Abstract
This paper reports the effects of the seed layers prepared by spin-coating and dip-coating methods on the morphology and density of ZnO nanowire arrays, thus on the performance of ZnO nanowire-based dye-sensitized solar cells (DSSCs). The nanowire films with the thick ZnO buffer layer (~0.8–1 μm thick) can improve the open circuit voltage of the DSSCs through suppressing carrier recombination, however, and cause the decrease of dye loading absorbed on ZnO nanowires. In order to further investigate the effect of TiO2buffer layer on the performance of ZnO nanowire-based DSSCs, compared with the ZnO nanowire-based DSSCs without a compact TiO2buffer layer, the photovoltaic conversion efficiency and open circuit voltage of the ZnO DSSCs with the compact TiO2layer (~50 nm thick) were improved by 3.9–12.5 and 2.4–41.7%, respectively. This can be attributed to the introduction of the compact TiO2layer prepared by sputtering method, which effectively suppressed carrier recombination occurring across both the film–electrolyte interface and the substrate–electrolyte interface.
Keywords: ZnO nanowires, Arrays, DSSC, Hydrothermal growth
Introduction
Dye-sensitized solar cells (DSSCs) based on a dye-sensitized wide-band-gap nanocrystalline semiconductor (typically TiO2) film have attracted widespread attention as a potential, cost-effective alternative to silicon solar cells since they were first introduced by O’Regan and Grätzel in 1991 [1]. As one of the key components of dye-sensitized solar cells, the photoelectrode, composed of nanocrystalline semiconductor materials accumulated on a transparent conducting glass, has a very important influence on the photovoltaic performance [2,3]. It is well known that the energy conversion efficiency of DSSCs depends on the electron transport in the photoelectrode. Therefore, one-dimensional structure such as rods or wires of semiconductor materials can greatly improve DSSCs efficiency by offering direct electrical pathways for photogenerated electrons, thus enhancing the electron transport in the photoelectrode. Recently, considerable efforts have been devoted to the synthesis of such 1D materials used as the photoelectrodes of DSSCs [4-7].
Among various emerging 1D nanomaterials, ZnO, a wide-band-gap (3.37 eV) semiconductor with a large exciton binding energy of 60 meV at room temperature, is a promising alternative semiconductor to TiO2. This is because that the band gap and the energetic position of the valence band maximum and conduction band minimum of ZnO are very close to that of TiO2 and that the wurtzite structure of ZnO favors the formation of ordered 1D structures, moreover, presenting better electron transport compared with TiO2[4]. Consequently, the solar cell using nanowire arrays as the photoelectrodes shows a higher conversion efficiency compared to those using the disorderedly structured ZnO films [4]. In order to further improve the cell efficiency, the effective approaches currently applied are to control the morphology of ZnO nanostructure films, which can significantly increase dye loading and light harvesting [8,9], and to modify the surface of ZnO nanostructure films that can suppress carrier recombination [10]. However, by introducing a blocking layer at the base of the ZnO films, the influence of the blocking layer on the performance of ZnO DSSCs is an ongoing debate [10].
In this study, we report that the ZnO nanowire films with high aspect ratios and different thicknesses of ZnO buffer layers, which formed at the base of the nanowire films during growth, were prepared from different ZnO seed preparation methods. We also show that carrier recombination in ZnO nanowire-based dye-sensitized solar cells can be effectively suppressed and the photovoltaic conversion efficiency enhanced by introducing the TiO2buffer layer prepared by sputtering method.
Experimental Section
Materials
Polyethyleneimine (PEI, M.W.: 600) was purchased from Aldrich and used as received.Cis-bis (isothiocyanato) bis (2,2′-bipyridy1-4,4′-dicarboxylate) ruthenium (II) bistetrabutylammonium (also called N719) was from Solaronix SA, Switzerland. Other chemicals (Beijing Chemical Co.) used in our experiments were of analytical reagent grade without further purification. Fluorine-doped tin oxide substrates (FTO TEC-8, LOF) were first cleaned through sonication in acetone/ethanol for 30 min and then hydrolyzed in boiling deionized water at 100 °C for 30 min followed by air-drying.
ZnO Nanowire Array Synthesis
ZnO nanowire arrays were made in aqueous solution, using a two-step process described elsewhere [4]. To study the effect of a thin compact TiO2 film on FTO substrate on the solar cell performance of ZnO array film, it was prepared at room temperature by using reactive DC magnetron sputtering.
Preparation of ZnO Seeds on FTO Substrates
In order to study the effect of ZnO crystal seed particles on the morphology and solar cell performance of ZnO array film, they were coated onto the FTO substrates by using two different methods.
1. Spin-coating method. Zinc acetate dehydrate [Zn(CH3COO)2·2H2O] was dissolved in the mixed solution of ethanolamine and 2-methoxyethanol. The concentrations of both Zn(CH3COO)2·2H2O and ethanolamine in the resulting solution are 0.75 M. The coating solution was spin-coated onto FTO substrates at 3,000 rpm for several times. The FTO substrates were subsequently annealed at 300 °C in air for 15 min in order to convert Zinc acetate to ZnO.
2. Dip-coating method. The FTO substrates were dip-coated in a 2.5 mM ethanolic solution of zinc acetate dehydrate. Following dip-coating, the zinc acetate films on the FTO substrates were annealed at 300 °C in air for 15 min.
Hydrothermal Deposition
ZnO nanowire arrays were grown by placing vertically the ZnO-seeded FTO substrates in solutions with 25 mM Zn(NO3)2, 25 mM hexamethylenetetramine (HMT) and 7.3 mM polyethyleneimine at 92.5 °C. In order to obtain a constant nanowire array growth rate, the solutions were refreshed during the reaction period (solution turnover time 2.5 h). Subsequently, the substrates were washed with water/ethanol and annealed at 400 °C for 30 min to remove any residual organics.
Cell Assembly
The resulting substrates were immersed in dry ethanol containing 0.3 mM of N719 for 40 min. To assemble the solar cells, a Pt-coated conducting glass was placed on the ZnO nanowire array films separated by a 50-μm thin membrane spacer. The assembled cell was then clipped together as an open cell. An electrolyte, which was made with 0.1 M LiI (Aldrich), 0.1 M I2(Aldrich), 0.6 M dimethylpropylimidazolium iodide (DMPImI, Aldrich) and 0.5 Mtert-butylpyridine (Aldrich) in dry acetonitrile (Aldrich), was injected into the open cell from the edges by capillarity.
Characterization
The morphology of the products was characterized with use of field-emission scanning electron microscopy (FESEM, Hitachi S-4800). XRD analysis was performed on a powder X-ray diffractometer (Rigaku D/max-2500 diffractometer using CuKα radiation,λ = 0.1542, 40 kV, 100 mA). Photocurrent–voltage measurements were performed using simulated AM 1.5 sunlight with an output power of 100 mW cm−2.
Results and Discussion
Effect of ZnO Crystal Seed Particles Prepared by Different Methods
In this study, we found that the different ZnO seed preparation methods strongly influenced the morphology and density of ZnO nanowire arrays, leading to the different performance of the DSSCs based on the ZnO nanowire films. Figure 1 shows the top-view and cross-sectional FESEM images of two samples prepared with 7.3 mM of PEI for 30 h on the FTO substrates with ZnO seed layers prepared by spin-coating and dip-coating. The mean values of the nanowire dimension, the array density and aspect ratio were estimated from a statistical evaluation of FESEM images and are summarized in Table 1. In order to avoid possible variations at their top, the diameters of the nanowires were measured slightly below the nanowire tip. Although they had the similar length, the diameter distributions between the well-aligned ZnO nanowires for samples A and B had a significant difference. The nanowire arrays for the samples A1, A2 and A3 had mean diameters ranging from 195 to 210 and 120 to 150 nm for samples B1, B2 and B3. The densities of ZnO nanowires for samples A1, A2 and A3 were 2.1, 2.2 and 2.0 × 109 wires cm−2, respectively, which are much higher than that of samples B1, B2 and B3 (1.5, 1.6, 1.2 × 109 wires cm−2). The different thicknesses of ZnO buffer layers, which formed at the base of the nanowire films during growth, were obtained from different preparation ZnO seed methods: ~0.8–1 μm for samples A1, A2 and A3; ~300–500 nm for samples B1, B2 and B3. From the resulting observations, we can conclude that the high density of the nanowires achieved is attributed to the larger number of ZnO seeds on the FTO surface prepared by several spin-coating times [11,12], and that, however, this seed preparation method results in a greater variation in nanowire diameter. The crystallinity of grown ZnO nanowire arrays on FTO substrate was investigated using X-ray diffraction. Because all the samples had the very similar XRD patterns, only the XRD pattern of sample B1 was shown in Fig. 2. Diffraction peaks in XRD pattern can be indexed as wurtzite hexagonal structure (JCPDS card No. 36-1451). With respect to the crystallographic orientation, the most intense peak of ZnO corresponds to the (0002) plane, indicating a strong preferential orientation along the (0001) direction. The resulting observation can be inferred from SEM observations (Fig. 1b, d, f, h, j and l). These results reveal that the nanowires, crystallized along the ZnO (0001) direction, were hexagonal prisms vertically aligned on the FTO substrate.
Figure 1.
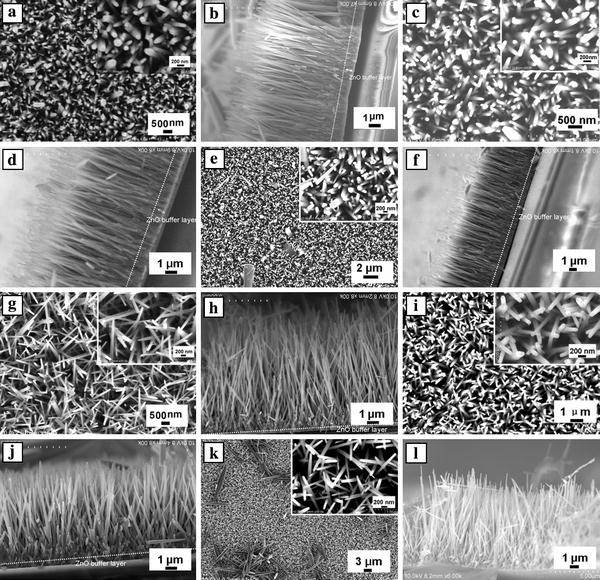
SEM images of ZnO nanowire arrays grown on FTO substrates with different ZnO seed obtained from (a–f) the spin-coating method, (g–k) the dip-coating method.a,c,e,g,iandkcorrespond to top-view observations,b,d,f,h,jandlcorrespond to cross-sectional views. The insets show high-magnification SEM images
Table 1.
Mean values of the nanowire dimensions, nanowire aspect ratio and array density for different ZnO seed preparation methods
| ZnO seed preparation methods | Diameter (nm) | Length (μm) | Nanowire aspect ratio | Density (×109 wires cm−2) |
|---|---|---|---|---|
| Spin-coating | ||||
| Sample A1 |
200 |
9.5 |
48 |
2.1 |
| Sample A2 |
195 |
9.1 |
47 |
2.2 |
| Sample A3 |
210 |
8.2 |
39 |
2.0 |
| Dip-coating | ||||
| Sample B1 |
120 |
9.5 |
79 |
1.5 |
| Sample B2 |
150 |
8.2 |
55 |
1.6 |
| Sample B3 | 130 | 9.8 | 75 | 1.2 |
Figure 2.
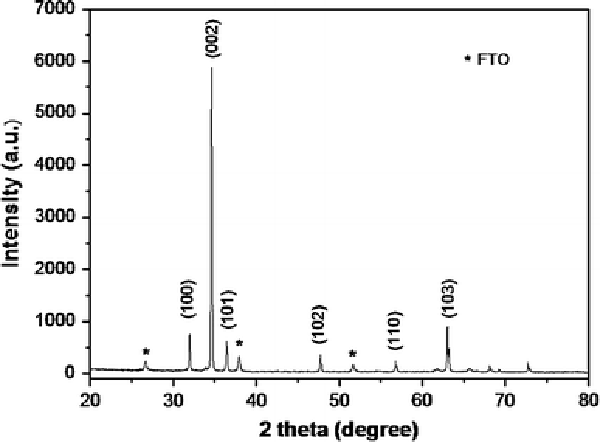
XRD pattern of the sample B1
The effect of the morphology and density of ZnO nanowire arrays on their DSSC performance was investigated, as shown in Fig. 3, while the parameters of dye-sensitized solar cell based on ZnO nanowire array films are listed in Table 2. Although the densities of nanowire arrays for samples B1, B2 and B3 were lower than that of samples A1, A2 and A3, the short circuit current density (Isc) increased from 1.61–1.97 to 1.93–2.25 mA cm−2, and in contrast, the open circuit voltage (Voc) decreased from 0.57–0.58 to 0.54–0.56 V on using samples B1, B2 and B3 as the photoanode compared with samples A1, A2 and A3. The fill factor showed little change for all samples. The samples B1, B2 and B3 based DSSCs demonstrated higher energy conversion efficiency (η) of 0.66–0.73% when compared to the samples A1, A2 and A3 based DSSCs of 0.57–0.69%. The increase in Isc may be due to that the ZnO nanowires with the thin ZnO buffer layer (~300–500 nm) had higher aspect ratios (~55–79) compared with that (~39–48) of the ZnO nanowires with ~0.8–1 μm of the ZnO buffer layer, which caused the increase of dye loading absorbed on ZnO nanowires [13]. However, the ZnO buffer layer can act as a blocking layer to suppress carrier recombination that can occur across both the film–electrolyte interface and the substrate–electrolyte interface [10]. Therefore, the thin ZnO buffer layer can less effectively suppress carrier recombination than the thick ZnO buffer layer for sample A, resulting in the maximum loss of Voc (~40 mV).
Figure 3.
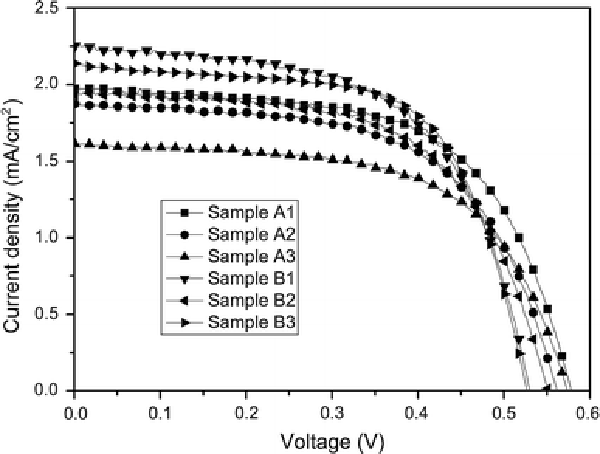
Current–voltage plots for ZnO DSSCs based on nanowire arrays prepared by different seed preparation methods
Table 2.
Parameters of dye-sensitized solar cell based on ZnO nanowire array films with different ZnO seed preparation methods
| Sample | Isc(mA/cm2) | Voc (V) | ff | η(%) |
|---|---|---|---|---|
| Sample A1 |
1.97 |
0.58 |
0.60 |
0.69 |
| Sample A2 |
1.87 |
0.57 |
0.60 |
0.64 |
| Sample A3 |
1.61 |
0.58 |
0.61 |
0.57 |
| Sample B1 |
2.25 |
0.54 |
0.59 |
0.72 |
| Sample B2 |
1.93 |
0.56 |
0.61 |
0.66 |
| Sample B3 | 2.14 | 0.54 | 0.63 | 0.73 |
Effect of TiO2Blocking Layer
In this section, we investigated the influence of a dense, thin TiO2blocking layer (about 50 nm thick, as can be seen from Fig. 4h, j and l) underneath the ZnO nanowire array film prepared by sputtering method on carrier recombination in ZnO DSSC. Figure 4shows the top-view and cross-sectional FESEM images of ZnO nanowire arrays prepared with 7.3 mM of PEI for 40 h on the bare FTO substrate and on the TiO2-coated FTO substrate with the ZnO seeds prepared by dip-coating. The mean values of the nanowire dimension, the array density and aspect ratio are summarized in Table 3. As shown in Fig. 4, fairly well-aligned nanowires, typically 160–170 nm wide with the length of 10.6–11 μm, and 120–135 nm wide with the length of 10.8–11.2 μm, grew onto the bare and TiO2-coated FTO substrates, respectively. The six ZnO nanowire films had a similar thickness of ZnO buffer layer (~500 nm). The density of ZnO nanowires for the bare FTO substrate were 1.4–1.6 × 109 wires cm−2, which are slightly higher than that of the TiO2-coated FTO substrate (1.1–1.5 × 109 wires cm−2).
Figure 4.
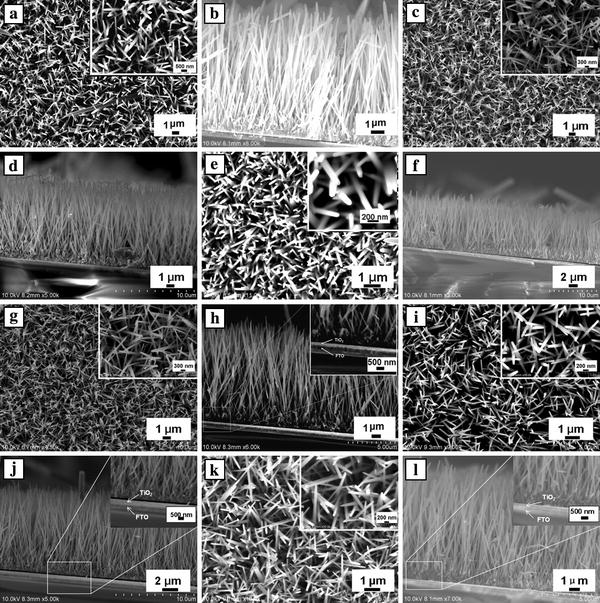
Top-view and cross-sectional SEM images of ZnO nanowire arrays prepared with 7.3 mM of PEI for 40 ha–fon the bare FTO substrate andg–lon the TiO2-coated FTO substrate. The insets show high-magnification SEM images
Table 3.
Mean values of the nanowire dimensions, nanowire aspect ratio and array density for nanowire arrays on the bare and TiO2-coated FTO substrates
| ZnO seed preparation methods | Diameter (nm) | Length (μm) | Nanowire aspect ratio | Density (×109 wires cm−2) |
|---|---|---|---|---|
| Bare FTO substrates | ||||
| Sample C1 |
170 |
11 |
65 |
1.4 |
| Sample C2 |
165 |
11 |
67 |
1.5 |
| Sample C3 |
160 |
10.6 |
66 |
1.6 |
| TiO2-coated FTO | ||||
| Sample D1 |
130 |
11 |
85 |
1.1 |
| Sample D2 |
120 |
11.2 |
93 |
1.2 |
| Sample D3 | 135 | 10.8 | 80 | 1.5 |
The effect of the TiO2 blocking layer on the photovoltaic performance of a DSSC was investigated. The current–voltage characteristics of the DSSCs for the bare and TiO2-coated FTO substrates are shown in Fig. 5. The parameters of the cells are summarized in Table 4. The DSSC derived from the nanowire arrays with the TiO2 blocking layer exhibited considerably improved Isc and Voc, compared to that of the DSSC without the TiO2 blocking layer. However, the change trend of the fill factor was complex. This is, Isc was increased by 4.2–25.1% from 3.31–3.77 to 3.72–4.14 mA cm−2, and Voc was increased by 3.9–12.5% from 0.48–0.51 to 0.53–0.55 V. As a result, η was improved by 2.4–41.7% from 0.72–0.84 to 0.86–1.02% by introducing the TiO2 blocking layer. The increase in Isc can be attributed to the increase in the aspect ratio of the nanowire arrays with and without the TiO2 blocking layer from about 65–67 to 80–93. Voc is known to be strongly dependent on the charge recombination reactions taking place on both the film–electrolyte interface and the substrate–electrolyte interface, a larger Voc value can be achieved through suppressing those reactions [14]. Therefore, the increase in Voc indicates that the compact TiO2 layer prepared by sputtering method can effectively suppress the charge recombination. This clearly shows an effective increase in η by introducing the TiO2 blocking layer.
Figure 5.
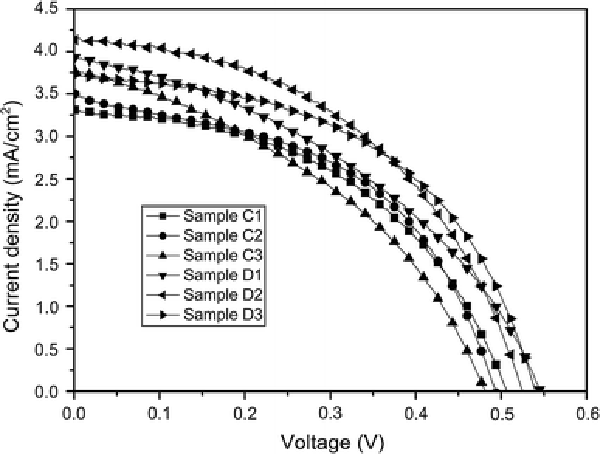
Current–voltage curves for ZnO DSSCs based on nanowire arrays grown onto the bare and TiO2-coated FTO substrates
Table 4.
Parameters of dye-sensitized solar cell based on ZnO nanowire array films on the bare and TiO2-coated FTO substrates
| Sample | Isc(mA/cm2) | Voc (V) | ff | η(%) |
|---|---|---|---|---|
| Sample C1 |
3.31 |
0.51 |
0.48 |
0.80 |
| Sample C2 |
3.50 |
0.49 |
0.49 |
0.84 |
| Sample C3 |
3.77 |
0.48 |
0.40 |
0.72 |
| Sample D1 |
3.93 |
0.55 |
0.40 |
0.86 |
| Sample D2 |
4.14 |
0.53 |
0.46 |
1.02 |
| Sample D3 | 3.72 | 0.54 | 0.50 | 1.01 |
Conclusion
In summary, the work presented here shows that the different ZnO seed preparation methods strongly influenced the morphology and density of ZnO nanowire arrays. The nanowire film growing from the ZnO seeds prepared by dip-coating had a thin ZnO buffer layer (~300–500 nm thick), which can less effectively suppress carrier recombination than the thick ZnO buffer layer (~0.8–1 μm thick) for spin-coating, resulting in the maximum loss ofVoc(about 40 mV). In order to further investigate the effect of a TiO2buffer layer on the performance of ZnO nanowire-based DSSCs, a TiO2blocking layer (about 50 nm thick) underneath the ZnO nanowire array film was prepared onto the FTO substrate by sputtering method. The two different ZnO nanowire films with and without the compact TiO2buffer layer (~50 nm) had the similar thickness of ZnO buffer layer (~300–500 nm) and were used to assemble the DSSCs. By introducing the compact TiO2layer (~50 nm thick), the photovoltaic conversion efficiency and open circuit voltage of the ZnO DSSCs were improved by 3.9–12.5 and 2.4–41.7%, respectively. This can be because that the compact TiO2layer effectively suppressed carrier recombination occurring across both the film–electrolyte interface and the substrate–electrolyte interface.
Contributor Information
Weiguang Yang, Email: wgyangd@gmail.com.
Farong Wan, Email: wanfr@mater.ustb.edu.cn.
Acknowledgments
The authors would like to acknowledge financial support for this work from the Beijing Municipal Education Commission.
References
- O’Regan B, Grätzel M. Nature. 1991. p. 737. [DOI]
- Nazeeruddin MK, Pechy P, Renouard T, Zakeeruddin SM, Humphry-Baker R, Comte P, Liska P, Cevey L, Costa E, Shklover V, Spiccia L, Deacon GB, Bignozzi CA, Gratzel M. J. 2001. p. 1613. COI number [1:CAS:528:DC%2BD3MXosFSmsw%3D%3D] [DOI] [PubMed]
- Grant CD, Schwartzberg AM, Smestad GP, Kowalik J, Tolbert L, Zhang JZ. J. 2002. p. 40. COI number [1:CAS:528:DC%2BD38Xislykt7w%3D] [DOI]
- Law M, Greene LE, Johnson JC, Saykally R, Yang PD. Nat. 2005. p. 455. COI number [1:CAS:528:DC%2BD2MXks1Cit7o%3D]; Bibcode number [2005NatMa...4..455L] [DOI] [PubMed]
- Kang SH, Chio S-H, Kang M-S, Kim J-Y, Kim H-S, Hyeon T, Sung Y-E. Adv. 2008. p. 54. COI number [1:CAS:528:DC%2BD1cXlt1Wgtbk%3D] [DOI]
- Jennings JR, Ghicov A, Peter LM, Schmuki P, Walker AB. J. 2008. p. 13364. COI number [1:CAS:528:DC%2BD1cXhtVymt7bP] [DOI] [PubMed]
- Adachi M, Murata Y, Takao J, Jiu J, Sakamoto M, Wang F. J. 2004. p. 14943. COI number [1:CAS:528:DC%2BD2cXovVKgtLw%3D] [DOI] [PubMed]
- Gao Y, Nagai M, Chang T-C, Shyue J-J. Cryst. 2007. p. 2467. COI number [1:CAS:528:DC%2BD2sXht1GmtrfO] [DOI]
- Jiang CY, Sun XW, Lo GQ, Kwong DL. Appl. 2007. p. 263501. Bibcode number [2007ApPhL..90z3501J] [DOI]
- Law M, Greene LE, Radenovic A, Kuykendall T, Liphardt J, Yang P. J. Phys. Chem. B. 2006. p. 22652. COI number [1:CAS:528:DC%2BD28XhtVKiurzJ] [DOI] [PubMed]
- Yamabi S, Imai H. J. 2002. p. 3773. COI number [1:CAS:528:DC%2BD38XptFSmt7o%3D] [DOI]
- Elias J, Tena-Zaera R, Levy-Clement C. Thin Solid Films. 2007. p. 8553. COI number [1:CAS:528:DC%2BD2sXhtFSrsrbP]; Bibcode number [2007TSF...515.8553E] [DOI]
- Gao Y, Nagai M, Chang T-C, Shyue J-J. Crys. 2007. p. 2467. COI number [1:CAS:528:DC%2BD2sXht1GmtrfO] [DOI]
- Cameron PJ, Peter LM. J. Phys. Chem. B. 2003. p. 14394. COI number [1:CAS:528:DC%2BD3sXptlOkur8%3D] [DOI]


