Abstract
Bacterial biofilms are associated with a large number of persistent and chronic infections. Biofilm-dwelling bacteria are particularly resistant to antibiotics and immune defenses, which makes it hard if not impossible to eradicate biofilm-associated infections. In the urinary tract, free iron is strictly limited but is critical for bacterial growth. Biofilm-associated Escherichia coli cells are particularly desperate for iron. An attractive way of inhibiting biofilm formation is to fool the bacterial regulatory system for iron uptake. Here, we demonstrate that biofilm formation can be impaired by the addition of divalent metal ions, such as Zn(II) and Co(II), which inhibit iron uptake by virtue of their higher-than-iron affinity for the master controller protein of iron uptake, Fur. Reduced biofilm formation of urinary tract-infectious E. coli strains in the presence of Zn(II) was observed in microtiter plates and flow chambers as well as on urinary catheters. These results further support that iron uptake is indeed crucial for biofilm formation, and thereby, targeting these uptake systems might be an effective way to eradicate biofilms caused by infectious strains.
Most bacteria live as complex communities adhered to surfaces rather than as planktonic isolated cells. These compact microbial consortia, referred to as biofilms, are commonly associated with many health problems (5, 6, 10, 37). It is estimated that biofilms contribute to more than 80% of human infections (8). Biofilms are ubiquitous: dental plaque, lung infections, and infections related to the use of medical devices, such as urinary catheters, are but a few common examples. Virtually all medical implants are prone to colonization and biofilm formation by pathogenic bacteria, and these biofilms often serve as a source for recurrent infections. Biofilm-linked infections are particularly problematic, because biofilm-associated bacteria can withstand host immune defenses, antibiotics, biocides, and hydrodynamic shear forces far better than the corresponding planktonic bacteria. These characteristics make biofilm-associated infections particularly recalcitrant to treatment, and it is a common and frustrating experience that after treatment, surviving biofilm-associated bacteria will carry on the infection.
Iron is essential for bacterial growth. Bacteria face iron-limiting conditions in the mammalian host, where free iron is strictly limited and normally bound to sequestering proteins such as transferrin and lactoferrin. To counter such iron-limiting conditions, bacteria use different highly efficient mechanisms of iron acquisition. A typical high-affinity iron uptake system consists of a low-molecular-mass Fe(III)-chelating compound, known as a siderophore, combined with its cognate membrane-located receptor (22). Such iron acquisition systems are generally regarded as important virulence or fitness factors. Escherichia coli, and in particular urinary tract-infectious E. coli, strains are well equipped with iron uptake systems and can express many of these systems under iron-limiting conditions. Urinary tract E. coli isolate 83972 has been shown to induce several iron systems, i.e., enterobactin, salmochelin, aerobactin, hemin, and yersiniabactin, during growth in vitro in iron-limiting medium and also in vivo in the human urinary tract (31, 33). The major iron regulator in E. coli is the ferric uptake regulator protein, Fur. It acts as a positive repressor; i.e., it represses the transcription of more than 90 genes in E. coli upon interactions with its corepressor, Fe(II), and causes derepression in the absence of Fe(II) (16). Metal binding increases the affinity of Fur for its DNA binding site by ∼1,000-fold. Fur also binds and shows activation by other divalent metal ions, such as Co(II), Mn(II), Zn(II), Cd(II), and Cu(II) (2, 9). Interestingly, while only Fe(II) binds with sufficient affinity to activate Fur significantly at physiological metal concentrations in E. coli, the affinity of Fur for Zn(II) and Co(II) is significantly greater than that for Fe(II) (25). Also, Fe(III) activates Fur for DNA binding (25).
The availability of iron has been shown to be important for the formation of biofilms by E. coli (15, 38). Urinary tract-infectious E. coli strains formed significantly less biofilm in the presence of an iron chelator and more biofilm with iron added to growth medium (15). Also, mutants lacking the iron uptake receptor responsible for iron acquisition through yersiniabactin showed impaired biofilm formation in iron-limiting medium (15). By virtue of its role in iron uptake regulation and the fact that metal ions such as Co(II) and Zn(II) have a higher affinity for Fur than for Fe(II), it should be possible to induce iron starvation with these metals. On this background, we have investigated the effect of metal ions on the biofilm formation of E. coli.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Six E. coli strains, 83972, VR50, VR89, VR91, VR95, and VR96; two Klebsiella pneumoniae strains, i222-86 and i3-89; and one Klebsiella oxytoca strain, i113-96, all urinary tract isolates, were used in this study and were described previously (11, 32). The yellow fluorescent version of 83972, 83972yfp, used for the flow cell experiment, was described previously (13). fyuA-carrying plasmid pVR3 was described previously (15).
All cultivations were performed in the minimal laboratory medium ABTG, i.e., AB minimal medium supplemented with 0.2% glucose, 0.02% Casamino Acids, and 1.0 μg/ml thiamine, or pooled human urine. Human urine was collected from a pool of 10 healthy female volunteers who had no history of urinary tract infection (UTI) or antibiotic use in the prior 2 months. For each experiment, urine was collected randomly from three to five of these individuals (pH 5.5 to 7.5). The urine was pooled (pH 6.5 to 7.0), filter sterilized, stored at 4°C, and used within the following 2 to 3 days. ZnSO4, Co(NO3)2, and FeCl3 were added at concentrations that did not affect the growth characteristics of the strains (500 μM, 500 μM, and 10 μM, respectively). For the Fur experiments, ABTG (with 0.2% glucose, 0.02% Casamino Acids, and 1.0 μg/ml thiamine) without iron was prepared. For the growth of VR50, 10 μl/ml pantothenic acid was added when grown in ABTG.
Biofilm formation in microtiter plates.
Cells were pregrown in ABTG or pooled human urine, and 10 μl was used to inoculate 1 ml of growth medium, with and without the addition of metal ions, in 24-well flat-bottom microtiter plates (Iwaki). The microtiter plates were incubated statically at 37°C overnight, and the biofilm was monitored by crystal violet staining as described previously (14). Each strain and each concentration were assayed in three to four wells on each plate, and all experiments were repeated at least three times. For each plate, two to three wells were used as blanks containing sterile growth medium with and without the addition of appropriate metal ions.
Biofilm formation in flow cell chambers.
Flow cell experiments were performed at 37°C in ABTG minimal medium essentially as described previously (11), with an additional overnight wash of the flow cell system with sterile MilliQ water. Biofilm formation with and without the addition of ZnSO4 (final concentration of 50 μM) was monitored at 16, 24, and 40 h postinoculation. Acquired pictures were processed for display by using IMARIS software (Bitplane), and the relative biofilm coverage of the substratum was estimated by using COMSTAT software. Each experiment in flow chambers was performed in triplicate channels and repeated twice.
Biofilm formation on catheters.
Biofilm formation was assayed under hydrodynamic conditions in 100% silicone Foley catheters (Unomedical). Pieces of catheter with a length of 3.5 cm were cut, sterilized in 0.5% sodium hypochlorite for 1 h, and subsequently washed extensively with sterile water. Each piece of catheter was put into a glass tube with 5.0 ml ABTG minimal medium with and without 500 μM ZnSO4. The tubes were then inoculated with 50 μl of a culture of VR50 freshly grown overnight. The tubes were left at 37°C in a water bath with shaking at 160 rpm for 24 h before analysis. The amount of adhered bacteria on the catheters was examined by viable counts. The catheter pieces were first washed in 50 ml phosphate-buffered saline (PBS) three times, and excess liquid was removed by capillarity on adsorbent paper to reduce the influence of nonattached or loosely attached bacteria. The catheter pieces were then placed into plastic tubes containing 5.0 ml PBS. Adhered bacteria were detached by sonication for 4 min at 42 Hz at room temperature, followed by vortexing at maximum velocity for 15 s and serial dilutions and plating onto LB agar plates. The sonication procedure did not adversely affect bacterial viability (this was confirmed by plating samples of bacterial liquid cultures before and after sonication). The efficient removal of the adherent bacteria was verified by microscopic examination. The experiment was performed in five replicates and repeated twice.
Construction of a fur plasmid.
The fur gene of 83972 was amplified by PCR (P788 [5′-GGCCGGATCCAGGACAGATTCCGCATGACCGACAACAATACCGCCC-3′] and P789 [5′-GGCCCGTCGACTTATTTGCCTTCGTGCGCATGTTCATC-3′]), containing BamHI and SalI restriction sites, and cloned into pACYC184. The resulting plasmid, pFur (pVR5), was transformed into strain 83972.
Creation of a fur knockout mutant.
A fur knockout mutant of 83972 was constructed by using the λRed recombinase gene replacement system (7). Briefly, the npt gene from plasmid pKD4 was amplified by using a primer set (P769 [5′-GGACAGATTCCGCATGACTGATAACAATACCGCCCTAAAGGTGTAGGCTGGAGCTGCTTCG-3′] and P770 [5′-CGTTCAGGCTGGCTTATTTGCCTTCGTGCGCATGTTCATCCATATGAATATCCTCCTTAG-3′]) containing 40-nucleotide homology extensions of the fur target gene. The PCR products were purified from agarose gels and transformed into 83972(pKD46), and kanamycin-resistant colonies were selected. λRed helper plasmid pKD46 was cured by growth at 37°C, and the correct double-crossover and recombination event was confirmed by PCR using primers P771 and P772 (5′-GCGCGCCATGGCTGATAACAATACCGCCCTAAAG-3′ and 5′-GGCCCGAATTCTTATTTGCCTTCGTGCGCATGTTCATC-3′, respectively).
RESULTS
Biofilm formation of UTI strains is impaired by the presence of the divalent cations of zinc and cobalt.
To investigate the effect of the addition of divalent metal ions to biofilm-forming cells, strains known to form a good biofilm were selected. The best biofilm-forming strains from two previous studies included six E. coli and three Klebsiella strains isolated from patients with symptomatic urinary tract infection (UTI) and asymptomatic bacteriuria (11, 32). The concentrations of Zn(II) and Co(II) were set at values not inhibiting the bacterial planktonic growth of the strains, i.e., 500 μM for both metal ions; no effect on the final optical density at 600 nm (OD600) was observed for any of the strains (P > 0.1 by t test). This is in accordance with previous reports on the metal toxicity of E. coli where MIC values of divalent cations such as Mn(II), Ni(II), Cu(II), Zn(II), and Cd(II) were in the millimolar range for planktonically grown E. coli cells, with Zn(II) having an MIC of 2.2 mM (18).
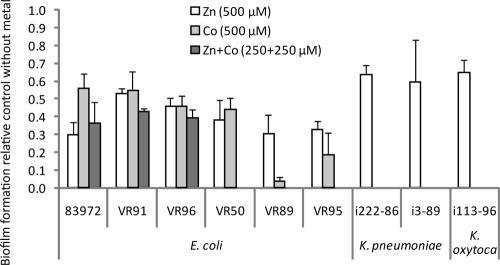
Biofilm formation was performed under non-iron-limiting conditions in microtiter plates with the minimal medium ABTG. It turned out that Zn(II) significantly reduced the biofilm formation of all nine isolates tested: by 47 to 70% for E. coli and 35 to 40% for Klebsiella sp. (Fig. 1). The same concentration of Co(II) reduced the biofilm formation of E. coli strains by 44 to 97%. The three strains least affected by Zn(II) and Co(II), i.e., 83972, VR91, and VR96, were also investigated for biofilm formation in the presence of both metal ions. The reduction in biofilm formation in medium containing 250 μM each Zn(II) and Co(II) was similar to that in medium with either metal. Thus, no synergistic effect of the addition of both metals to the biofilm-forming medium could be observed (Fig. 1).
FIG. 1.
Biofilm formation in microtiter plates of E. coli and Klebsiella sp. urinary tract isolates in minimal laboratory medium in the presence of Zn(II) (ZnSO4) and Co(II) [Co(NO3)2]. Values are shown relative to strains forming biofilms in the same medium without the addition of metal ions. Biofilm formation was determined by crystal violet staining. Values are means of at least three independent experiments, and error bars indicate standard deviations (σn − 1). All strains showed significantly reduced biofilm formation in the presence of Zn(II) or Co(II) (P < 0.001 by paired t test). Zn(II), Co(II), and the two metals in combination were tested for nine, six, and three of the strains, respectively.
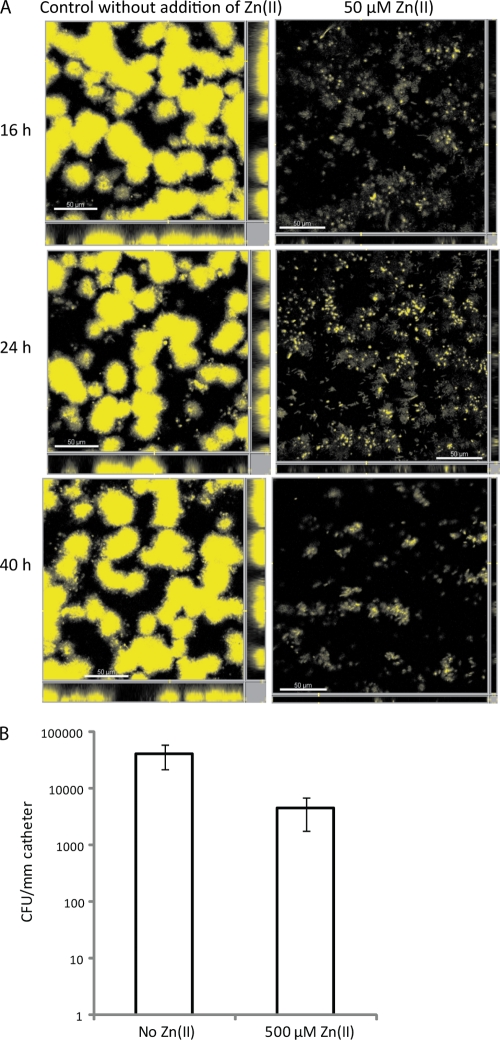
To investigate a different biofilm-forming condition and to better mimic conditions encountered in the human urinary tract and in catheters, the flow cell chamber system was employed. E. coli 83972 was tested in the same minimal medium as that in microtiter plates with and without the addition of 50 μM Zn(II). The biofilm formation of E. coli 83972 turned out to be considerably more reduced in the flow cell chamber than in the microtiter plate assay. One-tenth of the zinc concentration used in microtiter plates reduced biofilm up to 95% in flow cell chambers, compared with a 70% reduction in microtiter plates. The biofilm volumes of 83972 in the presence of Zn(II) were 14%, 9%, and 5% after 16, 24, and 40 h, respectively, compared with that formed in medium without Zn(II) (Fig. 2 A).
FIG. 2.
Addition of Zn(II) inhibits biofilm formation of UTI strain E. coli 83972 in flow chambers (A) and strain VR50 on urinary catheters (B). (A) Biofilm formation of strain 83972yfp in minimal medium with and without 50 μM ZnSO4 was examined in the flow cell chamber system and monitored by using scanning confocal laser microscopy at 16, 24, and 40 h postinoculation. Scale bars represent 50 μm. (B) Biofilm formation of strain VR50 in minimal medium with and without 500 μM ZnSO4 was examined on urinary catheters (100% silicone) by viable counts. Catheters were incubated for 24 h, washed, and sonicated, and serial dilutions were plated for determinations of CFU. Results displayed are means of five replicates, and error bars indicate standard deviations (σn− 1). VR50 formed significantly less biofilm on catheters in medium with Zn(II) than in medium with no additional zinc ions (P < 0.01 by t test).
Taken together, the addition of 500 μM Zn(II) and Co(II) greatly reduced the biofilm formation of E. coli and 500 μM Zn(II) reduced the biofilm formation of Klebsiella sp. urinary tract isolates grown under nonlimiting iron conditions under static conditions in microtiter plates. Furthermore, the addition of 50 μM Zn(II) greatly reduced the biofilm formation of E. coli 83972 under hydrodynamic conditions in the flow cell system.
Zinc reduces biofilm formation on urinary catheters.
The strain that forms most biofilm on both silicone and latex urinary catheters, i.e., VR50 (12), was used to investigate the role of additional zinc in biofilm formation on 100% silicone Foley catheters. Catheter pieces of 3.5 cm were inoculated with VR50 in ABTG minimal laboratory medium with and without 500 μM Zn(II), and the biofilm was developed for 24 h. Determinations of CFU per mm of catheter revealed that the addition of Zn(II) reduced the biofilm 9.4-fold (Fig. 2B), while the final OD600 for VR50 in medium with Zn(II) was 112% ± 11% of that of VR50 in medium without Zn(II) (P > 0.05 by t test). This demonstrates that Zn(II) reduces bacterial growth not only on polystyrene and glass, as shown in microtiter plates and in flow chambers, respectively, but also on silicone surfaces of urinary catheters.
Impaired biofilm formation in the presence of zinc or cobalt ions cannot be restored by the addition of iron.
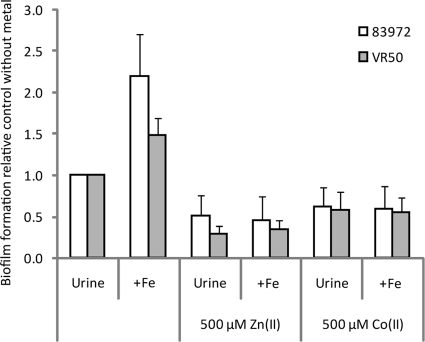
The effect of the iron concentration on E. coli biofilm formation was recently reported; the addition of iron during biofilm formation under iron-limiting conditions significantly increased the amount of biofilm formed, while the addition of an iron chelator reduced biofilm formation (15). Biofilm-forming cells seemed to be more dependent on iron concentrations and more sensitive to iron starvation than planktonically grown cells (15). Iron acquisition is regulated by Fur. In the presence of iron, Fur represses iron acquisition systems in E. coli while inducing them—by derepression—when the iron concentration is limited. Fur binds the divalent metal ions Zn(II) and Co(II) as well as Fe(II) and Fe(III). To elucidate whether the reduced biofilm formation observed in the presence of Zn(II) was dependent on or independent of iron availability, we investigated whether increasing the iron concentration could restore biofilm formation. This was done in an iron-limiting growth medium. While the addition of 10 μM iron indeed increased biofilm formation in the absence of Zn(II), no effect of additional iron could be seen in the presence of Zn(II) (Fig. 3). The addition of Zn(II) or Co(II) efficiently eradicated the increase in biofilm formation observed at higher iron concentrations; in media without Zn(II) and Co(II), the addition of iron increased biofilm formation by 120% and 48% for the two UTI strains 83972 and VR50, respectively, while no significant difference in biofilm formation could be observed when iron was added in the presence of one of the competing ions, Zn(II) or Co(II) (Fig. 3). In other words, the negative effect of Zn(II) and Co(II) on biofilm formation could not be relieved by the addition of iron, possibly due to the activation of Fur by these ions (Fur-Zn and Fur-Co) leading to an efficient repression of the iron uptake systems.
FIG. 3.
Biofilm formation in microtiter plates of the two E. coli UTI strains 83972 and VR50 in human urine with and without the addition of iron (10 μM FeCl3), zinc (ZnSO4), and cobalt [Co(NO3)2]. Values in the presence of metal ions are shown relative to those of the control (i.e., strain grown in urine without the addition of metal ions). Biofilm formation was determined by crystal violet staining. Values are means of at least three independent experiments, and error bars indicate standard deviations (σn− 1). All values were significantly different from those of the control (P < 0.001 by paired t test).
The addition of up to 500 μM (i.e., 10, 50, 100, 200, and 500 μM) iron did not restore the biofilm-forming capability in the presence of 500 μM Zn(II) or Co(II) (data not shown). Since Fur has a significantly higher affinity for Zn(II) and Co(II) than for Fe(II), a possible explanation could be that despite the high iron levels, the high levels of Zn(II) and Co(II) activate Fur, which tightly regulates the iron uptake systems, and consequently, not enough iron is imported into the cells for efficient biofilm formation.
Taken together, the results revealed that the addition of iron could not restore the impaired biofilm formation in the presence of Zn(II) or Co(II). Arguably, since the Fe uptake systems are shut down by Zn- or Co-activated Fur, the cells are unable to take up iron; i.e., they are starving in the midst of plenty.
Increased levels of active Fur inhibit biofilm formation.
Increasing the amount of active Fur in cells will lead to an enhanced repression of the transcription of iron acquisition systems regulated by Fur. A plasmid expressing the fur gene, pFur, was transformed into strain 83972, and biofilm formation in the absence and presence of Zn(II) and iron was investigated during growth under iron-limiting conditions. The overexpression of Fur in the absence of Zn(II) or iron did not affect biofilm formation (Fig. 4). However, the decrease in biofilm formation observed in the presence of Zn(II) was further reduced in the strain overexpressing Fur; the reduction in biofilm formation of the wild-type strain in the presence of Zn(II) was 64%, while in the Fur-expressing strain, the biofilm was reduced by 92% in the presence of Zn(II). The large reduction in biofilm formation of the Fur-expressing strain in the presence of Zn(II) could be explained by the presence of a higher level of active Fur (Fur-Zn) in the cells effectively repressing the iron uptake systems, leading to iron concentrations too small for efficient biofilm formation.
FIG. 4.
Biofilm formation of E. coli UTI strain 83972 overexpressing Fur in iron-limiting minimal medium with and without the addition of zinc (ZnSO4) and iron (FeCl3). Biofilm formation is shown relative to that of the control (i.e., native strain grown without the addition of metal ions) and was determined by crystal violet staining. Values are means of seven independent experiments, and error bars indicate standard deviations (σn− 1). Asterisks indicate significant differences in biofilm formation. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (paired t test).
In this iron-limiting growth medium, the addition of 10 μM iron increased biofilm formation by 39% for the wild-type strain (Fig. 4). Interestingly, increasing the expression of Fur in the cells grown with additional iron reduced biofilm formation by 61%. This reduction in the biofilm formation of 83972(pFur) in the presence of iron or zinc compared with that of 83972 in the same medium shows that an increased pool of Fur has a negative impact on biofilm formation in the presence of ions capable of activating Fur. Thus, the results reveal that increased levels of active Fur reduce biofilm formation, most likely through the repression of iron uptake systems.
A fur mutant showed no effect on biofilm formation in the presence of zinc.
A fur mutant will not be capable of shutting down the iron import systems thorough activated Fur, and its biofilm formation should therefore ultimately not be affected by additional Zn(II) in the medium. A fur mutant of 83972, 83972Δfur, was investigated for biofilm formation in ABTG minimal medium (with no iron added) and a natural iron-limiting medium, viz., human urine. Mutant strain 83972Δfur showed no difference in biofilm formation capability compared with that of the wild-type strain in either medium; the biofilm formation of the mutant was 109% ± 43% compared with that of the wild type (P = 0.94 by paired t test). Also, in the presence of additional zinc (500 μM), the fur mutant showed no significant difference in biofilm formation. The biofilm formation of 83972Δfur in medium with Zn(II) was 84% ± 24% of that of the wild-type strain in medium without additional Zn(II) (P = 0.11 by paired t test). Given that the biofilm formation of wild-type strain 83972 in the presence of Zn(II) was 30% of that in medium without Zn(II) (Fig. 1), there was a 2.8-fold increase in biofilm for the fur mutant compared with the wild type in the presence of Zn(II) (P < 0.05 by t test). This shows that the fur mutant was significantly less affected by the addition of Zn(II) than the wild-type strain. The large variation in biofilm formation of the fur mutant compared with the biofilm formation of the wild type (i.e., ±43%) could be explained by the fact that fur not only affects iron metabolism but also controls several additional functions in the cell, such as succinate, fumarate, and acetate metabolism as well as oxidative and acid stresses (17), which could affect biofilm formation. It was shown previously that Fur, apart from iron uptake, also indirectly regulates intracellular iron storage and utilization and that fur mutants have a low intracellular iron content (1). E. coli compensates for iron starvation in two ways: by inducing iron transport systems and by reducing the cellular demand for iron, which in turn could be achieved by repressing iron-requiring systems and derepressing alternative systems, such as replacing SodB with SodA (Mn containing) and FumA with the iron-free FumC (27, 29). Since the biofilm growth mode appears more sensitive to iron content than planktonic growth (15), this indicates that a biofilm-forming fur mutant encounters even greater iron stress than the corresponding wild-type strain.
DISCUSSION
Urinary tract infection (UTI) constitutes a serious health problem that affects millions of people each year. UTI is the most common infection in patients with indwelling urinary catheters, and the incidence of UTI in this patient group is ∼100% within a month. Between 15% and 25% of patients in general hospitals will have a urinary catheter in place sometime during their stay (37). Urinary catheters are also extensively used in aged-care facilities. Unfortunately, catheters are extremely prone to bacterial biofilm formation. In the United States, catheter-associated UTI was estimated to cause close to 1 million additional hospital days per year (34). E. coli and Klebsiella are the top two species in UTI and are generally excellent biofilm formers (12). Iron is essential for bacterial growth, and bacteria like E. coli and Klebsiella species possess a battery of iron uptake systems to cope with low-iron environments like urine. Fur is the central regulator of these systems. Fur has a 10,000-fold-greater affinity for Zn(II) than for Fe(II) (25). An exponentially growing E. coli cell contains ∼1.2 × 106 atoms of iron (28), whereof about 1% can be found in the free or loosely bound state (21). Fur is present in ∼5,000 copies per cell in log-phase cells (39). Iron influx approaches its maximum at an extracellular iron concentration of ∼1 μM (36). Elevated levels of metals other than iron could interfere with normal iron regulation by activating Fur inappropriately, thus shutting down iron import and leading to iron starvation. This phenomenon is illustrated by the observation that Mn(II) is toxic for fur+, but not for fur mutant, E. coli strains (17). Notably, Fur has a higher affinity for Zn(II) and Co(II) than for Fe(II); the dissociation constants of Fur to Zn(II) and Co(II) are 1.4 × 10−10 and 1.5 × 10−7 M, respectively, compared with 1.2 × 10−6 M for that to Fe(II) (25). In our hands, strain 83972 overexpressing Fur made significantly less biofilm in medium with excess Zn(II), most likely due to activated Fur shutting down the iron uptake systems.
We have previously shown that biofilm-associated bacteria like E. coli are particularly sensitive to iron starvation in environments with low concentrations of free iron, e.g., urine (15). Based on this, we predicted that interference with iron uptake would seriously impede biofilm formation. An attractive way of doing this is to fool the bacterial regulatory system for iron uptake, i.e., Fur. The activation of Fur by high-affinity Fur-chelating metals like Zn(II) and Co(II) will result in a shutdown of the Fur-controlled iron uptake systems like the enterobactin, ferric dicitrate, and aerobactin systems; in fact, all known iron transport systems in E. coli are repressed by active Fur, with the uptake of Fe(III) through the siderophore uptake systems as well as the uptake of Fe(II) occurring mainly through the FeoABC system (3). According to this tenet, the bacteria will act like there is plenty of iron while, in fact, there is very little, with dire consequences for biofilm formation. In effect, our hypothesis was fruitful, and concentrations of Zn(II) and Co(II) that did not inhibit planktonic growth did in fact inhibit biofilm formation. Even the addition of massive amounts of iron could not alleviate the inhibitory effect, due to the 10,000-fold-greater affinity of Fur for Zn(II) than for Fe(II); that is, the uptake systems were kept in shutdown mode, and the bacteria could not profit from the extra iron.
Also, it is possible that the expression of siderophore uptake systems may have effects on biofilm formation other than increasing the intracellular iron availability. For example, the Fur-regulated siderophore receptor Iha, found in pathogenic E. coli isolates, is known to promote adherence to epithelial cells (30, 35), but the iha gene has not been associated with in vitro biofilm formation (19, 26). One siderophore uptake receptor that has been shown to be involved in biofilm formation is the fyuA gene (which, like the other iron uptake systems, carries a Fur box and, thus, is repressible by active Fur), encoding the ferric yersiniabactin uptake receptor FyuA. It has been shown that the expression of fyuA is required for the efficient biofilm formation of UTI strains, and strains overexpressing FyuA showed significantly increased biofilm formation in an iron-limiting medium (15); however, although the decrease in biofilm formation of fyuA mutants could be alleviated by the addition of extra iron, the exact role of FyuA in biofilm formation is not known, and whether the effect is due purely to a decrease in the intracellular iron concentration or also a result of any additional mechanism remains to be elucidated. Here, strain 83972, containing an fyuA-carrying plasmid, was not capable of restoring biofilm formation in the presence of Zn(II) (data not shown), indicating that this gene is efficiently repressed by activated Fur.
From the results in the present study, it cannot be excluded that the regulation of Fur through the addition of Zn(II) and Co(II) might have additional downstream effects on putative adhesins and other factors involved in biofilm formation. Fur not only regulates iron uptake and storage but also controls several additional functions in the cell, such as succinate, fumarate, and acetate metabolism; chemotaxis; flagellum expression; as well as oxidative and acid stresses, and plays a role in pathogenesis (4, 17, 23). Iron, through the activation of Fur, has been reported to repress colonization factor antigen I (CFA/I) fimbriae (20) and to regulate type 1 fimbria expression (24, 38) in E. coli. However, the strain mainly employed in the present study, 83972, does not carry the genes encoding CFA/I fimbriae, is defunct in type 1 fimbrial expression, and is nonmotile in iron-limiting medium.
The present information might be exploited for devices, such as urinary tract catheters, that are particularly prone to biofilm fouling. Here, one can envision that matrix coating with zinc-containing substances should reduce biofilm formation and, subsequently, UTI.
Acknowledgments
We thank Birthe Jul Bondo for expert technical assistance.
This work was supported by grants from the Danish Medical Research Foundation (grant 271-06-0555) and Lundbeckfonden (grant R17-A1603).
Footnotes
Published ahead of print on 23 April 2010.
REFERENCES
- 1.Abdul-Tehrani, H., A. J. Hudson, Y. S. Chang, A. R. Timms, C. Hawkins, J. M. Williams, P. M. Harrison, J. R. Guest, and S. C. Andrews. 1999. Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J. Bacteriol. 181:1415-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagg, A., and J. B. Neilands. 1987. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26:5471-5477. [DOI] [PubMed] [Google Scholar]
- 3.Braun, V. 2003. Iron uptake by Escherichia coli. Front. Biosci. 8:1409-1421. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter, B. M., J. M. Whitmire, and D. S. Merrell. 2009. This is not your mother's repressor: the complex role of fur in pathogenesis. Infect. Immun. 77:2590-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 6.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114-122. [DOI] [PubMed] [Google Scholar]
- 9.de Lorenzo, V., S. Wee, M. Herrero, and J. B. Neilands. 1987. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J. Bacteriol. 169:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrières, L., V. Hancock, and P. Klemm. 2007. Biofilm exclusion of uropathogenic bacteria by selected asymptomatic bacteriuria Escherichia coli strains. Microbiology 153:1711-1719. [DOI] [PubMed] [Google Scholar]
- 12.Ferrières, L., V. Hancock, and P. Klemm. 2007. Specific selection for virulent urinary tract infectious Escherichia coli strains during catheter-associated biofilm formation. FEMS Immunol. Med. Microbiol. 51:212-219. [DOI] [PubMed] [Google Scholar]
- 13.Fexby, S., T. Bjarnsholt, P. O. Jensen, V. Roos, N. Hoiby, M. Givskov, and P. Klemm. 2007. A biological Trojan horse: antigen 43 provides specific bacterial uptake and survival in human neutrophils. Infect. Immun. 75:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock, V., L. Ferrières, and P. Klemm. 2007. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol. Lett. 267:30-37. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, V., L. Ferrières, and P. Klemm. 2008. The ferric yersiniabactin uptake receptor FyuA is required for efficient biofilm formation by urinary tract infectious Escherichia coli in human urine. Microbiology 154:167-175. [DOI] [PubMed] [Google Scholar]
- 16.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 17.Hantke, K. 1987. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol. Gen. Genet. 210:135-139. [DOI] [PubMed] [Google Scholar]
- 18.Harrison, J. J., H. Ceri, C. A. Stremick, and R. J. Turner. 2004. Biofilm susceptibility to metal toxicity. Environ. Microbiol. 6:1220-1227. [DOI] [PubMed] [Google Scholar]
- 19.Kanamaru, S., H. Kurazono, A. Terai, K. Monden, H. Kumon, Y. Mizunoe, O. Ogawa, and S. Yamamoto. 2006. Increased biofilm formation in Escherichia coli isolated from acute prostatitis. Int. J. Antimicrob. Agents 28(Suppl. 1):S21-S25. [DOI] [PubMed] [Google Scholar]
- 20.Karjalainen, T. K., D. G. Evans, D. J. Evans, Jr., D. Y. Graham, and C. H. Lee. 1991. Iron represses the expression of CFA/I fimbriae of enterotoxigenic E. coli. Microb. Pathog. 11:317-323. [DOI] [PubMed] [Google Scholar]
- 21.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. U. S. A. 93:13635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez, J. L., A. Delgado-Iribarren, and F. Baquero. 1990. Mechanisms of iron acquisition and bacterial virulence. FEMS Microbiol. Lett. 75:45-56. [DOI] [PubMed] [Google Scholar]
- 23.Massé, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McHugh, J. P., F. Rodriguez-Quinones, H. Abdul-Tehrani, D. A. Svistunenko, R. K. Poole, C. E. Cooper, and S. C. Andrews. 2003. Global iron-dependent gene regulation in Escherichia coli. J. Biol. Chem. 278:29478-29486. [DOI] [PubMed] [Google Scholar]
- 25.Mills, S. A., and M. A. Marletta. 2005. Metal binding characteristics and role of iron oxidation in the ferric uptake regulator from Escherichia coli. Biochemistry 44:13553-13559. [DOI] [PubMed] [Google Scholar]
- 26.Naves, P., G. del Prado, L. Huelves, M. Gracia, V. Ruiz, J. Blanco, G. Dahbi, M. Blanco, M. del Carmen Ponte, and F. Soriano. 2008. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 45:86-91. [DOI] [PubMed] [Google Scholar]
- 27.Niederhoffer, E. C., C. M. Naranjo, K. L. Bradley, and J. A. Fee. 1990. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J. Bacteriol. 172:1930-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunoshiba, T., F. Obata, A. C. Boss, S. Oikawa, T. Mori, S. Kawanishi, and K. Yamamoto. 1999. Role of iron and superoxide for generation of hydroxyl radical, oxidative DNA lesions, and mutagenesis in Escherichia coli. J. Biol. Chem. 274:34832-34837. [DOI] [PubMed] [Google Scholar]
- 29.Park, S. J., and R. P. Gunsalus. 1995. Oxygen, iron, carbon, and superoxide control of the fumarase fumA and fumC genes of Escherichia coli: role of the arcA, fnr, and soxR gene products. J. Bacteriol. 177:6255-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid, R. A., P. I. Tarr, and S. L. Moseley. 2006. Expression of the Escherichia coli IrgA homolog adhesin is regulated by the ferric uptake regulation protein. Microb. Pathog. 41:207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roos, V., and P. Klemm. 2006. Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect. Immun. 74:3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roos, V., E. M. Nielsen, and P. Klemm. 2006. Asymptomatic bacteriuria Escherichia coli strains: adhesins, growth and competition. FEMS Microbiol. Lett. 262:22-30. [DOI] [PubMed] [Google Scholar]
- 33.Roos, V., G. C. Ulett, M. A. Schembri, and P. Klemm. 2006. The asymptomatic bacteriuria Escherichia coli strain 83972 out-competes UPEC strains in human urine. Infect. Immun. 74:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamm, W. E. 1991. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am. J. Med. 91:65S-71S. [DOI] [PubMed] [Google Scholar]
- 35.Tarr, P. I., S. S. Bilge, J. C. Vary, Jr., S. Jelacic, R. L. Habeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thulasiraman, P., S. M. Newton, J. Xu, K. N. Raymond, C. Mai, A. Hall, M. A. Montague, and P. E. Klebba. 1998. Selectivity of ferric enterobactin binding and cooperativity of transport in Gram-negative bacteria. J. Bacteriol. 180:6689-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren, J. W. 2001. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 17:299-303. [DOI] [PubMed] [Google Scholar]
- 38.Wu, Y., and F. W. Outten. 2009. IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J. Bacteriol. 191:1248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]