of the Ribosome Database Project II (RDPII) (4). The designed oligonucleotides were synthesized and labeled at the 5′ end with fluorescein isothiocyanate (FITC) or 3-iodocyanine (Cy3) dye by Thermo (Ulm, Germany). These labeled probes were evaluated with paraformaldehyde (PFA)-fixed sludge. The formamide (FA) concentration for optimum probe stringency was determined empirically by performing a series of FISH experiments at 5% FA increments from 15% formamide at set hybridization and wash temperatures (17). Subsequently, evaluations of these novel designed probes were operated by using them simultaneously with a generally accepted PAOmix probe set with different labeled dyes (5).
FISH analyses.
Sludge samples collected from the SBR at the end of the anaerobic phase for FISH were fixed with a 4% paraformaldehyde-phosphate-buffered saline (PBS) solution (137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4 [pH 7.2]) for 4 h at 4°C. Fixed sludge samples were washed twice in PBS, resuspended in a PBS-ethanol solution (1:1, vol/vol), and then stored at −20°C. Fixed cells were homogenized by using a homogenizer (Dispergierantrieb Top10 basic; IKA, Germany) at 45 s for 3 cycles at maximum speed prior to the FISH experiments. Homogenized cells were spotted onto poly-l-lysine-coated slides (Sigma) and dried for 20 min at 45°C. After the slides were dried, the cells were dehydrated for 3 min in a graded ethanol series with the ethanol concentration increasing from 50 to 96% ethanol in H2O. The specific FISH probes Acc444, Acc184, Acc72, and Acc119 (3 ng/μl each) that targeted the four “Ca. Accumulibacter” clades and their corresponding helpers or competitors were used for FISH hybridization. FISH experiments were carried out according to methods described previously by Amann et al. (1) and Hugenholtz et al. (17). Nonlabeled FISH probes were always prepared to examine autofluorescent bacteria.
FISH/MAR analyses.
Combined FISH and MAR were used to evaluate acetate and phosphorus uptake by four “Ca. Accumulibacter” clades according to procedures described previously (13, 24, 26, 37), with a slight modification. Sludge samples collected from the SBR at the end of the aerobic and anaerobic phases were incubated with 20 μCi [1-14C]sodium acetate (Amersham, United Kingdom) and 33Pi (Perkin-Elmer) anaerobically and aerobically, respectively (18). All anaerobic preparations of the sludge samples were carefully flushed with O2-free N2. For FISH/MAR analyses, FISH hybridization methods were the same as those described above for FISH analyses except for the use of coverslips instead of poly-l-lysine-coated slides. Subsequent steps of the MAR protocol were performed as detailed previously by Lee et al. (26). Kodak type NTB was used as an autoradiographic liquid emulsion according to the instructions provided by the manufacturer. For negative controls, sludge samples pasteurized at 80°C for 10 min were used in parallel in all experiments. No MAR-positive signals were found in the control samples, even after 7 days of exposure time.
We used a Zeiss Axiophot epifluorescence microscope (Carl Zeiss, Germany), which was equipped with an HBO100 mercury vapor short-arc lamp, an AxioCam MRm digital camera (Carl Zeiss, Germany), and band path filter sets 10 and 20 for FITC and Cy3, respectively. Photographs were taken and prepared with the software package Axiovision, Ver4.5 (Carl Zeiss, Germany). All specimens for FISH analysis were mounted in Citifluor and were viewed and recorded under proper filter sets. Nomarski photographs were also taken simultaneously. For FISH/MAR combination experiments, coverslips were placed upside down. Views of probe-targeted cells were first identified under fluorescence excitation, and bright-view photographs of the same field using black-silver particles for the MAR signals were then collected.
Flow cytometric cell sorting.
FISH and fluorescence-activated cell sorting (FACS) were carried out with modifications of methods described previously (35, 42, 43, 46). Briefly, sludge samples collected from the SBR at the end of the anaerobic phase were fixed in the same way as described above for FISH experiments. Four FITC-labeled FISH probes, Acc444, Acc184, Acc72, and Acc119 (5 ng/μl, each), that targeted four “Ca. Accumulibacter” clades and their corresponding helpers or competitors were used for FACS (Table 1). After harvesting of the fixed cells by centrifugation, approximately 107 to 108 cells were resuspended in a hybridization buffer containing 0.9 M NaCl, 20 mM Tris-HCl, 0.01% sodium dodecyl sulfate, 35% formamide (pH 7.2), and each respective FISH probe and its correspondent helpers or competitor. The suspended solutions were homogenized by using the homogenizer at 45 s for 5 cycles at maximum speed and incubated for 4 h at 46°C. The cells were centrifuged and then washed in 100 μl hybridization buffer at 48°C for 20 min. The cells were then pelleted by centrifugation and resuspended in ice-cold PBS buffer.
TABLE 1.
FISH oligonucleotides used in this study
| Probe | Sequence (5′-3′) | Target organism | Helper(s) and/or competitor(s) | Reference |
|---|---|---|---|---|
| EUB338Ia | GCTGCCTCCCGTAGGAGT | Eubacteria | 1 | |
| EUB338IIa | GCAGCCACCCGTAGGTGT | Eubacteria | 7 | |
| EUB338IIIa | GCTGCCACCCGTAGGTGT | Eubacteria | 7 | |
| PAO651a | CCCTCTGCCAAACTCCAG | “Ca. Accumulibacter” | 5 | |
| PAO462a | CCGTCATCTACWCAGGGTATTAAC | “Ca. Accumulibacter” | 5 | |
| PAO846a | GTTAGCTACGGCACTAAAAGG | “Ca. Accumulibacter” | 5 | |
| Acc444 | CCCAAGCAATTTCTTCCC | Acc-SG1 | HAcc462 and HAcc426 | This study |
| HAcc466b | CATCTACTCAGGGTATTAA | Acc-SG1 | This study | |
| HAcc426b | CGCCGAAAGAGCTTTACA | Acc-SG1 | This study | |
| Acc184 | GCTCCCAGAACGCAAGGT | Acc-SG2 | CAcc184 | This study |
| CAcc184c | GCTCCCAGAGCGCAAGGT | Acc-SG2 | This study | |
| Acc72 | GAGTGTTACCACCCCGTG | Acc-SG3 | This study | |
| Acc119 | GGATACGTTCCGATGCTT | Acc-SG4 | HAcc99, HAcc139, and CAcc119 | This study |
| HAcc99b | CTCACCCGTCCGCCACTC | Acc-SG4 | This study | |
| HAcc139b | GCTACGTTATCCCCCACTC | Acc-SG4 | This study | |
| CAcc119c | GGGCACGTTCCGATGCAT | Acc-SG4 | This study |
Used as EUBmix and PAOmix, respectively.
Helper probes are designated with an H.
Competitor probes are designated with a C.
For single-cell sorting, the FITC-labeled cells were sonicated at 15% amplitude for 30 s in a sonifier (Sonopuls GM3200; Bandelin, Germany) and were then filtered successively through a gauze filter (pore size, 35 μm) (Falcon type 2235 tube with a strainer cap; BD, NJ) and filter paper (pore size, 10 μm; Millipore, Billerica, MA) to remove large aggregates. Flow cytometric analysis and cell sorting were performed by using a FACSAria II (BD) instrument equipped with a 488-nm laser, which was used for measurements of forward scatter (FSC), side scatter (SSC) (488-nm-band-pass filter for detection), and probe-conferred fluorescence (530-nm-band-pass filter). Fresh sheath fluid produced by BD was used, and new clean sheath fluid was introduced before sample sorting. Cells were analyzed in the logarithmic mode using FSC, SSC, and FITC fluorescence excited by a 488-nm laser. Sorting gates were constructed by using different combinations of FSC and SSC to exclude cell aggregate regions and sort each “Ca. Accumulibacter” clade with high purity (Fig. 1). Based upon preliminary work defining the FSC and SSC properties, different gating regions were defined and limited to very specific regions of the FSC and SSC for specific cell sorting. Candidate populations were selected based upon well-defined gating regions and high FITC fluorescence intensities. Samples were prepared to ensure that analytical event rates never exceeded 3,000 events per second. Sorted cells were collected into sterile 5-ml tubes (BD Falcon) and were stored at −80°C. Instrument control and data analysis were carried out by using FACS DiVa software (BD).
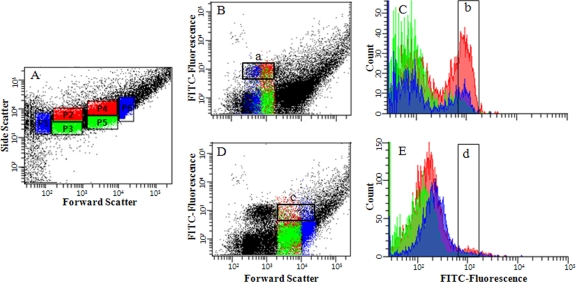
FIG. 1.
Single-cell sorting process of members of four “Ca. Accumulibacter” clades hybridized with the respective specific FISH probes using a flow cytometer (this figure shows the single-cell sorting process for members of Acc-SG3 labeled with probe Acc72). (A) Sorting gates were constructed based on the combinations of FSC and SSC (P1 to P6). Each gate region was set to separate cells with high FITC fluorescence intensity (square boxes labeled a, b, c, and d indicate sorted cells with high FITC intensity). (B and D) Bivariate dot plots based on forward scatter and FITC intensity. (C and E) Cell counts based on FITC fluorescence intensities of the square boxes labeled a and in B and D. Sorted cells of the P1, P2, and P3 regions with high FITC intensity were confirmed through 16S rRNA gene clone libraries and RFLP analysis.
Selection of the sorted candidate populations for analysis of “Ca. Accumulibacter” ppk1 gene homologs.
“Ca. Accumulibacter” clade cells from the sorted “Ca. Accumulibacter” candidate populations for the analysis of “Ca. Accumulibacter” ppk1 gene homologs were screened with a community analysis of cells sorted by the combination of different gating regions and high FITC fluorescence intensity. Genomic DNA from the sorted candidate cells was extracted by boiling at 100°C for 10 min using an Instagene matrix (Bio-Rad). Constructions of 16S rRNA gene clone libraries and their RFLP analyses were carried out according to the procedures described above. All representative clones with unique RFLP patterns were sequenced, and the resulting sequences were compared with the 16S rRNA gene sequences shown in Fig. 2. Sorted “Ca. Accumulibacter” clade cells with the highest frequency of the sorted candidate populations were selected for subsequent analysis of “Ca. Accumulibacter” ppk1 gene homologs.
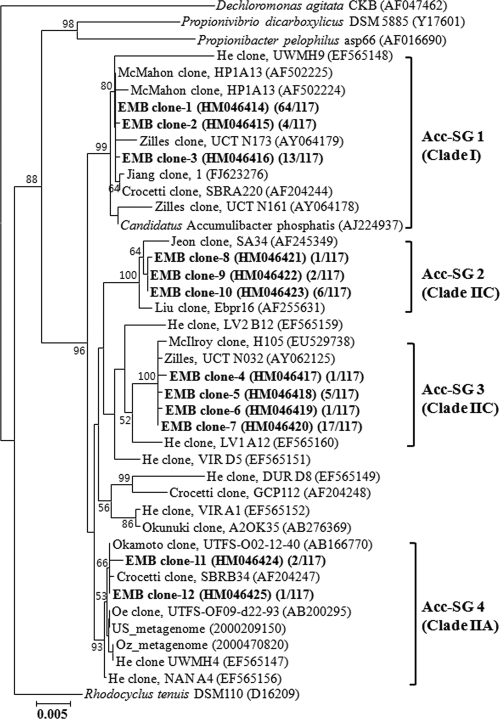
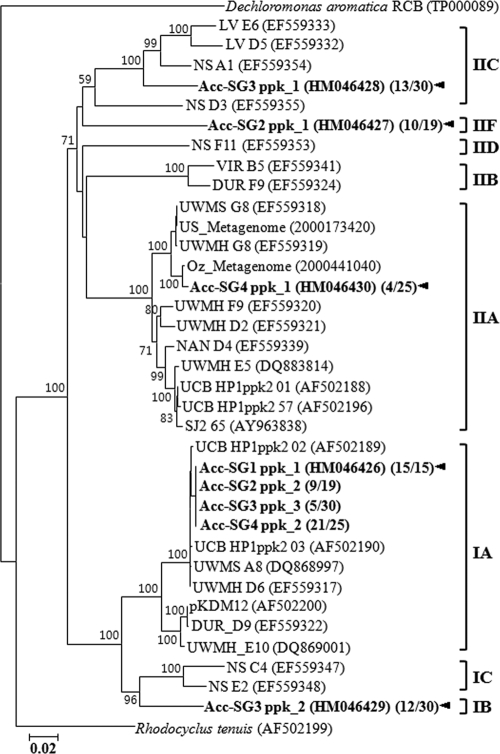
FIG. 2.
Phylogenetic analysis of “Candidatus Accumulibacter”-related 16S rRNA gene sequences. Sequences found in this study (boldface type) were contrasted with those from reference sequences in the GenBank database. Numbers in parentheses indicate frequencies of clones exhibiting the same fragment patterns as the results of the RFLP analysis. Acc-SG1, Acc-SG2, Acc-SG3, and Acc-SG4 designate “Ca. Accumulibacter” clades, and specific FISH probes Acc444, Acc184, Acc72, and Acc119, targeting members of the “Ca. Accumulibacter” clades, were designed, respectively. Bootstrap values are shown in percentages of 1,000 replicates when greater than 50%. Clade names in parentheses were described on the basis of the clade names assigned previously (15). Dechloromonas agitata CKB was used as an outgroup. The scale bar indicates the number of changes per nucleotide position.
Analysis of “Ca. Accumulibacter” ppk1 gene homologs.
Amplification and analysis of “Candidatus Accumulibacter” ppk1 gene homologs from four sorted “Ca. Accumulibacter” candidate cells with the highest frequency of each “Ca. Accumulibacter” clade were carried out with modifications of methods described previously (15, 32). Approximately 10,000 to 50,000 sorted cells of members of the four “Ca. Accumulibacter” clades were pelleted by centrifugation and washed in distilled water (DW). Genomic DNA was extracted by boiling at 100°C for 10 min using an Instagene matrix (Bio-Rad). “Ca. Accumulibacter” ppk1 gene homologs were amplified by using the “Ca. Accumulibacter”-specific primers ACCppk1-254F (TCACCACCGACGGCAAGAC) and ACCppk1-1376R (ACGATCATCAGCATCTTGGC) as described previously (15, 32). The resulting PCR amplicons were cloned into the pCR2.1 vector (TA cloning; Invitrogen). After blue-white screening of the colonies, approximately 20 clones from the respective libraries were verified with ACCppk1-254F and ACCppk1-1376R, and their PCR amplicons were analyzed by using the RFLP approach described above. Clones were grouped according to their RFLP patterns, and representative clones with unique RFLP patterns were sequenced. The retrieved ppk1 gene homologs were compared to available sequences with the BLASTX network service at the NCBI website. Sequences belonging to “Ca. Accumulibacter” ppk1 gene homologs were aligned, and a phylogenetic tree was constructed by using the PROTDIST and Neighbor modules available in the PHYLIP software package, version 3.62 (8).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the 16S rRNA and ppk1 gene sequences determined in this study are HM046414 to HM046425 and HM046426 to HM046430, respectively.
RESULTS
SBR performance.
Two anaerobic-aerobic SBRs for EBPR were continuously operated for more than 6 months under identical conditions with sodium acetate as the sole carbon source, and they showed complete EBPR performance. The two reactors showed good EBPR efficiencies with rapid acetate consumption and orthophosphate (PO4-P) release under anaerobic conditions and polyphosphate accumulation under subsequent aerobic conditions (data not shown). Soluble PO4-P values reached over 80 mg/liter at the end of the anaerobic phase, and there was complete PO4-P uptake at the end of the aerobic phase. FISH analysis using PAOmix probes against EUBmix probes showed that microorganisms labeled with PAOmix probes (“Ca. Accumulibacter”) dominated the EBPR sludge, comprising more than 50% of cells labeled with the EUBmix probes (data not shown). However, “Ca. Accumulibacter” microorganisms with different morphologies were mixed, and their population densities changed over the operation time. Sludge samples containing PAOmix probe-positive cells with different morphologies were used for subsequent molecular analyses and FACS.
Phylogenetic analysis of the 16S rRNA gene clone library.
To analyze “Ca. Accumulibacter” clades with different morphologies, 16S rRNA gene clone libraries were constructed by using sludge samples obtained from SBRs over approximately 6 months of operation. At this time, mixed-liquor-suspended solids (about 3,200 mg/liter) and the profiles of PO4-P and carbon compounds were relatively constant throughout the cycles. A total of approximately 250 bacterial 16S rRNA gene clones were selected randomly and were evaluated by RFLP analysis. Representative clones (92 clones) with unique RFLP patterns were partially sequenced (approximately 500 nucleotides), and the resulting sequences were submitted to GenBank for BlastN searches. “Ca. Accumulibacter”-related 16S rRNA gene clones made up approximately 47% (117 of 250 clones) of the total number of clones. Therefore, representative 16S rRNA gene clones (12 of 92 clones) related to “Candidatus Accumulibacter” were more completely sequenced (more than 1,420 nucleotides) and were analyzed phylogenetically. These sequences were clearly classified into four major phylogenetic “Ca. Accumulibacter” clades (Acc-SGs), as shown in Fig. 2. All “Ca. Accumulibacter” clones obtained from this study shared 97.5 to 99.9% 16S rRNA gene sequence similarities. Among them, Acc-SG1 and Acc-SG3 clones were most distantly related, with 97.5 to 97.6% sequence similarities. However, within the same “Ca. Accumulibacter” clades, clones were very closely related to each other, sharing 99.7 to 99.9% sequence similarities.
In our study, the most dominant “Ca. Accumulibacter” clones (81 of 117 clones) were affiliated with Acc-SG1, which contained the type 16S rRNA gene sequence, clone R6, of “Candidatus Accumulibacter phosphatis” (16). Clones belonging to members of Acc-SG3 (24 of 117 clones) were the next most dominant clones. Previous studies reported that Acc-SG3, related to 16S rRNA gene sequences, originated from various geographic locations, including Australia, the United States, France, and Japan (5, 6, 28, 32, 48). Clones related to Acc-SG2 (9 of 117 clones) and Acc-SG4 (3 of 117 clones) made up a relatively small percentage of the clone libraries. It was surprising that Acc-SG4-related clones had the smallest percentage because they were affiliated with 16S rRNA gene sequences from a metagenomic analysis of “Ca. Accumulibacter,” which were predominant in two EBPR reactors from the United States and Australia (designated US-Metagenome and Oz-Metagenome) (12).
FISH probe design and evaluation.
The specific oligonucleotide FISH probes Acc444, Acc184, Acc72, and Acc119, targeting members of Acc-SG1, Acc-SG2, Acc-SG3, and Acc-SG4, respectively, were designed to further clarify the phylogenies of different morphotypes hybridized by PAOmix probes. Additionally, probe analysis using the RDPII “Probe Match” tool also showed that the four designed FISH probes had sufficient specificities to each “Ca. Accumulibacter” clade (4) and targeted all “Ca. Accumulibacter” 16S rRNA gene sequences retrieved in this study. The Acc444 and Acc119 probes, which targeted the “Ca. Accumulibacter” clades of Acc-SG1 and Acc-SG4, respectively, were used with two unlabeled oligonucleotide helpers, which covered adjacent regions and helped to open the secondary structure of the rRNA molecules (10). Additionally, HAcc466 could not be used with a PAO462 probe of PAOmix (5) because there was one overlapping nucleotide base. Thus, where HAcc466 was applied, PAOmix indicated the mixture of PAO651 and PAO846 in this study. It was confirmed that the mixture of only PAO651 and PAO846 was sufficient to cover all “Ca. Accumulibacter” 16S rRNA gene sequences shown in Fig. 2 by checking the coverage abilities of the two FISH probes. The Acc184 and Acc119 probes, which targeted the “Ca. Accumulibacter” clades Acc-SG2 and Acc-SG4, respectively, were used with unlabeled competitors to improve their FISH probe specificities (10). The FISH probe sequences, competitors, and helpers used in this study are listed in Table 1.
Optimal FA concentrations for FISH analysis and FACS were determined by using increments of FA concentrations for PFA-fixed sludge since no pure culture was available. The optimal FA concentration was approximately 35% for all FISH probes. Nonprobe controls were always prepared to examine autofluorescent bacteria because autofluorescent cells existed in our sludge in a small proportion. However, because their fluorescence intensities and morphologies were quite different from those of “Ca. Accumulibacter,” these cells could be recognized and easily excluded throughout the study. FISH analysis showed that all bacterial cells responding to probes of Acc444, Acc184, Acc72, and Acc119, were also positive for PAOmix probes in sludge samples, indicating that the four “Ca. Accumulibacter” clades retrieved in this study were authentic phylogenetic clades of “Candidatus Accumulibacter” (data not shown).
FISH and FISH/MAR analyses.
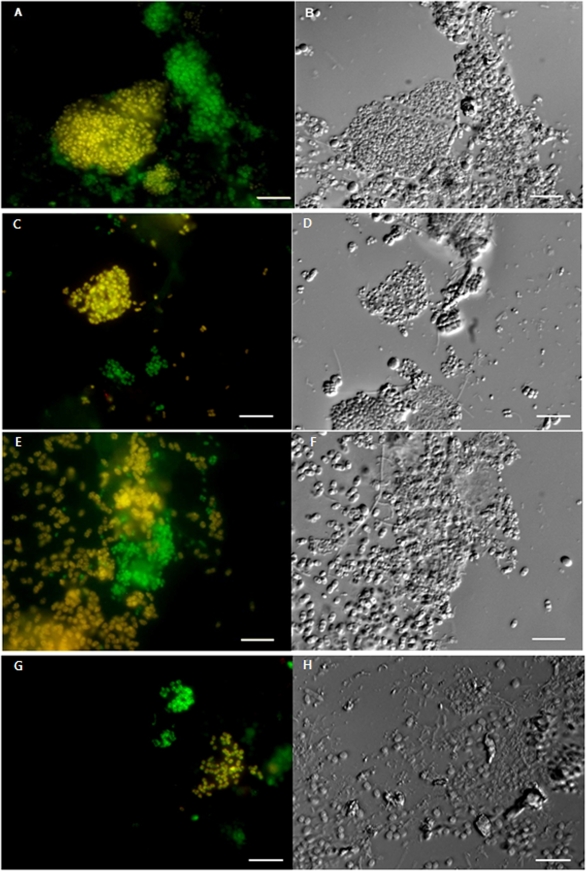
Experiments with “Ca. Accumulibacter” FISH probes revealed that four “Ca. Accumulibacter” clades with different morphologies existed together in the sludge, although their population densities fluctuated over the operational time (data not shown). Probe Acc444-targeted cells (members of Acc-SG1) were coccus-type cells (approximately 1 μm in size) (Fig. 3 A and B) and were always found in SBR sludge with somewhat constant and dominant population densities (approximately 30 to 35% of EUBmix binding cells). Bacterial cells responding to probe Acc72 (members of Acc-SG3) were the second major “Ca. Accumulibacter” clade (approximately 5 to 15% of EUBmix binding cells), and their population densities fluctuated greatly with operation time, although the reactors were operated identically. Probe Acc72-positive cells had coccobacillus cell types approximately 2 to 3 μm in size, which clearly differentiated them from other “Ca. Accumulibacter” clades (Fig. 3E and F). Nomarski photographs of the same fields showed additional evidence for their morphological differences (Fig. 3B, D, F, and H). Bacterial cells of Acc-SG2 members that were positive with probe Acc184 existed as the minority (approximately 1 to 5% of EUBmix binding cells) and had a morphology that was almost similar to that of Acc-SG1 members (Fig. 3C and D). Probe Acc119-targeted cells (members of Acc-SG4), which were affiliated with 16S rRNA gene sequences from a metagenomic analysis of “Ca. Accumulibacter,” were always present in very small numbers (<1% of EUBmix binding cells), as described above for the phylogenetic analysis of the 16S rRNA gene clone library. Their cell morphologies were also the same as those of members of Acc-SG1 (Fig. 3G and H).
FIG. 3.
FISH and Nomarski images of “Ca. Accumulibacter” clades showing different morphologies and sizes in activated sludge. (A, C, E, and G) FISH images show “Ca. Accumulibacter” cells responding to the PAOmix (fluorescein [green]) and the “Ca. Accumulibacter” clade-specific (Cy3 [red]) probes Acc444, Acc184, Acc72, and Acc119, which targeted members of Acc-SG1 (A), Acc-SG2 (C), Acc-SG3 (E), and Acc-SG4 (G), respectively. Yellow cells (overlay of red and green) indicate cells of “Ca. Accumulibacter clades” that were labeled with PAOmix and the “Ca. Accumulibacter” FISH probe. (B, D, F, and H) Nomarski photomicrographs of the same fields with FISH views. Members of Acc-SG1 (A), Acc-SG2 (C), and Acc-SG4 (G) were coccus shaped, at approximately 1 μm in size. Members of Acc-SG3 (E) were coccobacillus shaped, at approximately 2 to 3 μm in size. Scale bars for all panels are 10 μm.
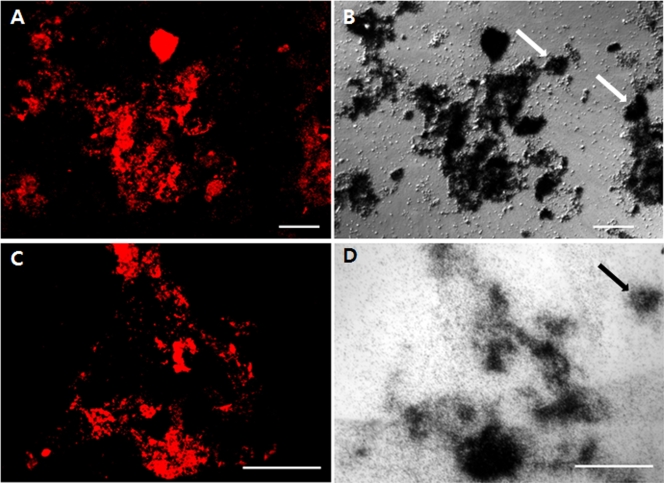
FISH/MAR combination analyses demonstrated that all bacterial cells responding to PAOmix, which covered all bacteria that were positive with probes of Acc444, Acc184, Acc72, and Acc119, were able to take up sodium acetate in the anaerobic phase and, subsequently, to accumulate orthophosphate in the aerobic phase (Fig. 4), indicating that all respective “Ca. Accumulibacter” clades were involved in the EBPR process. However, FISH/MAR experiments clearly showed that the sludge samples contained PAOmix-negative cells, which were able to take up acetate in the anaerobic period and to accumulate polyphosphate in the aerobic period (Fig. 4).
FIG. 4.
FISH and MAR images of activated sludge. (A and C) FISH images showing bacteria hybridized with PAOmix probes (PAO462, PAO651, and PAO844) (Cy3 [red]). (B and D) Nomarski images showing MAR-positive cells taking up [14C]sodium acetate under anaerobic conditions (B) and 33Pi under aerobic conditions (D). Arrows in B and D indicate MAR-positive microorganisms not hybridizing with PAOmix probes. Scale bars for all panels are 100 μm.
FACS analysis.
Although population densities of members of Acc-SG2, Acc-SG3, and Acc-SG4 were generally very low, relatively enriched sludge samples through FISH investigations during the SBR operations were taken for flow cytometric cell sorting. Well-dispersed sludge samples with relatively high population densities were hybridized with the specific FITC-labeled FISH probes Acc444, Acc184, Acc72, and Acc119, targeting the four “Ca. Accumulibacter” clades Acc-SG1, Acc-SG2, Acc-SG3, and Acc-SG4, respectively, and their corresponding helpers or competitors. Nonlabeled control samples were used to determine background levels. From preliminary tests, it was found that FA-fixed sludge samples were strongly inclined to form cell aggregates. Therefore, although dispersion by sonication and successive filtrations using 35- and 10-μm-pore-size filters of FISH probe-labeled cells were performed just before flow cytometric cell sorting, FACS analyses of members of Acc-SG2, Acc-SG3, and Acc-SG4 with relatively low population densities failed, except for members of Acc-SG1 with high population densities. Sorted cells of minor “Ca. Accumulibacter” clades were contaminated with large amounts of probe Acc444-targeted members of Acc-SG1. It was inferred that contaminations were caused by the formation of cell aggregates. Therefore, to exclude cell aggregates, several sorting gates were constructed on the basis of different combinations of FSC and SSC to find the separated cell region, and sorted cells were collected from each sorting gate. (See Fig. 1A for the sorting process of probe Acc72-targeted members of Acc-SG3. In this case, six sorting gates, P1 to P6, were constructed.)
Based on bivariate plots of FITC fluorescence versus cell counts, the sorted “Ca. Accumulibacter” candidate populations showing high fluorescence frequencies were chosen for evaluation of sorting efficiencies (P1, P2, and P3 fractions were chosen for probe Acc72-targeted members of Acc-SG3) (Fig. 1Ba). The selected “Ca. Accumulibacter” candidate populations were evaluated by using RFLP of 16S rRNA genes of sorted cells, and finally, one “Ca. Accumulibacter” candidate population was chosen for the analysis of “Ca. Accumulibacter” ppk1 gene homologs (the P2 fraction was finally selected for probe Acc72-targeted members of Acc-SG3) (Fig. 1Bb). Through this process, relatively high purities of sorted cells belonging to each “Ca. Accumulibacter” clade were obtained. Based on RFLP of 16S rRNA genes, the population densities of the four probe-targeted “Ca. Accumulibacter” cells of Acc-SG1, Acc-SG2, Acc-SG3, and Acc-SG4 were approximately >90%, >30%, >65%, and ∼10%, respectively. However, the sorted cells of the Acc-SG2, Acc-SG3, and Acc-SG4 clades still contained members of Acc-SG1.
Analysis of “Ca. Accumulibacter” ppk1 gene homologs.
To retrieve “Ca. Accumulibacter” ppk1 gene homologs from four sorted samples belonging to each “Ca. Accumulibacter” clade, a “Ca. Accumulibacter” ppk1-specific PCR primer set (32) was applied to the extracted genomic DNAs of sorted cells, and their clone libraries were constructed. Approximately 20 white clones from the respective libraries were randomly selected and analyzed by using RFLP analysis by HhaI and HaeIII double digestion. Representative clones with all different fragment patterns were sequenced and described as ppk1 gene homologs through a BLASTX search of the GenBank database. Their sequence comparisons and relationships were subsequently analyzed via the construction of a phylogenetic tree (Fig. 5).
FIG. 5.
Phylogram indicating inferred relatedness of ppk1 gene homologs from the sorted “Candidatus Accumulibacter” clades. Sequences found in this study were contrasted with those from reference sequences in the GenBank database, and the ppk1 genes were classified on the basis of the clade names assigned previously (39). Bootstrap values are shown in percentages of 1,000 replicates when greater than 50%. Numbers in parentheses indicate frequencies of colonies exhibiting the same restriction patterns of ppk1 gene homologs in libraries constructed from the respective sorted cells of “Ca. Accumulibacter” clades. Arrowheads indicate the inferred ppk1 gene sequences corresponding to four “Ca. Accumulibacter” clades. The scale bar indicates the number of changes per nucleotide position.
Analyses of sorted cells responding to probe Acc444 (members of Acc-SG1) produced just one kind of ppk1 gene homolog (15 of 15), which was affiliated with clade IA of “Ca. Accumulibacter” ppk1 genes, classified previously by He et al. (15). The production of just one kind of ppk1 gene homolog means tight relatedness between the 16S rRNA gene and ppk1 gene sequences. Probe Acc184-targeted sorted cells (members of Acc-SG2) produced two ppk1 gene homologs with similar portions. However, because one ppk1 gene homolog (9 of 19) was identical to that of probe Acc444-targeted sorted cells (members of Acc-SG1), it was inferred that the ppk1 gene homolog might be derived from the contamination of members of Acc-SG1, and the ppk1 gene homolog could be excluded easily from the relatedness between the 16S rRNA gene sequence and the ppk1 gene homolog. Another ppk1 gene homolog (10 of 19), which might be derived from probe Acc184-targeted members of Acc-SG2, was distantly affiliated with clade IIF of “Ca. Accumulibacter” ppk1 gene homologs.
Surprisingly, probe Acc72-targeted sorted cells (members of Acc-SG3) with distinctive morphologies produced three ppk1 gene homologs: two major ppk1 gene homologs (13 and 12 of 30) and one minor ppk1 gene homolog (5 of 30). The minor ppk1 gene homolog was also identical to that of probe Acc444-targeted sorted cells (members of Acc-SG1), suggesting that the minor homolog might originate from the contamination of members of Acc-SG1. Therefore, it was inferred that probe Acc72-targeted “Ca. Accumulibacter” cells comprised two copies of ppk1 gene homologs, which were affiliated with different clades, clades IB and IIC, of “Ca. Accumulibacter” ppk1 gene homologs with 83.9% DNA sequence identity. Probe Acc119-targeted sorted cells (members of Acc-SG4) with very low population densities generated two kinds of ppk1 gene homologs. The major ppk1 gene homolog (21 of 25) was identical to that of probe Acc444-targeted members of Acc-SG1. The minor ppk1 gene homolog (4 of 25) was affiliated with clade IIA of “Ca. Accumulibacter” ppk1 genes. Although probe Acc119-targeted sorted cells (members of Acc-SG4) produced two kinds of ppk1 gene homologs, the major ppk1 gene homolog was easily excluded from the possibility of being Acc-SG4-related ppk1 gene homologs because the sorted cells contained large amounts of members of Acc-SG1. Metagenomic analysis of “Ca. Accumulibacter” also supported the relationship between 16S rRNA sequences of members of Acc-SG4 and their ppk1 gene sequence (12).
DISCUSSION
This paper describes the analysis of fine-scale population structures of “Candidatus Accumulibacter phosphatis” using FISH and flow cytometric cell sorting with EBPR sludge. Through previous studies, many 16S rRNA gene sequences related to “Ca. Accumulibacter” have been retrieved from either laboratory-scale reactors or full-scale plants performing EBPR (5, 6, 15, 16, 20, 28, 32, 48). Interestingly, it has been shown that 16S rRNA gene sequences were able to be classified into several phylogenetic clades, and several clades of “Ca. Accumulibacter” were found at the same time from a single sludge source (3, 5, 9, 15, 28, 32, 39, 48). Our study identified four different “Ca. Accumulibacter” clades, Acc-SG1, Acc-SG2, Acc-SG3, and Acc-SG4, on the basis of 16S rRNA gene sequences (Fig. 2). However, population densities of “Ca. Accumulibacter” were quite different from those of previous studies (12, 15); namely, members of Acc-SG4 were predominant in other studies and metagenomic analyses (US-Metagenome and OZ-Metagenome sludge), but they were minor members, at less than 1%, in our sludge. Although we operated the SBR reactors with the same medium and conditions with SBR operation for US-Metagenome sludge, members of Acc-SG4 were not enriched (data not shown). This result suggests that members of Acc-SG4 might have different ecotypic and phenotypic properties from those of other studies, although they formed a same cluster in the phylogenetic tree. On the other hand, members of Acc-SG1 were predominant and remained somewhat constant in our sludge, but they were minor in the metagenomic analyses. Recently, Flowers et al. (9) showed that “Ca. Accumulibacter” cells of clade IA, which might be members of Acc-SG1 and have nitrate reduction ability, were enriched.
The specific FISH probes Acc444, Acc184, Acc72, and Acc119, which targeted four “Ca. Accumulibacter” clades, and some helpers and competitors were designed by using the ARB program to investigate morphologies and physiological characteristics. However, when probes Acc444 and Acc119, which targeted members of Acc-SG1 and Acc-SG4, respectively, were used independently, their FISH signals were too weak and were not recognized with a digital camera and an epifluorescence microscope. Therefore, adjacent helper oligonucleotides were added to improve their signal intensities (10), and their fluorescence intensities increased significantly. FISH analyses showed that our sludge samples contained “Ca. Accumulibacter” cells with two different morphotypes (Fig. 2.). Members of clades Acc-SG1, Acc-SG2, and Acc-SG4 were coccus shaped (about 1 μm in size), while members of Acc-SG3 were large and coccobacillus shaped (about 2 to 3 μm in size), which clearly differentiated them from other members. Kong et al. (22) previously identified two morphologically different (also stated as cocci and coccobacilli) “Ca. Accumulibacter” cells responding to probe PAO651 in laboratory-scale EBPR reactors. Recently, Carvalho et al. (3) reported two different morphotypes of “Ca. Accumulibacter” targeted by PAOmix probes from denitrifying EBPR sludge, which were termed as having coccus and rod morphologies. Those researchers proposed that “Ca. Accumulibacter” cells with a coccus morphotype were not able to use nitrate as an electron acceptor but were able to use oxygen and, possibly, nitrite. “Ca. Accumulibacter” cells with a rod morphotype were proposed to be PAOs, able to use nitrate, nitrite, and oxygen. However, because previous studies did not provide 16S rRNA gene sequence information, the two morphotypic cells were not able to correlate with the members of “Ca. Accumulibacter” clades Acc-SC1, Acc-SC2, Acc-SC3, and Acc-SC4 of this study.
FISH/MAR combination analyses showed that “Ca. Accumulibacter” cells responding to PAOmix, which covered members of all four PAO clades, hybridized with FISH probes Acc444, Acc184, Acc72, and Acc119, were involved in EBPR. However, samples of activated sludge contained PAOmix-negative cells that were able to take up acetate in the anaerobic period and/or to accumulate polyphosphate in the aerobic period (Fig. 3), which suggests that other PAOs not belonging to “Ca. Accumulibacter” might be present in our sludge, as reported previously. Kong et al. (24) previously reported that another group of bacteria belonging to “Ca. Actinobacteria” that have been found in some EBPR systems could be PAOs. Other researchers have shown that not all “Ca. Accumulibacter” cells took up 33Pi during the aerobic stage (23, 27); Wong et al. (45) found that a considerable proportion of PAOmix probe-labeled cells appeared to contain little or no poly(P) granules with an approach utilizing dual staining with FISH/DAPI (4′,6-diamidino-2-phenylindole).
Previous studies have shown that polyphosphate kinase (ppk1) genes involved in the production of polyphosphate are useful for elucidating fine “Ca. Accumulibacter” diversity as a genetic marker because ppk1 genes are more diverse than are 16S rRNA gene sequences, and “Ca. Accumulibacter” has only one copy of the ppk1 gene (15, 33). He and colleagues (15) grouped the ppk1 genes into five distinct clades, designated clades I, IIA, IIB, IIC, and IID, and laboratory-scale reactors have shown only clades IA, IIA, and IID (39, 44). Using FISH and flow cytometric cell sorting, we attempted to link the 16S rRNA gene sequences of four “Ca. Accumulibacter” clades and their ppk1 genes in the “Ca. Accumulibacter” lineages. Flow cytometric cell sorting has been used in environmental microbiology because it is possible to analyze complex microbial cell populations rapidly on a single-cell basis (35, 38, 41, 46). However, our flow cytometric cell sorting of the four “Ca. Accumulibacter” clades labeled with FISH probes failed, except for members of Acc-SG1 that had a high population density. Although dispersion by sonication and successive filtrations were carried out just before flow cytometric cell sorting, PFA-fixed “Ca. Accumulibacter” cells formed aggregates rapidly, unlike unfixed bacteria. Therefore, to exclude cell aggregates, several sorting gates were constructed on the basis of different combinations of FSC and SSC. Finally, candidate cells of each “Ca. Accumulibacter” clade at a high purity were able to be collected from a specific sorting gate for analysis of “Ca. Accumulibacter” ppk1 gene homologs (Fig. 1).
“Ca. Accumulibacter” ppk1 gene homologs were retrieved from four sorted samples by using a “Ca. Accumulibacter” ppk1-specific PCR primer set. Surprisingly, two different ppk1 gene homologs were retrieved from probe Acc72-targeted “Ca. Accumulibacter” cells (members of Acc-SG3) with rod morphotypes. They were affiliated with clades IB and IIC of “Ca. Accumulibacter” ppk1 gene homologs with a relatively low DNA sequence identity (83.9%), suggesting that members of Acc-SG3 may have experienced a lateral gene transfer of ppk1 gene homologs. However, the possibility that the Acc72-enriched cells contained a mixture of several discrete populations, each carrying a different single ppk1 allele, cannot be ruled out. This is the first report showing two copies of “Ca. Accumulibacter” ppk1 gene homologs and showing the presence of a clade IIC ppk1 gene homolog from laboratory-scale reactors. Sorted cells responding to probe Acc444 (members of Acc-SG1) produced clade IA ppk1 gene homologs of “Ca. Accumulibacter” strains. “Ca. Accumulibacter” cells containing the clade IA ppk1 gene homolog were predominant in our study, but they were minor members in metagenomic analyses (US-Metagenome and OZ-Metagenome sludge) (13). Recently, Flowers et al. (9) investigated “Ca. Accumulibacter” cells of clade IA using a FISH probe with just one nucleotide difference from our probe Acc444 in a flanking region, indicating that the relationship between 16S rRNA gene sequences of clade IA and ppk1 gene homologs was consistent with our results. However, the morphology and nitrate reduction ability of clade IA “Ca. Accumulibacter” cells were not consistent with those reported previously by Carvalho et al. (3). In conclusion, this study and previous results suggest that “Ca. Accumulibacter” clades may have been adapted to different ecological environments and represent various physiological properties such as denitrifying PAOs. Knowledge of their differences will ultimately benefit the control and optimization of EBPR systems.
Acknowledgments
These efforts were supported by grants from the MEST to the Environmental Biotechnology National Core Research Center (grant R15-2003-012-02002-0) and to the 21C Frontier Microbial Genomics and Application Center Program (grant MG05-0104-4-0), Ministry of Science and Technology, South Korea.
We thank Hyun Cho and the Center for Research Facilities at Chung-Ang University for their help with the FACS analysis.
Footnotes
Published ahead of print on 23 April 2010.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond, P. L., P. Hugenholtz, J. Keller, and L. L. Blackall. 1995. Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors. Appl. Environ. Microbiol. 61:1910-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho, G., P. C. Lemos, A. Oehmen, and M. A. M. Reis. 2007. Denitrifying phosphorus removal: linking the process performance with the microbial community structure. Water Res. 41:4383-4396. [DOI] [PubMed] [Google Scholar]
- 4.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, A. S. Kulam-Syed-Mohideen, D. M. McGarrell, A. M. Bandela, E. Cardenas, G. M. Garrity, and J. M. Tiedje. 2007. The Ribosomal Database Project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res. 35:D169-D172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crocetti, G. R., P. Hugenholtz, P. L. Bond, A. Schuler, J. Keller, D. Jenkins, and L. L. Blackall. 2000. Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl. Environ. Microbiol. 66:1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabert, P., B. Sialve, J. P. Delgenes, R. Moletta, and J. J. Godon. 2001. Characterisation of the microbial 16S rDNA diversity of an aerobic phosphorus-removal ecosystem and monitoring of its transition to nitrate respiration. Appl. Microbiol. Biotechnol. 55:500-509. [DOI] [PubMed] [Google Scholar]
- 7.Daims, H., A. Brühl, R. Amann, K. H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 2002. PHYLIP (phylogeny inference package), version 3.6a. Department of Genetics, University of Washington, Seattle, WA.
- 9.Flowers, J. J., S. He, S. Yilmaz, D. R. Noguera, and K. D. McMahon. 2009. Denitrification capabilities of two biological phosphorus removal sludges dominated by different “Candidatus Accumulibacter” clades. Environ. Microbiol. Rep. 1:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs, B. M., F. O. Glöckner, J. Wulf, and R. Amann. 2000. Unlabeled helper oligonucleotides increase the in situ accessibility to 16S rRNA of fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 66:3603-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuhs, G. W., and M. Chen. 1975. Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater. Microb. Ecol. 2:119-138. [DOI] [PubMed] [Google Scholar]
- 12.Garcia Martin, H., N. Ivanova, V. Kunin, F. Warnecke, K. W. Barry, A. C. McHardy, C. Yeates, S. He, A. A. Salamov, E. Szeto, E. Dalin, N. H. Putnam, H. J. Shapiro, J. L. Pangilinan, I. Rigoutsos, N. C. Kyrpides, L. L. Blackall, K. D. McMahon, and P. Hugenholtz. 2006. Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities. Nat. Biotechnol. 24:1263-1269. [DOI] [PubMed] [Google Scholar]
- 13.Ginige, M. P., P. Hugenholtz, H. Daims, M. Wagner, J. Keller, and L. L. Blackall. 2004. Use of stable-isotope probing, full-cycle rRNA analysis, and fluorescence in situ hybridization-microautoradiography to study a methanol-fed denitrifying microbial community. Appl. Environ. Microbiol. 70:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammes, F., M. Berney, Y. Wang, M. Vital, O. Koster, and T. Egli. 2008. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 42:269-277. [DOI] [PubMed] [Google Scholar]
- 15.He, S., D. L. Gall, and K. D. McMahon. 2007. “Candidatus Accumulibacter” population structure in enhanced biological phosphorus removal sludges as revealed by polyphosphate kinase genes. Appl. Environ. Microbiol. 73:5865-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesselmann, R. P. X., C. Werlen, D. Hahn, J. R. van der Meer, and A. J. B. Zehnder. 1999. Enrichment, phylogenetic analysis and detection of a bacterium that performs enhanced biological phosphate removal in activated sludge. Syst. Appl. Microbiol. 22:454-465. [DOI] [PubMed] [Google Scholar]
- 17.Hugenholtz, P., G. W. Tyson, and L. L. Blackall. 2002. Design and evaluation of 16S rRNA-targeted oligonucleotide probes for fluorescence in situ hybridization. Methods Mol. Biol. 179:29-42. [DOI] [PubMed] [Google Scholar]
- 18.Jeon, C. O., and J. M. Park. 2000. Enhanced biological phosphorus removal in a sequencing batch reactor supplied with glucose as a sole carbon source. Water Res. 34:2160-2170. [Google Scholar]
- 19.Jeon, C. O., W. Park, P. Padmanabhan, C. DeRito, J. R. Snape, and E. L. Madsen. 2003. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. U. S. A. 100:13591-13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon, C. O., D. S. Lee, and J. M. Park. 2003. Microbial communities in activated sludge performing enhanced biological phosphorus removal in a sequencing batch reactor. Water Res. 37:2195-2205. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. M., N. T. Le, B. S. Chung, J. H. Park, J.-W. Bae, E. L. Madsen, and C. O. Jeon. 2008. Influence of soil components on the biodegradation of benzene, toluene, ethylbenzene, and o-, m-, and p-xylenes by the newly isolated bacterium Pseudoxanthomonas spadix BD-a59. Appl. Environ. Microbiol. 74:7313-7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong, Y., M. Beer, G. N. Rees, and R. J. Seviour. 2002. Functional analysis of microbial communities in aerobic:anaerobic sequencing batch reactors fed with different phosphorus/carbon ratios. Microbiology 148:2299-2307. [DOI] [PubMed] [Google Scholar]
- 23.Kong, Y., J. L. Nielsen, and P. H. Nielsen. 2004. Microautoradiographic study of Rhodocyclus-related polyphosphate-accumulating bacteria in full-scale enhanced biological phosphorus removal plants. Appl. Environ. Microbiol. 70:5383-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong, Y., J. L. Nielsen, and P. H. Nielsen. 2005. Identity and ecophysiology of uncultured actinobacterial polyphosphate-accumulating organisms in full-scale enhanced biological phosphorus removal plants. Appl. Environ. Microbiol. 71:4076-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-147. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York, NY.
- 26.Lee, N., P. H. Nielsen, K. H. Andreasen, S. Juretschko, J. L. Nielsen, K. H. Schleifer, and M. Wagner. 1999. Combination of fluorescent in situ hybridization and microautoradiography—a new tool for structure-function analyses in microbial ecology. Appl. Environ. Microbiol. 65:1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, N., P. H. Nielsen, H. Aspegren, M. Henze, K. H. Schleifer, and J. L. Jansen. 2003. Long-term population dynamics and in situ physiology in activated sludge systems with enhanced biological phosphorus removal operated with and without nitrogen removal. Syst. Appl. Microbiol. 26:211-227. [DOI] [PubMed] [Google Scholar]
- 28.Liu, W.-T., A. T. Nielsen, J.-H. Wu, C.-S. Tsai, Y. Matsuo, and S. Molin. 2001. In situ identification of polyphosphate- and polyhydroxyalkanoate-accumulating traits for microbial populations in a biological phosphorus removal process. Environ. Microbiol. 3:110-122. [DOI] [PubMed] [Google Scholar]
- 29.Lu, S., M. Park, H. S. Ro, D. S. Lee, W. Park, and C. O. Jeon. 2006. Analysis of microbial communities using culture-dependent and culture-independent approaches in an anaerobic/aerobic SBR reactor. J. Microbiol. 44:155-161. [PubMed] [Google Scholar]
- 30.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maszenan, A. M., R. J. Seviour, B. K. C. Patel, P. Schumann, Y. Burghardt, Y. Tokiwa, and H. M. Stratton. 2000. Three isolates of novel polyphosphate accumulating gram positive cocci, obtained from activated sludge, belong to a new genus, Tetrasphaera gen. nov., and description of two new species, Tetrasphaera japonica sp. nov. and Tetrasphaera australiensis sp. nov. Int. J. Syst. Evol. Microbiol. 50:593-603. [DOI] [PubMed] [Google Scholar]
- 32.McMahon, K. D., M. A. Dojka, N. R. Pace, D. Jenkins, and J. D. Keasling. 2002. Polyphosphate kinase from activated sludge performing enhanced biological phosphorus removal. Appl. Environ. Microbiol. 68:4971-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahon, K. D., S. Yilmaz, S. He, D. L. Gall, D. Jenkins, and J. D. Keasling. 2007. Polyphosphate kinase genes from full-scale activated sludge plants. Appl. Microbiol. Biotechnol. 77:167-173. [DOI] [PubMed] [Google Scholar]
- 34.Mino, T., M. C. M. Van Loosdrecht, and J. J. Heijnen. 1998. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 32:3193-3207. [Google Scholar]
- 35.Miyauchi, R., K. Oki, Y. Aoi, and S. Tsuneda. 2007. Diversity of nitrite reductase genes in “Candidatus Accumulibacter phosphatis”-dominated cultures enriched by flow-cytometric sorting. Appl. Environ. Microbiol. 73:5331-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura, K., A. Hiraishi, Y. Yoshimi, M. Kawaharasaki, K. Matsuda, and Y. Kamagata. 1995. Microlunatus phosphovorus gen. nov. sp. nov., a new Gram-positive polyphosphate accumulating bacterium isolated from activated sludge. Int. J. Syst. Bacteriol. 45:17-22. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen, J. L., D. Christensen, M. Kloppenborg, and P. H. Nielsen. 2003. Quantification of cell-specific substrate uptake by probe-defined bacteria under in situ conditions by microautoradiography and fluorescence in situ hybridization. Environ. Microbiol. 5:202-211. [DOI] [PubMed] [Google Scholar]
- 38.Orphan, V. J. 2009. Methods for unveiling cryptic microbial partnerships in nature. Curr. Opin. Microbiol. 12:231-237. [DOI] [PubMed] [Google Scholar]
- 39.Peterson, S. B., F. Warnecke, J. Madejska, K. D. McMahon, and P. Hugenholtz. 2008. Environmental distribution and population biology of Candidatus Accumulibacter, a primary agent of biological phosphorus removal. Environ. Microbiol. 10:2692-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stante, L., C. M. Cellamare, F. Malaspina, G. Bortone, and A. Tiche. 1997. Biological phosphorus removal by pure culture of Lampropedia spp. Water Res. 31:1317-1324. [Google Scholar]
- 41.Stepanauskas, R., and M. E. Sieracki. 2007. Matching phylogeny and metabolism in the uncultured marine bacteria, one cell at a time. Proc. Natl. Acad. Sci. U. S. A. 104:9052-9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallner, G., R. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 43.Wallner, G., B. Fuchs, S. Spring, W. Beisker, and R. Amann. 1997. Flow sorting of microorganisms for molecular analysis. Appl. Environ. Microbiol. 63:4223-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilmes, P., A. F. Andersson, M. G. Lefsrud, M. Wexler, M. Shah, B. Zhang, R. L. Hettich, P. L. Bond, N. C. Verberkmoes, and J. F. Banfield. 2008. Community proteogenomics highlights microbial strain-variant protein expression within activated sludge performing enhanced biological phosphorus removal. Int. Soc. Microb. Ecol. 2:853-864. [DOI] [PubMed] [Google Scholar]
- 45.Wong, M.-T., T. Mino, R. J. Seviour, M. Onuki, and W.-T. Liu. 2005. In situ identification and characterization of the microbial community structure of full-scale enhanced biological phosphorous removal plants in Japan. Water Res. 39:2901-2914. [DOI] [PubMed] [Google Scholar]
- 46.Zehr, J. P., S. R. Bench, J. C. Brandon, I. Hewson, F. Niazi, T. Shi, J. Tripp, and J. P. Affourtit. 2008. Globally distributed uncultivated oceanic N2-fixing cyanobacteria lack oxygenic photosystem II. Science 322:1110-1112. [DOI] [PubMed] [Google Scholar]
- 47.Zeng, R. J., A. M. Saunders, Z. Yuan, L. L. Blackall, and J. Keller. 2003. Identification and comparison of aerobic and denitrifying polyphosphate-accumulating organisms. Biotechnol. Bioeng. 83:140-148. [DOI] [PubMed] [Google Scholar]
- 48.Zilles, J. L., J. Peccia, M.-W. Kim, C.-H. Hung, and D. R. Noguera. 2002. Involvement of Rhodocyclus-related organisms in phosphorus removal in full-scale wastewater treatment plants. Appl. Environ. Microbiol. 68:2763-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]