Abstract
Background
Recurrent angioedema may affect the skin or, less commonly, the tongue, gastrointestinal tract, and larynx. Angioedema is a clinical sign that can be produced by a variety of diseases. Asphyxiation due to edematous obstruction of the upper airway is rare, but, for the affected patients, it is a permanent risk.
Methods
Review of the literature based on a selective search and the authors’ decades of experience treating patients with angioedema in a dedicated ambulatory care unit.
Results
Hereditary angioedema due to C1 esterase inhibitor deficiency has been intensively studied, and nearly all steps in its pathogenesis are known, from the causative gene defect all the way to the clinical presentation of angioedema. Bradykinin is the main mediator in this pathway. New treatment options (icatibant; C1-inhibitor concentrate for self-administration and long-term treatment) have helped patients considerably. In recent years, a new type of hereditary angioedema has been described, resulting not from a lack of C1 inhibitor, but rather from mutations of coagulation factor XII or other, as yet unidentified genetic abnormalities. There are major differences in the pharmacological treatment of the different diseases that cause angioedema. In an emergency, when severe upper airway obstruction can be life-threatening, immediate treatment is needed to keep the upper airway open.
Conclusion
In patients with recurrent angioedema, the diagnostic classification of the underlying disorder as a particular type of hereditary or acquired angioedema is a prerequisite for appropriate treatment.
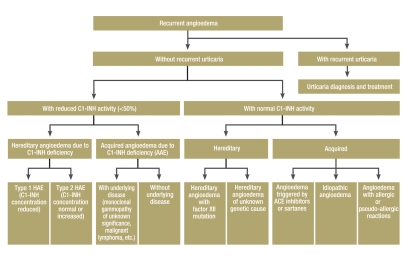
Angioedema (also known as Quincke disease) is the name given to edema lasting 1–7 days that recurs at irregular intervals. Target organs are the skin, tongue, glottis and larynx, gastrointestinal tract, and sometimes other soft organs. The clinical symptom referred to as angioedema forms part of a variety of disease entities (Box 1, Figure 1). In Germany, according to the present author’s estimate, several thousand patients suffer from one of the forms of recurrent angioedema. Cases of sudden asphyxiation are rare, but do occur every now and again (1). This review aims to draw attention to the various clinical features of recurrent angioedema and the practical steps for dealing with it, and to report the most recent developments in this field. The literature search was carried out on PubMed (search terms: angioedema, C1-inhibitor deficiency).
Box 1. Forms of angioedema with and without C1 inhibitor deficiency.
-
Hereditary angioedema due to C1 inhibitor deficiency
Type 1 (reduced activity and plasma concentration of C1 inhibitor)
Type 2 (reduced activity with normal or increased plasma concentration of C1 inhibitor)
-
Hereditary angioedema with normal C1 inhibitor
Hereditary angioedema due to mutation of the factor XII gene
Hereditary angioedema of unknown genetic cause
Angioedema due to acquired C1 inhibitor deficiency
Angioedema triggered by ACE inhibitors or other medical drugs
Recurrent angioedema in patients with chronic urticaria
Recurrent idiopathic angioedema
Angioedema as part of an allergic or pseudoallergic reaction
Figure 1.
Flow diagram for the diagnosis of recurrent angioedema
Hereditary angioedema due to C1-inhibitor deficiency
In this disease, almost all of the pathogenetic steps between the causative genetic defect and the clinical symptom of angioedema are now understood. In addition to the treatments known hitherto, some new therapy options exist that intervene at various stages in the pathogenesis.
Epidemiology
The prevalence of hereditary angioedema (HAE) due to C1-inhibitor deficiency (HAE-C1-INH) is around 1 in 50 000 (2). In Germany about 1200 patients have been diagnosed with this disease. About equal numbers of men and women are affected, so far as we know, but on average the disease is more severe in women (3).
Genetics
HAE-C1-INH has an autosomal dominant pattern of inheritance. The gene that codes for the C1-esterase inhibitor (C1-INH) is located on the long arm of chromosome 11 in subregion q12–q13.1 and consists of 8 exons and 7 introns. New techniques for identifying mutations have shown more than 200 mutants to date (e1, e2). Patients with type 1 HAE (85% of patients) have one normally expressed C1-INH gene and one abnormal or deleted gene that is not expressed. Patients with type 2 HAE also have one normal gene; the other one is abnormal and is expressed, leading to synthesis of dysfunctional C1-INH. Type 2 HAE (15% of patients) arises due to point mutations in the C1-INH gene. New mutations are present in around 20% of patients.
Pathogenesis
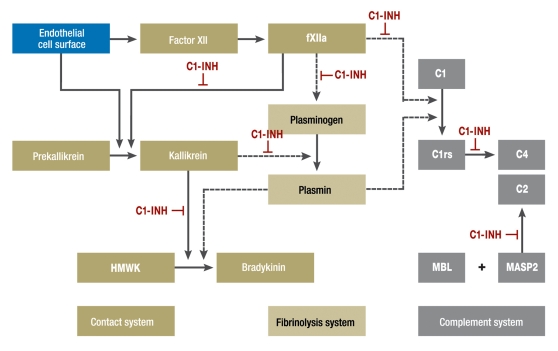
C1-INH (box 2) controls the spontaneous autoactivation of the first complement component (C1) and activated C1. A deficiency in functional C1-INH leads to activation of the initial phase of the complement system, and this results in a permanent reduction of plasma levels of C4. It is now known, however, that it is the inhibitory effect of C1-INH not on the complement system, but on the kallikrein–kinin system that has the essential pathogenetic role in HAE-C1-INH (figure 2). C1-INH is responsible for the inhibition of the greater part of plasma kallikrein and factor XIIa and is thus the most important regulator of activation of the kallikrein–kinin system. During acute attacks of HAE, kallikrein is insufficiently inhibited because of the deficiency in C1-INH, the kallikrein–kinin system (contact system) becomes activated, and at the end of the cascade there is an increased amount of bradykinin, the main mediator of increased vascular permeability and hence of the edema seen in HAE-C1-INH.
Box 2. C1-Esterase inhibitor (C1-INH).
-
Properties
Glycoprotein
Single chain
478 amino acids
Molecular weight 105 000 kDa
-
Where formed
Formed mostly in hepatocytes, to a small extent also in blood monocytes, skin fibroblasts, and endothelial cells of the umbilical cord
-
Classification
Belongs to the serpin family of serine protease inhibitors
-
Functions
Main inhibitor of various complement system proteases (C1r, C1s, mannan-binding, lectin-associated serine proteases [MASP] 1 and 2)
Main inhibitor of kallikrein and factor XIIa, the proteases of the kallikrein–kinin system
Lesser inhibitor of plasmin, a protease of the fibrinolytic system, and of factor XIa, a protease of the coagulation system
Figure 2.
Interactions and potential interactions between the contact, complement, and fibrinolysis systems. Abbreviations: C1-INH, C1-esterase inhibitor; fXIIa, activated coagulation factor XII; HMWK, high-molecular-weight kininogen; MBL, mannan-binding lectin; MASP2, mannan-binding lectin-associated protease 2
Clinical symptoms
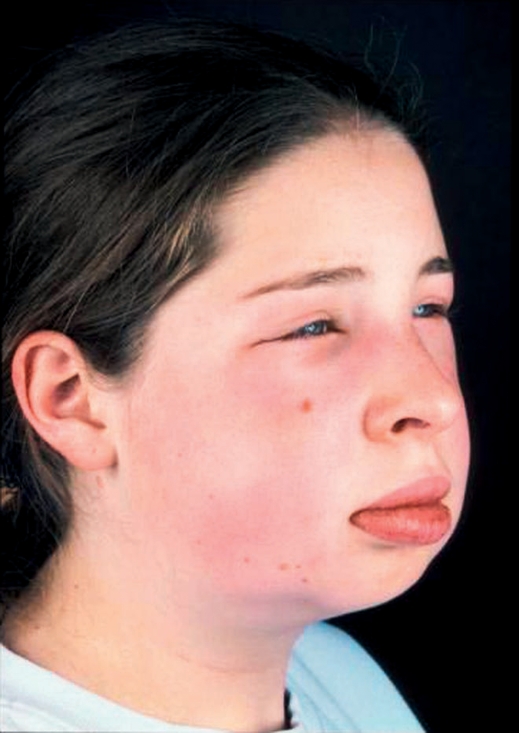
Clinically, HAE-C1-INH is characterized by recurring swelling of the skin (extremities, face, genitals), gastrointestinal attacks (painful abdominal cramps, sometimes cardiovascular symptoms, vomiting, and diarrhea), and by edema of the larynx and other organs (photo) (3– 5). Death is most likely to occur in patients whose symptoms have not been diagnosed (1, 5).
Photo.
Facial swelling in hereditary angioedema
Course
HAE-C1-INH manifests itself most often in the first decade of life, frequently in the second, and in a few patients even later. Recurring attacks of edema follow, the frequency of which varies greatly from patient to patient. In one study series, about 70% of patients had 12 attacks or fewer per year, and 30% more than 12 attacks (2).
Triggers of edema attacks
Some attacks are triggered by trauma, pressure, psychological stress situations, menstruation, ovulation, or infectious diseases. The tendency to edema attacks can be very greatly increased by ingestion of ACE inhibitors; these are contraindicated in patients with HAE. ATII receptor blockers can also increase a tendency to edema attacks, although more rarely. In the same way, attacks may become more frequent in women receiving estrogen in contraceptives or as hormone replacement therapy (e4).
Diagnostic work-up
Laboratory tests for a patient in whom HAE-C1-INH is suspected on clinical grounds should include the following:
C1-INH concentration
C1-INH activity
C4
If acquired C1-INH deficiency is suspected, also C1q, CH50, and auto-antibodies against C1-INH.
The diagnosis of HAE-C1-INH is made on the basis of recurrent skin swellings, abdominal pain attacks, laryngeal edema, and a positive family history in combination with the relevant laboratory findings, i.e., reduced C1-INH activity and (usually) reduced C1-INH concentrations together with reduced plasma levels of C4. Genetic testing is possible. Family testing, at least of close blood relatives, is required.
Treatment
HAE-C1-INH is a complex disease and, if severe, impacts on the life of patients in many different ways. Given the nature of the disease, for many patients medical therapy continues over many years, decades, or for their whole life. The goals of treatment are:
To prevent asphyxiation of the patient
To alleviate symptoms.
Since sudden asphyxiation (within hours) can happen at any age and almost always occurs without warning, taking on the treatment of a patient with HAE-C1-INH is a great responsibility. It is essential to give the patient comprehensive and detailed knowledge of the symptoms, especially the initial symptoms, of laryngeal edema (among other things: the feeling of a lump in the throat, difficulty swallowing, voice changes, onset of dyspnea), and the patient should also have a plan of what needs to be done when any of these symptoms occur. In the same way, other family members must be informed about the disease and the procedures that are needed. Since all these procedures are important and time-consuming, it is advisable to make use of the experience of an HAE treatment center. Ideally the HAE patient will be looked after close to home by his or her primary care physician in collaboration with an HAE treatment center. The two largest treatment centers in Germany, which now have decades of experience in treating patients with HAE, are the Dermatology Clinic at the University of Mainz and the Pediatric Department at the University of Frankfurt.
Treatment for acute edema attacks
Indications for treatment
Slight swellings of the hands and feet do not generally require treatment. Facial swellings in HAE should be treated, because quite often they are followed by laryngeal edema. Abdominal attacks, if mild, can be adequately treated with antispasmodic suppositories. However, most abdominal attacks are very painful and require treatment with C1-inhibitor concentrate or icatibant. Patients with HAE in the head area with edema of the pharynx or larynx represent an emergency, because of the imminent risk of asphyxiation, and should be admitted immediately for inpatient treatment. Treatment for laryngeal edema depends on how far advanced the edema is. Patients with life-threatening dyspnea should be intubated without delay, using a fiberoptic bronchoscope if necessary, and in the most extreme case a coniotomy (cricothyrotomy) may be performed. The medical therapy of choice is treatment with a C1-INH concentrate or icatibant.
C1-INH concentrate
Human C1-INH concentrate given intravenously has been proved a safe and highly effective treatment for acute attacks. It has been used in Germany for 30 years (e5). A series of observation studies have demonstrated its safety and effectiveness in treating laryngeal edema (6, e6), abdominal pain attacks (7), and skin swellings (8) in HAE-C1-INH. A randomized, controlled study was published in 1996 (9). Another study, relating to the licensing of Berinert in the USA, was published recently (10). Adverse effects are rare (fewer than 1 per 1000 uses) and relate to allergic/anaphylactic reactions and/or raised temperature.
For acute attacks, an early injection is recommended. Some patients now inject themselves with the preparation, or have it injected by a close relative, after appropriate instruction (self-treatment at home) (11, e7).
Icatibant
Icatibant is a bradykinin B2 receptor antagonist, similar in structure to bradykinin. An acute attack of HAE due to C1-INH deficiency can be treated by antagonizing the binding of bradykinin to the receptor. One non-controlled (12) and two controlled, randomized studies (licensing [phase III] studies with 130 patients, publication in preparation) have shown subcutaneously injected icatibant to be a safe and effective treatment for acute attacks of HAE-C1-INH. The first subjective improvement of symptoms was noticed after an average of 48 minutes; the first clinical improvement was observed after a median of 2 to 2.5 hours. Adverse effects include reactions at the injection site such as redness, wheal formation, and pain (noted in more than 1 in 10 uses) and a series of minor reactions including nausea, abdominal pain, and blocked nose (noted in fewer than 1 in 10 but more than 1 in 100 uses). Icatibant has been licensed for use in the countries of the European Union since July 2008.
Fresh frozen plasma
Because of its C1-INH content, fresh frozen plasma (FFP) is also effective in acute attacks of HAE (13). However, FFP also contains proteins of the kallikrein–kinin system, and these could also lead to increased production of bradykinin, which could aggravate the attack. Because of this and other drawbacks, treatment with FFP should if possible be avoided in Germany, where C1-INH concentrate and icatibant are available.
Further drugs
Ecallantide is a synthetic kallikrein inhibitor that has been shown in several clinical studies to be highly effective in acute HAE attacks. It is now licensed for use in the USA. A recombinant C1-INH has also been developed, obtained from the milk of transgenic rabbits. This too showed very good efficacy in HAE-C1-INH attacks. Corticosteroids, antihistamines, and epinephrine or epinephrine derivatives are not effective.
Long-term treatment to prevent edema attacks
Attenuated androgens
For long-term prophylaxis, androgen derivatives may be used, especially danazol, stanozolol, and oxandrolone. These androgens are highly effective. In a study published in 2008, 46% of patients receiving danazol were either completely symptom-free or had one attack or less per year; the average annual frequency of attacks was 33.3 before and 5.4 during danazol treatment (14). However, when used over the long term, attenuated androgens have many possible adverse effects, such as weight gain (in about 40% of those treated), menstrual abnormalities (in about 30% of women treated) and virilization (in about 40% of women treated), hepatotoxicity, and hepatocellular tumors, so that risks and benefits must be carefully weighed (14, e8). Regular follow-up visits are required to monitor for all unwanted effects (14).
Tranexamic acid
Two antifibrinolytic agents have been shown to be effective in the long-term treatment of HAE-C1-INH: epsilon amino-caproic acid and tranexamic acid, which is better tolerated. In adults the efficacy of tranexamic acid is generally much lower than that of attenuated androgens.
C1-INH concentrate
C1-INH concentrate can also be used for long-term prophylaxis (15, 16, e9). This treatment should be reserved for particularly severe cases, however.
Prognosis
Before the advent of suitable diagnostic and therapeutic modalities, HAE carried a high mortality; in some families as high as 25% to 50%. The cause of death was almost always asphyxiation due to laryngeal edema. Today deaths are rare, but they do occur (1).
Other angioedema entities
Hereditary angioedema with normal C1-INH
In this form of angioedema, also hereditary (type 3 HAE), those affected—almost always women—show normal plasma levels of C1-INH (17, e10). Oral contraceptives, pregnancy, and hormone replacement therapy often play a particular role as triggering or aggravating factors. Mutations of the factor XII gene have been demonstrated in some patients (18, e11).
Angioedema due to acquired C1-INH deficiency
This form of angioedema is seen in patients whose C1-INH deficiency is due to increased C1-INH catabolism. Accordingly, C1q is usually decreased. The symptoms are the same as those of HAE-C1-INH (19). In a significant proportion of these patients there is an underlying B-cell disorder, e.g., monoclonal gammopathy of unknown significance or malignant lymphoma, and it can often happen that these are discovered through the angioedema diagnosis. In some patients auto-antibodies against C1-INH are found (e12).
Angioedema due to ACE inhibitors or other drugs
About 0.1% to 2.2% of patients treated with ACE inhibitors develop recurrent angioedema, often facial swelling or edema of the tongue (20, 21, e13). Several cases of death by asphyxiation following closure of the upper airways have been reported (22, e14). The interval between the start of drug treatment and the appearance of the first angioedema can be months or several years, so that the causal connection between the angioedema and the triggering ACE inhibitors is sometimes recognized late. Because of the enormously high use of ACE inhibitors in Germany, cases of ACE-inhibitor-related angioedema are not rare. ATII-receptor blockers can also trigger the same forms of angioedema, although more rarely. Aspirin and numerous other drugs are also known to be triggers.
Recurrent angioedema in patients with chronic urticaria
More than 50% of patients with chronic recurrent urticaria report the occasional or frequent occurrence of angioedema (23). Angioedema can therefore also be symptom of this disease. Angioedema in chronic recurrent urticaria usually responds well to corticosteroids and antihistamines. Because of the prevalence of chronic recurrent urticaria (about 1% to 5% of the population are affected), urticaria-associated angioedema is particularly frequent (e15, e16).
Recurrent idiopathic angioedema
Recurrent idiopathic angioedema is the name given to cases of angioedema that cannot be identified as any of the other forms of angioedema: this is a diagnosis of exclusion. In these cases, none of the patient’s relatives are affected, C1-INH deficiency is not present, and the angioedema cannot be determined to be caused by drugs or any other triggers. Knowledge about the pathogenesis and treatment of this form of angioedema is still very limited (23, 24).
Angioedema as an allergic or pseudo-allergic reaction
Angioedema, usually in the form of facial swelling, also occurs as a symptom of acute allergic or pseudo-allergic reaction (24, 25). In these cases it is usually, though not always, associated with urticaria or, more rarely, the symptoms of anaphylactic shock. This form of angioedema also responds to corticosteroids and antihistamines. Usually it is a one-off occurrence; recurrences only occur on repeated exposure. The usual triggers are foods, medical drugs, and insect stings and bites.
Practical procedure with angioedema
The following hints can be helpful in the treatment of patients with suspected angioedema:
If an angioedema patient presents during the interval between two swellings, the time to the next swelling should if possible be used for the diagnostic work-up, so that the type of the angioedema can be identified. This allows the appropriate treatment to be given when the next swelling occurs; the wrong therapy can lead to life-threatening situations.
If a patient presents with acute angioedema, the first thing is to assess how threatening the symptoms are. Laryngeal edema and edema of the tongue are potentially life-threatening. Acute life-threatening situations with high-grade dyspnea require immediate interventions to secure the airways (intubation, percutaneous puncture of the trachea or the cricothyroid ligament if required, or, if necessary, cricothyrotomy or tracheotomy).
Facial or lip swelling is not threatening, but it should be remembered that laryngeal edema can follow facial swelling. Swellings of the extremities are not life-threatening, and neither are painful abdominal attacks.
Drug treatment varies for the different forms of angioedema, so the patient should always be asked whether any particular form of angioedema has already been diagnosed. Patients with HAE-C1-INH usually carry an emergency card that will give information about the treatment required. It is absolutely essential to look for and use this information: the present author is aware of numerous cases of suffocation and “near-misses” that occurred in patients, particularly those with HAE-C1-INH, because the clear information they were carrying about their diagnosis and treatment was ignored. The important point is that cortisone and antihistamines are definitely ineffective in some forms of angioedema (HAE-C1-INH, HAE with normal C1-INH, angioedema due to acquired C1-INH deficiency, angioedema due to ACE inhibitors) and of dubious efficacy in others. Inefficacy can lead to the loss of valuable time for any emergency procedures that may be needed.
If no existing information is available, an attempt to treat with high-dose corticosteroids and antihistamines is justified, but the patient must be observed afterwards. Once the acute symptoms have subsided, a diagnostic work-up for angioedema should be carried out. Further information is given in Box 3.
Box 3. Useful internet addresses.
German Society for Angioedema Research (GSAR), clinical issues and research into angioedema (www.angioedema.de).
There is a patient association in Germany for patients with hereditary angioedema (www. schwellungen.de) and also an international patient organization for C1-inhibitor deficiencies (www.haei.org).
Key Messages.
Angioedema consists of attacks of edema in the skin or other organs that are limited in duration to a few days.
Since the glottis and larynx can also be affected, although this is rare, attacks of angioedema can be life-threatening.
It is important to diagnose which form of angioedema is present, since therapies and in some cases prophylactic treatments can differ.
Hereditary angioedema is usually caused by functional deficiency of C1 inhibitor due to mutation of the relevant gene, but may also occur in various forms without C1 inhibitor deficiency.
New treatment possibilities for hereditary angioedema due to C1 inhibitor deficiency include icatibant (Firazyr), a novel bradykinin B2 receptor antagonist, and self-treatment at home with C1 inhibitor concentrate and long-term treatment with C1 inhibitor concentrate once a week or more often
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
The author declares that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Bork K, Siedlecki K, Bosch S, Schopf RE, Kreuz W. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc. 2000;75:349–354. doi: 10.4065/75.4.349. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni A, Cicardi M. Hereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine (Baltimore) 1992;71:206–215. doi: 10.1097/00005792-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119:267–274. doi: 10.1016/j.amjmed.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 4.Bork K, Staubach P, Eckardt AJ, Hardt J. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol. 2006;101:619–627. doi: 10.1111/j.1572-0241.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- 5.Bork K, Barnstedt SE. Laryngeal edema and death from asphyxiation after tooth extraction in four patients with hereditary angioedema. J Am Dent Assoc. 2003;134:1088–1094. doi: 10.14219/jada.archive.2003.0323. [DOI] [PubMed] [Google Scholar]
- 6.Bork K, Barnstedt SE. Treatment of 193 episodes of laryngeal edema with C1 inhibitor concentrate in patients with hereditary angioedema. Arch Intern Med. 2001;161:714–718. doi: 10.1001/archinte.161.5.714. [DOI] [PubMed] [Google Scholar]
- 7.Bork K, Meng G, Staubach P, Hardt J. Treatment with C1 inhibitor concentrate in abdominal pain attacks of patients with hereditary angioedema. Transfusion. 2005;45:1774–1784. doi: 10.1111/j.1537-2995.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 8.Bork K, Staubach P, Hardt J. Treatment of skin swellings with C1-inhibitor concentrate in patients with hereditary angio-oedema. Allergy. 2008;63:751–757. doi: 10.1111/j.1398-9995.2007.01577.x. [DOI] [PubMed] [Google Scholar]
- 9.Waytes AT, Rosen FS, Frank MM. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med. 1996;334:1630–1634. doi: 10.1056/NEJM199606203342503. [DOI] [PubMed] [Google Scholar]
- 10.Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009;1248:01–08. doi: 10.1016/j.jaci.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Bygum A, Andersen KE, Mikkelsen CS. Self-administration of intravenous C1-inhibitor therapy for hereditary angioedema and associated quality of life benefits. Eur J Dermatol. 2009;19:147–151. doi: 10.1684/ejd.2008.0603. [DOI] [PubMed] [Google Scholar]
- 12.Bork K, Frank J, Grundt B, Schlattmann P, Nussberger J, Kreuz W. Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant) J Allergy Clin Immunol. 2007;119:1497–1503. doi: 10.1016/j.jaci.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Prematta M, Gibbs JG, Pratt EL, Stoughton TR, Craig TJ. Fresh frozen plasma for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2007;98:383–388. doi: 10.1016/S1081-1206(10)60886-1. [DOI] [PubMed] [Google Scholar]
- 14.Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100:153–161. doi: 10.1016/S1081-1206(10)60424-3. [DOI] [PubMed] [Google Scholar]
- 15.Bork K, Witzke G. Long-term prophylaxis with C1-inhibitor (C1 INH) concentrate in patients with recurrent angioedema caused by hereditary and acquired C1-inhibitor deficiency. J Allergy Clin Immunol. 1989;83:677–682. doi: 10.1016/0091-6749(89)90082-1. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Saguer I HC, Fischer D, Ettingshausen CE, Kreuz W. Prophylactic treatment with pasteurised C1 inhibitor in hereditary angioedema (HAE—a prospective 32 months follow up) Blood. 1999;94(10) Sup 1 Abstract # 1032: 2339. [Google Scholar]
- 17.Bork K, Barnstedt SE, Koch P, Traupe H. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet. 2000;356213-217:213–217. doi: 10.1016/S0140-6736(00)02483-1. [DOI] [PubMed] [Google Scholar]
- 18.Dewald G, Bork K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun. 2006;343:1286–1289. doi: 10.1016/j.bbrc.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 19.Zingale LC, Castelli R, Zanichelli A, Cicardi M. Acquired deficiency of the inhibitor of the first complement component: presentation, diagnosis, course, and conventional management. Immunol Allergy Clin North Am. 2006;26:669–690. doi: 10.1016/j.iac.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Sabroe RA, Black AK. Angiotensin-converting enzyme (ACE) inhibitors and angio-oedema. Br J Dermatol. 1997;136:153–158. [PubMed] [Google Scholar]
- 21.Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51:1624–1630. doi: 10.1161/HYPERTENSIONAHA.108.110270. [DOI] [PubMed] [Google Scholar]
- 22.Dean DE, Schultz DL, Powers RH. Asphyxia due to angiotensin converting enzyme (ACE) inhibitor mediated angioedema of the tongue during the treatment of hypertensive heart disease. J Forensic Sci. 2001;46:1239–1243. [PubMed] [Google Scholar]
- 23.Frigas E, Park M. Idiopathic recurrent angioedema. Immunol Allergy Clin North Am. 2006;26:739–751. doi: 10.1016/j.iac.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065–1070. doi: 10.1503/cmaj.060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberger PA. Anaphylactic and anaphylactoid causes of angioedema. Immunol Allergy Clin North Am. 2006;26:753–767. doi: 10.1016/j.iac.2006.09.002. [DOI] [PubMed] [Google Scholar]
- e1.Gösswein T, Kocot A, Emmert G, Kreuz W, Martinez-Saguer I, Aygoren-Pursun E, et al. Mutational spectrum of the C1INH (SERPING1) gene in patients with hereditary angioedema. Cytogenet Genome Res. 2008;121:181–188. doi: 10.1159/000138883. [DOI] [PubMed] [Google Scholar]
- e2.Pappalardo E, Caccia S, Suffritti C, Tordai A, Zingale LC, Cicardi M. Mutation screening of C1 inhibitor gene in 108 unrelated families with hereditary angioedema: functional and structural correlates. Mol Immunol. 2008;45:3536–3544. doi: 10.1016/j.molimm.2008.05.007. [DOI] [PubMed] [Google Scholar]
- e3.Göring HD, Bork K, Späth PJ, Bauer R, Ziemer A, Hintner H, et al. Untersuchungen zum hereditären Angioödem im deutschsprachigen Raum. Hautarzt. 1998;49:114–122. doi: 10.1007/s001050050710. [DOI] [PubMed] [Google Scholar]
- e4.Bork K, Fischer B, Dewald G. Recurrent episodes of skin angio-edema and severe attacks of abdominal pain induced by oral contraceptives or hormone replacement therapy. Am J Med. 2003;114:294–298. doi: 10.1016/s0002-9343(02)01526-7. [DOI] [PubMed] [Google Scholar]
- e5.Bork K. Pasteurized C1 inhibitor concentrate in hereditary angio-edema: pharmacology, safety, efficacy and future directions. Expert Review of Clinical Immunology. 2008;4:13–20. doi: 10.1586/1744666X.4.1.13. [DOI] [PubMed] [Google Scholar]
- e6.Bork K, Hardt J, Schicketanz KH, Ressel N. Clinical studies of sudden upper airway obstruction in patients with hereditary angio-edema due to c1 esterase inhibitor deficiency. Arch Intern Med. 2003;163:1229–1235. doi: 10.1001/archinte.163.10.1229. [DOI] [PubMed] [Google Scholar]
- e7.Longhurst HJ, Bork K. Hereditary angioedema: causes, manifestations and treatment. Br J Hosp Med (Lond) 2006;67:654–657. doi: 10.12968/hmed.2006.67.12.22439. [DOI] [PubMed] [Google Scholar]
- e8.Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angio-edema: comparison of treated and untreated patients. J Allergy Clin Immunol. 1997;99:194–196. doi: 10.1016/s0091-6749(97)70095-2. [DOI] [PubMed] [Google Scholar]
- e9.Levi M, Choi G, Picavet C, Hack CE. Self-administration of C1-inhibitor concentrate in patients with hereditary or acquired angio-edema caused by C1-inhibitor deficiency. J Allergy Clin Immunol. 2006;117:904–908. doi: 10.1016/j.jaci.2006.01.002. [DOI] [PubMed] [Google Scholar]
- e10.Bork K, Gul D, Hardt J, Dewald G. Hereditary angioedema with normal C1 inhibitor: clinical symptoms and course. Am J Med. 2007;120:987–992. doi: 10.1016/j.amjmed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- e11.Bork K, Wulff K, Hardt J, Witzke G, Staubach P. Hereditary angio-edema caused by missense mutations in the factor XII gene: clinical features, trigger factors, and therapy. J Allergy Clin Immunol. 2009;124:129–134. doi: 10.1016/j.jaci.2009.03.038. [DOI] [PubMed] [Google Scholar]
- e12.Alsenz J, Bork K, Loos M. Autoantibody-mediated acquired deficiency of C1 inhibitor. N Engl J Med. 1987;316:1360–1366. doi: 10.1056/NEJM198705283162202. [DOI] [PubMed] [Google Scholar]
- e13.Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26:725–737. doi: 10.1016/j.iac.2006.08.001. [DOI] [PubMed] [Google Scholar]
- e14.Ulmer JL, Garvey MJ. Fatal angioedema associated with lisinopril. Ann Pharmacother. 1992;26:1245–1246. doi: 10.1177/106002809202601012. [DOI] [PubMed] [Google Scholar]
- e15.Jiamton S, Swad-Ampiraks P, Kulthanan K, Suthipinittharm P. Urticaria and angioedema in Siriraj medical students. J Med Assoc Thai. 2003;86:74–81. [PubMed] [Google Scholar]
- e16.Gaig P, Olona M, Munoz Lejarazu D, Caballero MT, Dominguez FJ, Echechipia S, Garcia Abujeta JL, Gonzalo MA, Lleonart R, Martinez Cocera C, Rodriguez A, Ferrer M. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14:214–220. [PubMed] [Google Scholar]