Abstract
Context
Randomized trials suggest adjuvant chemotherapy is effective for elderly patients with stage III colon cancer. However, the elderly are less likely to receive this therapy than younger patients, perhaps because of concern about adverse effects.
Objective
To evaluate adjuvant chemotherapy use and outcomes for older patients with stage III colon cancer from well-defined population-based settings and healthcare systems.
Design
Observational study of adjuvant chemotherapy use and outcomes by age, using Poisson regression to estimate the number of adverse events adjusted for demographic and clinical factors, including comorbid illness and specific elements of chemotherapy regimens documented with clinically detailed medical record reviews and patient and surrogate surveys.
Setting
Five geographically defined regions (Alabama, Iowa, Los Angeles County, Northern California, and North Carolina), five integrated health-care delivery systems, and 15 Veterans hospitals.
Patients
All 675 patients diagnosed with stage III colon cancer during 2003-2005 who underwent surgical resection were followed up to 15 months post-diagnosis.
Main outcome measures
Chemotherapy regimen, dose, duration and annualized mean number of adverse events stratified by age.
Results
Half of the 202 patients >=75 years received adjuvant chemotherapy compared with 87% of 473 younger patients (diff 37%, 95% CI 30%-45%). Among adjuvant chemotherapy users, 14 (14%) of patients >=75 years and 178 (44%) of younger patients received a regimen containing oxaliplatin (diff 30%, 95% CI 21%-38%). Older patients were less likely to continue. By 150 days, 99 (40%) patients >= 65 years and 68 (25%) younger patients had discontinued chemotherapy (diff 15%, 95% CI 7%-23%). Overall, 162 (24%) patients had at least one adverse clinical event, with more events among patients treated with vs. without adjuvant chemotherapy (mean 0.394 vs. 0.160, diff 0.234, 95% CI 0.11-0.36, p<0.001). Among adjuvant chemotherapy users, adjusted rates of late clinical adverse events show a reverse U-distribution with lower rates for patients >= 75 years (0.277) versus for younger patients (0.345 for 18-54, 0.519 for 55-64, and 0.446 for 65-75 years, p=0.008 for any age effect).
Conclusions
Older patients in the community receive less toxic and shorter chemotherapy regimens, and those treated had fewer adverse events than younger patients. The effect of these differences on clinical outcomes is not clear.
Keywords: stage III colon caner, colorectal neoplasia, adjuvant chemotherapy, adverse events, community settings
Randomized trials have shown reductions in cancer death and recurrence in patients with stage III colon cancer treated with adjuvant chemotherapy.1-4 A pooled analysis of trials comparing 5-fluorouracil (5-FU) and leucovorin or levamisole against no adjuvant chemotherapy reported a 24% reduction in mortality and a 32% reduction in disease recurrence across all age categories of chemotherapy users, indicating that the effectiveness of adjuvant chemotherapy with 5-FU-based regimens is similar in elderly and younger patients.5 Based upon selected patients accrued to clinical trials, several analyses have shown a disease-free survival advantage associated with the addition of oxaliplatin to standard 5-FU and leucovorin; but with increased toxicity which does not appear to vary by age.4, 6, 7
In practice elderly patients with stage III colon cancer are much less likely to receive adjuvant chemotherapy8-14 despite evidence that adjuvant therapy with 5-FU is effective across the spectrum of age. Physicians cite the lack of RCTs evaluating the effectiveness of adjuvant chemotherapy for large numbers of patients over age 80 as an important reason for not treating the elderly. Additionally, physicians often cite comorbid conditions and drug toxicities, in conjunction with the additional effort and expense of treating older patients, as the most common reasons for not treating the elderly with adjuvant chemotherapy.11, 13, 15 Patients in clinical trials are systematically different from those in the community, where most decisions about chemotherapy are made. Compared with patients diagnosed nationally with stage III colon cancer, trial patients are younger, more likely to be white, and less likely to have comorbidities or functional impairment than community-dwelling adults.8, 11
Therefore, we analyzed the use of adjuvant chemotherapy and clinical adverse events by age in a large multi-regional cohort of patients with stage III colon cancer. We accounted for multiple dimensions of patients’ burden of illness, intensity of chemotherapy regimen, and other clinical variables that might affect adverse events in these patients.
METHODS
Study design
The Cancer Care Outcomes Research and Surveillance (CanCORS) study examined care delivered to population- and health system-based cohorts of patients, including 4713 patients newly diagnosed with colorectal cancer between 2003 and 2005 and followed for up to 15 months.16 Patients were living in Northern California, Los Angeles County, North Carolina, or Alabama, or received care in one of five large health maintenance organizations or 15 Veterans Administration hospitals.11, 15-19 Human subjects committees at all participating institutions approved the study. All interviewed participants provided verbal consent based on interviewer scripts approved by relevant IRBs, and all living patients provided written consent for medical record review.
Study sample
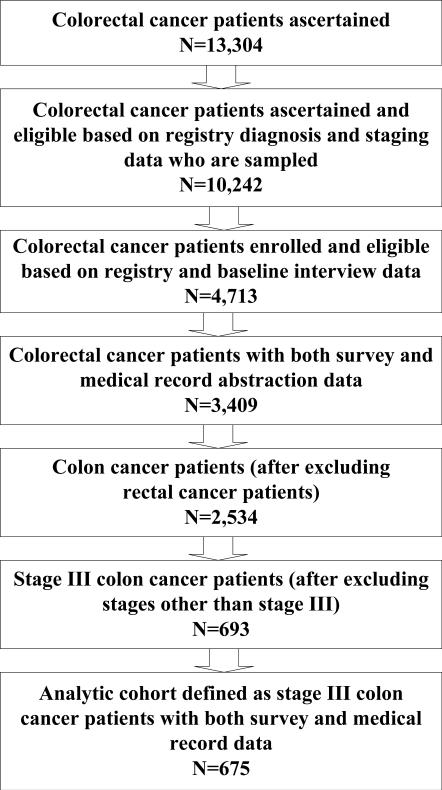
This analysis included all 675 patients with stage III colon cancer who underwent surgical resection and had survey and medical record data (Figure 1). We used data from a baseline patient survey approximately four months after diagnosis and from the review of medical records from multiple providers from three months before to 15 months after diagnosis.16 Surveys were conducted in English, Spanish, and Chinese and included four options: a 45 minute full survey (71%), a 20-minute brief survey for patients too sick to complete the full one (13%), and two surrogate surveys, one for patients alive but too sick to participate (9%) and one for patients deceased at the time of the baseline survey (7%). Self-reported patient demographics including age, gender, race/ethnicity, income and marital status were included to control for sociodemographic factors related to access and utilization. We used data from medical records to assign AJCC collaborative stage20 to 76% of study patients; where complete stage data were not available from medical records, we obtained collaborative stage data from participating cancer registries.
Figure 1.
Cohort Characteristics
Preexisting Burden of Illness
We assessed comorbidity from 3 months before diagnosis to the time of initial treatment from the medical record using the ACE-27 instrument.21, 22 Patients’ recalled health status during the four weeks prior to diagnosis was obtained from the baseline survey.17 Other measures of patient-level burden of illness included a history of prior cancer and assignment of a do-not-resuscitate order prior to the first hospitalization with an admission date >30 days after surgical resection.
Adverse Events
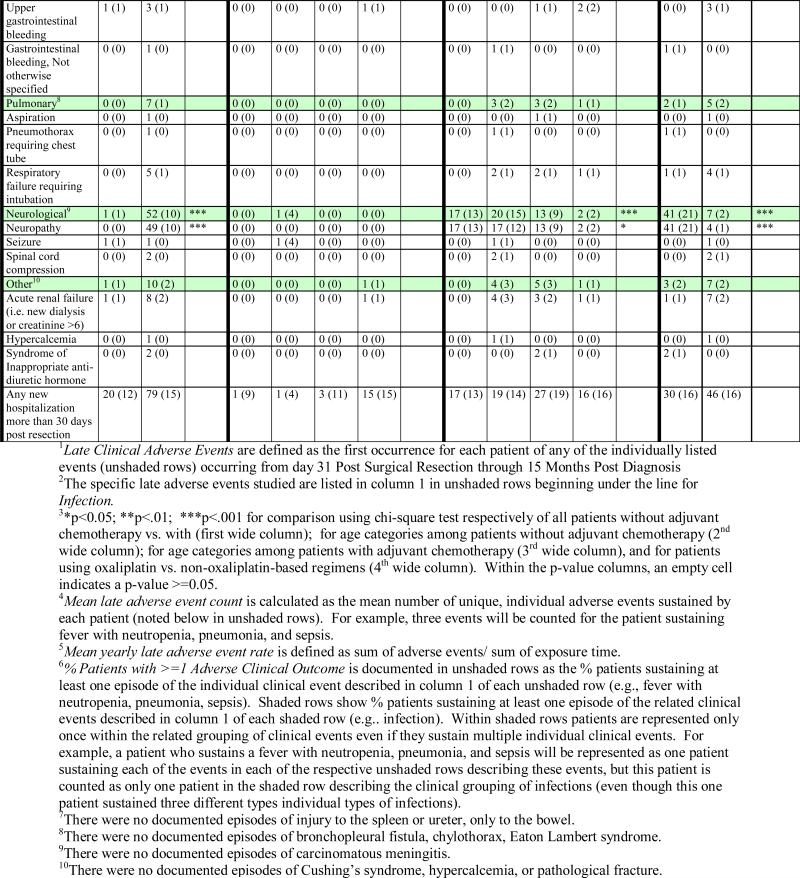
We defined adverse clinical events as the first occurrence of each of a subset of 39 clinical diagnoses that could reliably be abstracted from the medical record and that were important enough to adversely affect the patient's process of care, quality of life and/or survival as described in Table 3. Events were included regardless of whether the events could be directly attributed to treatment.
Table 3.
Late1 Clinical Adverse Events Day 31 Post Surgical Resection through 15 Months Post Diagnosis for Patients with Stage 3 Colon Cancer (Unadjusted)

|

|
Using the same list of clinical diagnoses, we defined early and late adverse events. Those that occurred prior to 30 days after surgical resection were considered early and were used as predictor variables. Events that occurred between 31 days after surgical resection and 15 months after diagnosis were used as a surrogate for events attributable to chemotherapy and considered late. (See Appendix B for complete listing of late adverse events.) We defined outcomes as annualized late adverse event rates, calculated by dividing the sum of each patient's unique clinical events by their total number of days alive subsequent to 30 days after resection.
Adjuvant Chemotherapy use
Chemotherapy was defined as adjuvant if the first dose was administered within 6 months after surgical resection and prior to any cancer recurrence. To characterize the type of chemotherapy, we classified initial chemotherapy regimens into oxaliplatin-containing, non-oxaliplatin-containing, and unknown. Chemotherapy initiation was categorized as days from surgical resection to first chemotherapy. Patients were considered to have received reduced-dose chemotherapy if their initial regimen included at least one dose of: 5FU bolus <350 mg/m2; 5FU continuous <600 mg/m2; capecitabine <850 mg/m2; or oxaliplatin <75 mg/m2. Duration of treatment was categorized by specifying the proportion of patients discontinuing chemotherapy by a specified date (e.g. before six months, See Figure 2), and also as a continuous variable counting the number of days from first to last chemotherapy dose.
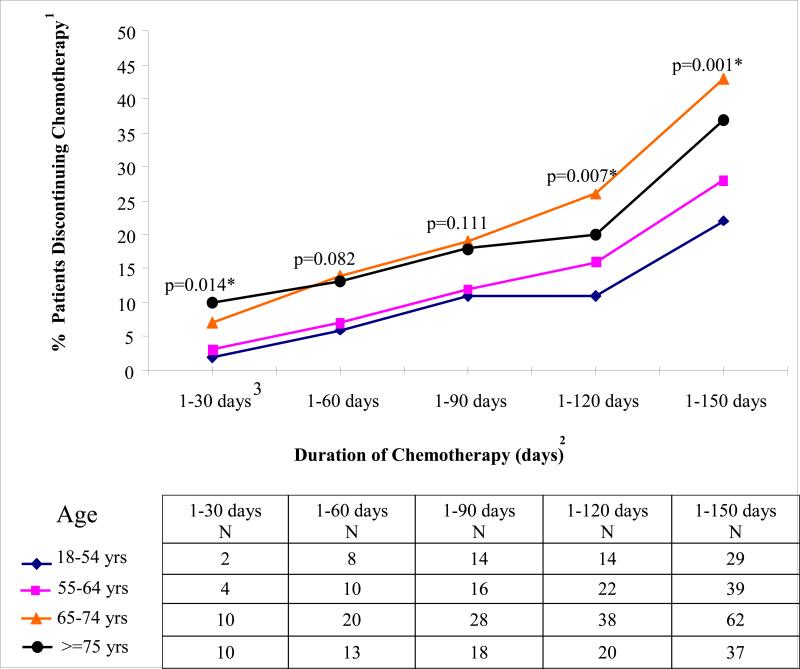
Figure 2. Cumulative Proportions of Patients Discontinuing Chemotherapy by Specified Day by Age.
1Y-axis shows % patients discontinuing chemotherapy at specified time period.
2X-axis shows the time window during which adjuvant chemotherapy is discontinued. For each age-specific curve, the data points show the % patients who have discontinued adjuvant chemotherapy by the end of the specified time window. Across all of the time windows listed in the x-axis, the n associated with the denominator is constant and includes all patients who initiate adjuvant chemotherapy (n=513).
3For example, within the time window from 1-30 days following adjuvant chemotherapy initiation, across respectively increasing age categories, 2, 3, 7, and 10% of patients have discontinued treatment (p=0.014).
*p-values indicate differences in probability of chemotherapy discontinuation by age for surviving patients as of 30, 60, 90, 120, and 150 days after chemotherapy initiation using Cox proportional hazard model. As noted, older patients are significantly more likely to discontinue chemotherapy at all time points (i.e., 30, 60, 90, 120, and 150 days). Note that the % patients having last chemotherapy date as of 180 days does not differ by age.(not shown).
Validity of Record Abstraction
We validated the accuracy of medical record abstraction by comparing 146 medical record abstractions with gold standard records, specified by the research team. The mean agreement score was 0.825 (SD 0.222).
Statistical analyses
We used univariate analyses to describe study patients, their chemotherapy initiation, initial regimen, dose, duration, and adverse event rates using CanCORS core 1.07 and medical record abstraction 1.9 data sets. All significance tests were two-sided at the 0.05 level. Analyses used SAS 9.1.3 and Stata 9.2.
We used a Poisson model to describe the count of unique patient-level adverse events with exposure defined as their total number of days alive subsequent to 30 days post-resection.23 Finally, we used recycled predictions, a method that produces adjustments in the event rate scale,24 to estimate the yearly late adverse event rates of patients with vs. without chemotherapy stratified by age.
Independent variables included age; gender; race/ethnicity; income; marital status; burden of illness (survey type, pre-diagnosis health status, comorbidity, early adverse events, prior cancer, and early DNR order); initial adjuvant chemotherapy regimen (with or without oxaliplatin, missing regimen, or none), chemotherapy initiation date, reduced dose chemotherapy, chemotherapy duration less than six months, and number of days from first to last chemotherapy. We treated sites as fixed rather than random effects, because we studied a limited number of sites that were purposively selected rather than sampled from a larger number of sites. The model also adjusted for calendar time trends and days from diagnosis to survey.
We compared patients age ≥75 with patients in younger age categories and tested for interactions between age and survey type, pre-diagnosis health status, comorbidity, and postoperative adverse events; between age and chemotherapy type (oxaliplatin vs. not); and between comorbidity and chemotherapy (oxaliplatin vs. not). Two statistically significant interactions were included in the model: youngest age category*no adjuvant chemotherapy and surrogate survey*non-oxaliplatin chemotherapy use.
Sensitivity analyses
We assessed whether results were sensitive to the model chosen by fitting an alternative model using inverse probability of treatment weights based on propensity score for receiving any chemotherapy, and separately for receiving individual chemotherapy regimens.25, 26 No statistically significant differences between results of the primary and alternate model were observed. Results were also similar when we constrained the close of the observation window for adverse outcomes to six months after surgical resection and again separately by the date of the last documented medical record visit. Finally, results were similar when we omitted do-not-resuscitate order (DNR) from the model, and when we omitted deceased patients (7%) whose baseline survey was completed by a surrogate.
RESULTS
Study Cohort
Within the study cohort, those 75 years and older were less likely to be non-white [47 (23%) vs. 169 (36%)], to be married or living with a partner [103 (51%) vs. 326 (69%)], and to report annual income more than $20,000 compared with younger patients [144 (71%) vs.370 (78%), p<0.001 for all three comparisons, Table 1). Fewer than 48 (10%) patients <75 years were unable to complete the survey because of sickness or death, while 66 (33%) patients aged 75 or older were unable (p<0.001). 30 (15%) patients at least 75 years had no comorbidity, a lower proportion than noted in other age groups [62 (44%), 51 (32%), and 33 (19%) respectively for three younger age cohorts, p<.001). Early clinical adverse events were more prevalent among patients at least 75 years with mean score 0.54 for >=75 vs. 0.35 for patients <75 years, (diff 0.19, 95% CI 0.05-0.34).
Table 1.
Patient characteristics (n=675)
| 18-54 yrs N=142 (21%) | 55-64 N=160 (24%) | 65-74 N=171 (25%) | ≥75 yrs1 N=202 (30%) | p-value2 | |
|---|---|---|---|---|---|
| Used adjuvant chemotherapy [%]3 | 131 (92%) | 137 (86%) | 144 (84%) | 101 (50%) | <.001 |
| Did not use adjuvant chemotherapy [%]4 | 11 (8%) | 23 (14%) | 27 (16%) | 101 (50%) | |
| Demographics | |||||
| Gender (% male) | 72 (51%) | 107 (67%) | 105 (61%) | 97 (48%) | 0.23 |
| Married or living together [%] | 101 (71%) | 113 (71%) | 112 (66%) | 103 (51%) | <.001 |
| White [%] | 80 (56%) | 100 (63%) | 124 (73%) | 155 (77%) | <.001 |
| Hispanic [%] | 20 (14%) | 10 (6%) | 11 (6%) | 9 (4%) | |
| Asian [%] | 9 (6%) | 8 (5%) | 4 (2%) | 16 (8%) | |
| African American (or other) [%] | 33 (23%) | 42 (26%) | 32 (19%) | 22 (11%) | |
| Completing full survey [%] | 125 (88%) | 125 (78%) | 117 (68%) | 106 (52%) | <.001 |
| Completing brief survey [%] | 13 (9%) | 22 (14%) | 23 (13%) | 30 (15%) | |
| Patients with survey completed by surrogate as patient was too sick to complete [%] | 2 (1%) | 7 (4%) | 19 (11%) | 36 (18%) | |
| Patients with survey completed by surrogate after patient deceased [%] | 2 (1%) | 6 (4%) | 12 (7%) | 30 (15%) | |
| Income < $20,000 [%] | 23 (16%) | 32 (20%) | 48 (28%) | 58 (29%) | <.001 |
| Income $20-40,000 [%] | 28 (20%) | 43 (27%) | 54 (32%) | 73 (36%) | |
| Income >$40,000 [%] | 91 (64%) | 85 (53%) | 69 (40%) | 71 (35%) | |
| Burden of Illness | |||||
| Median (Interquartile range) Pre-diagnosis health status5 | 45 (34-58) | 44 (33-54) | 48 (37,58) | 46 (35-58) | 0.12 |
| No comorbidity [%]6 | 62 (44%) | 51 (32%) | 33 (19%) | 30 (15%) | <.001 |
| Mild comorbidity [%] | 55 (39%) | 62 (39%) | 78 (46%) | 90 (45%) | |
| Moderate comorbidity [%] | 17 (12%) | 28 (18%) | 31 (18%) | 47 (23%) | |
| Severe comorbidity [%] | 8 (6%) | 19 (12%) | 29 (17%) | 35 (17%) | |
| Early clinical adverse event score [Mean, 95% CI]7 | 0.35 (.23-.46) | 0.41 (0.26-0.57) | 0.29 (0.20-0.39) | 0.54 (0.39-0.69) | .0015 |
| Prior cancer [%]8 | 8 (6%) | 13 (8%) | 21 (12%) | 53 (26%) | <.001 |
| Patients with do-not-resuscitate order documented prior to 1st hospitalization [%]9 | 0 (0%) | 0 (0%) | 1 (1%) | 4 (2%) | 0.02 |
| Characteristics of Chemotherapy for Subset of Patients Receiving Adjuvant Chemotherapy (n=513) | N=131 | N=137 | N=144 | N=101 | p-value2 |
| Number of days from surgical resection to chemo initiation [Mean (SD), Median (Interquartile range)]10 | 46 (22) 41 (34-52) | 47 (23) 42 (33-54) | 46 (17) 43 (34-54) | 51(26) 45 (36-61) | 0.32 |
| Patients with reduced initial chemotherapy regimen dose [%]11 | 25 (19%) | 27 (20%) | 26 (18%) | 14 (14%) | 0.30 |
| Patients with oxaliplatin-containing initial chemotherapy regimen [%] (n=193) | 72 (55%) | 58 (42%) | 49 (34%) | 14 (14%) | <.001 |
| Patients with non-oxaliplatin containing initial chemotherapy regimen [%] (n=293) | 52 (40%) | 72 (53%) | 88 (61%) | 81 (80%) | <.001 |
| Patients with missing type chemotherapy regimen [%] (n=27) | 7 (5%) | 7 (5%) | 7 (5%) | 6 (6%) | 0.90 |
Amongst 202 patients >=75 years, 93 patients were 75-79 years, and 109 patients were >=80 years. 60 (65%) patients aged 75-79 and 41 (38%) patients aged 80 or more initiated adjuvant chemotherapy.
p-values are generated as follows for comparisions of variables by age. Cochran-Armitage Trend tests when comparing dichotomous variables, Chi-square tests when comparing categorical variables, Poisson tests when comparing count variables, and F-tests from ANOVA when comparing continuous variables.
Used adjuvant chemotherapy is defined as patients with first dose of chemotherapy administered within 6 months after surgical resection and prior to any cancer recurrence (defined by medical record abstraction).
Did not use adjuvant chemotherapy includes 12 patients who used chemotherapy not categorized as adjuvant: six patients initiated chemotherapy > 6 months following surgical resection and six initiated chemotherapy only after medical record documentation of a colon cancer recurrence. Among these 12 patients, four used oxaliplatin-containing and eight used non-oxalplatin containing regimens.
Pre-diagnosis health status scale is constructed from patient retrospective report of health status during the four weeks prior to diagnosis using five of 12 items from the SF-12 scale. Limited on moderate activities; limited on climbing flights of stairs; accomplished less because of physical health; limited in the kind of work because of physical health; Pain interfered with normal work. Mean score for the cohort is 44 (SD 12), minimum value (i.e., worst measured health status) 17 and maximum value (i.e., best measured health status) 58. A higher score is better health status.
Comorbidity was defined using the Adult Comorbidity Evaluation (ACE-27), a validated medical record-based system that assigns each patient a four-category comorbidity score (none, mild, moderate, or severe) based upon severity noted across multiple body systems, from three months prior to diagnosis to initial treatment.24, 25 A comorbidity score, assigned to each patient according to their highest level of severity noted for each body system, had mean 1.19 (SD 0.973), median 1.00, range 0-3 with 3 representing highest degree of comorbidity.
Mean early clinical adverse events score is derived as the sum of unique adverse outcomes per patient during the window from 90 days prior to diagnosis through 30 days post surgical resection.
% Pts with prior cancer is defined as medical record documentation of cancer prior to diagnosis of incident colon cancer.
Do Not Resuscitate order documented in medical record prior to first hospitalization associated with admission date >=31 days post surgical resection.
Mean and median number of days from surgical resection to chemotherapy initiation for patients with adjuvant chemotherapy. Patients without adjuvant chemotherapy use are not included in this calculation.
Patients are categorized as receiving reduced chemotherapy dose if their initial regimen included at least one dose of: 5FU bolus < 350 mg/m2; 5FU continuous < 600 mg/m2; Capecitabine < 850 mg/m2; Oxaliplatin < 75 mg/m2; or Irinotecan < 150 mg/m2.
Patient Selection for Adjuvant Chemotherapy
Overall, patients receiving adjuvant chemotherapy were significantly less burdened with comorbid illness. Among adjuvant chemotherapy users, 150 (29%) had no comorbidity compared with 26 (16%) among patients not receiving chemotherapy (diff 13%, 95% CI 6-20, Table 2).
Table 2.
Burden of Illness1 Characteristics for Adjuvant Chemotherapy Users and Non-Users, Overall and by Age Categories (Unadjusted)
| ALL PATIENTS | PATIENTS AGE 18-54 YEARS | PATIENTS AGE 55-64 YEARS | PATIENTS AGE 65-74 YEARS | PATIENTS AGE ≥75 YEARS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALL | ALL | p- value2 |
No chemo | Chemo | p- value for 18-542 |
No chemo | Chemo | p- value for 55-642 |
No chemo | Chemo | p-value for 65-742 |
No chemo | Chemo | p-value for 75+2 |
|
| Patient characteristic |
No Chemo N=162 |
Yes Chemo N=513 |
18-54 N=11 |
18-54 N=131 |
55-64 N=23 |
55-64 N=137 |
65-74 N=27 |
65-74 N=144 |
75+ N=101 |
75+ N=101 |
|||||
| Mean (95% CI) pre-diagnosis health status | 43 (41-45) | 45 (44-46) | 0.082 | 45 (37-53) | 44 (41-46) | 0.72 | 39 (34-45) | 43 (41-45) | 0.16 | 44 (40-48) | 46 (44-48) | 0.34 | 43 (41-46) | 46 (44-48) | 0.047 |
| No comorbidity | 26 (16%) | 150 (29%) | <0.001 | 4 (36%) | 58 (44%) | 0.30 | 4 (17%) | 47 (34%) | 0.09 | 4 (15%) | 29 (20%) | 0.83 | 14 (14%) | 16 (16%) | 0.70 |
| Mild comorbidity | 66 (41%) | 219 (43%) | 4 (36%) | 51 (39%) | 8 (35%) | 54 (39%) | 12 (44%) | 66 (46%) | 42 (42%) | 48 (48%) | |||||
| Moderate comorbidity | 36 (22%) | 87 (17%) | 1 (9%) | 16 (12%) | 5 (22%) | 23 (17%) | 5 (19%) | 26 (18%) | 25 (25%) | 22 (22%) | |||||
| Severe comorbidity | 34 (21%) | 57 (11%) | 2 (18%) | 6 (5%) | 6 (26%) | 13 (9%) | 6 (22%) | 23 (16%) | 20 (19%) | 15 (15%) | |||||
| Full survey3 | 86 (53%) | 387 (75%) | <.0001 | 9 (82%) | 116 (89%) | 0.33 | 18 (78%) | 107 (78%) | 0.99 | 18 (67%) | 99 (69%) | 0.40 | 41 (41%) | 65 (64%) | <.0001 |
| Brief survey | 17 (10%) | 71 (14%) | 1 (9%) | 12 (9%) | 3 (13%) | 19 (14%) | 2 (7%) | 21 (15%) | 11 (11%) | 19 (19%) | |||||
| Surrogate survey (alive + deceased) | 59 (36%) | 55 (11%) | 1 (9%) | 3 (2%) | 2 (9%) | 11 (8%) | 7 (26%) | 24 (17%) | 49 (49%) | 17 (17%) | |||||
| Mean (95% CI) early clinical adverse events score6 | 0.59 (0.42-0.77) | 0.35 (0.28-0.41) | <.0001 | 0.27 (0.04-0.59) | 0.35 (0.23-0.47) | 0.66 | 0.43 (0.00-0.92) | 0.41 (0.24-0.58) | 0.86 | 0.56 (0.19-0.92) | 0.24 (0.15-0.33) | 0.012 | 0.67 (0.43-0.92) | 0.41 (0.25-0.57) | 0.009 |
| % Pts w Prior cancer | 41 (25%) | 54 (11%) | <.0001 | 2 (18%) | 6 (5%) | 0.12 | 1 (4%) | 12 (9%) | 0.69 | 6 (22%) | 15 (10%) | 0.110 | 32 (32%) | 21 (21%) | 0.110 |
| DNR before 1st hospitalization | 4 (2.47%) | 0.19 | 0.013 | 0 | 0 | -- | 0 | 0 | -- | 1 (4%) | 0 | 0.160 | 3 (3%) | 1 (1%) | 0.620 |
Burden of illness is the term used to describe pre-diagnosis health status, comorbidity, type of survey, early clinical outcomes, prior cancer, and do-not-resuscitate order prior to 1st hospitalization following resection surgery.
p-values are generated as follows for comparisons of variables by age: Fisher Exact tests for dichotomous variables and categorical variables; Poisson tests for count variables, and F-tests from ANOVA for continuous variables.
Surveys were conducted to optimize patient participation including the 45 minute full survey (71%), the 20-minute brief survey for patients too sick to complete the full (13%), and a surrogate survey for patients alive but too sick (9%) and for patients deceased at the time of the baseline survey (7%).
Initial Chemotherapy Regimen
Overall, 513 (75%) of the 675 stage III colon cancer patients received any adjuvant chemotherapy. Half of the 202 patients >=75 years received adjuvant chemotherapy compared with 87% of 473 younger patients (diff 37%, 95% CI 30%-45%, p<.001). Among adjuvant chemotherapy users, 14 (14%) of patients >=75 years and 178 (44%) of younger patients used an oxaliplatin-containing regimen (diff 30%, 95% CI 21%-38%).
Chemotherapy Initiation, Dose and Duration
Patients initiated adjuvant therapy a median of 47.3 days from surgery (51 days for patients at least 75 years and 46 days for those<75 years). Overall, 18% of patients had at least one drug in their initial regimen delivered at a reduced dose and this did not vary according to age. Although the recommended duration of stage III adjuvant chemotherapy regimens is at least 24 weeks,27-29 by 21 weeks (150 days) from adjuvant chemotherapy initiation, more than one-quarter of patients had discontinued treatments. Patients age 65 years and older were more likely than younger patients to discontinue chemotherapy at all follow-up times. This effect was statistically significant within 30 and beyond 90 days after initiating chemotherapy (Figure 2). For example, by 150 days, 99 (40%) patients >= 65 years and 68 (25%) younger patients had discontinued chemotherapy (diff 15%, 95% CI 7%-23%).
Rate of Late Clinical Adverse Events
Table 3 summarizes the frequency of adverse events according to treatment. Overall, 162 patients (24%) had at least one late adverse event. Late events occurred in more than twice as many patients receiving versus not receiving adjuvant chemotherapy [142 (28%) vs. 21 (13%), diff 15%, 95% CI 8-21, Table 3]. The mean number of unique adverse events was also higher for adjuvant chemotherapy users versus non-users (0.39 vs. 0.16,, diff 0. 23, 95%, CI 0.11-0.36).
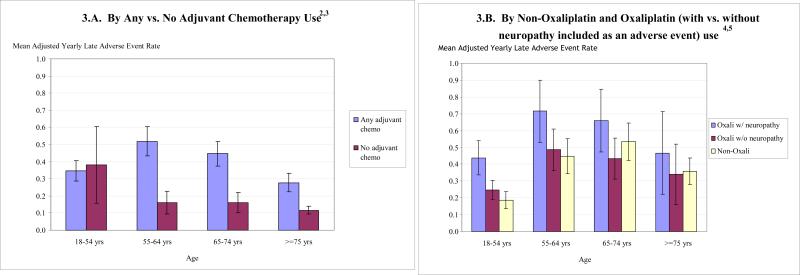
Late adverse events were associated with adjuvant chemotherapy (both oxaliplatin and non-oxaliplatin regimens) as well as surrogate survey type, early DNR order, and female gender (all p<0.05), after adjustment for other variables in the model (See Appendix B). Among adjuvant chemotherapy users (light colored bar in Figure 3.A), adjusted rates of late clinical adverse events show a reverse U-distribution across increasing age categories with the oldest patients having a lower adverse event rate than patients in the other age categories (0.345, 0.519, 0.446, 0.277; p=0.0080 for any age effect, and p=0.0125 for analysis of the effect of age categories beyond that explained by the youngest age category*no chemotherapy interaction). Regardless of whether the model included adjuvant chemotherapy as a single indicator variable, or as oxaliplatin vs. non-oxaliplatin regimens, adjuvant chemotherapy significantly predicted late adverse events (0.372 with adjuvant vs. 0.203 without adjuvant chemotherapy, diff 0.169, 95% CI 0.067-0.271). Across all tested age regimens, the inverted-U pattern of late adverse effects was preserved.
Figure 3. Adjusted Yearly Late Adverse Event Rates1.
1Poisson Models are adjusted for: gender (male); married or living together; race/ethnicity (Hispanic, Black, Asian with White as reference group); survey type (brief, surrogate- with full survey as reference group); age (<55, 55-64, 65-74 years- with >=75 years as reference group); income ($20,000 and $20-40,000- with >$40,000 as reference group); pre-diagnosis health status; comorbidity (none, mild, moderate- with severe as reference group); early (from 90 days before to <=30 days post-surgical resection); history of prior cancer; early do-not-resuscitate order; study sites; calendar date for colon cancer diagnosis; adjuvant chemotherapy (vs. none); number of days from diagnosis to chemotherapy initiation; reduced dose adjuvant chemotherapy; chemotherapy duration >=6 months; and count of # of days from 1st to last chemotherapy dose). For Figure 3.A, adjuvant chemotherapy is defined as any adjuvant chemotherapy with no adjuvant chemotherapy as reference group. For Figure 3.B, adjuvant chemotherapy is defined as oxaliplatin-containing, non-oxaliplatin-containing, or missing regimen vs. none. Regardless of whether the model included adjuvant chemotherapy as a single dummy variable (Figure 3.A) or as regimen-specific dummies (not shown), adjuvant chemotherapy significantly predicts late adverse events.
2 Among adjuvant chemotherapy users (light colored bar in Figure 3.A), adjusted rates of late clinical adverse events show a reverse U-distribution across increasing age categories with the oldest patients having a lower adverse event rate than patients in the other age categories (0.345, 0.519, 0.446, 0.277; p=0.0080 for any age effect, and p=0.0125 for analysis of the effect of age categories beyond that explained by the youngest age category*no chemotherapy interaction). This p-value corresponds to a multiple degree of freedom likelihood ratio test for which there is no difference statistic and corresponding confidence interval,, similar to an F-test in an ANOVA model.
3Among non-chemotherapy users (lower square-marked curve in Figure 3.A), late adverse events are highest among youngest patients (p=0.0125).
4Figure 3.B shows adjusted yearly late adverse event rates are significantly higher for patients using oxaliplatin (upper diamond-marked solid curve in Figure 3.B) as compared with patients using non-oxaliplatin based regimens (lower triangle-marked solid curve in Figure 3.B) across all four age categories (p<.001).
5Adjusted yearly late adverse event rates differ significantly among oxaliplatin users (upper diamond-marked solid curve vs. square-marked hyphenated line, p = 0.015) according to whether neuropathy is included or excluded in the definition of the adverse events. Adjusted yearly late adverse event rates for oxaliplatin users vs. non-users are higher when neuropathy is included in the model (upper diamond-marked solid curve in Figure 3.B vs. lower triangle-marked solid curve in Figure 3.B). Once neuropathy is excluded from the list of late adverse events, there is no difference between oxaliplatin and non-oxaliplatin users (No difference between middle square-marked dotted curve and lower triangle-marked solid curve in Figure 3.B).
Late adverse event rates were 50% higher with oxaliplatin vs. non-oxaliplatin containing regimens (0.580 vs. 0.403, diff 0.177, 95% CI 0.071-0.283, Figure 3B). The higher rates of late adverse events with oxaliplatin were accounted for by the higher rates of neuropathy among oxaliplatin users.
DISCUSSION
We analyzed adverse events in stage III colon cancer patients in relation to adjuvant chemotherapy treatment and age. Patients in our study were enrolled from defined populations and healthcare systems throughout the United States. Therefore these results describe care across diverse settings and patients in academic centers and community practices, complementing descriptions of adverse events in randomized controlled trials in which patients are highly selected.
From these diverse settings and patients, we found that use of adjuvant chemotherapy differed substantially from evidence-based recommendations, especially for older patients. Despite evidence from selected patients accrued to clinical trials showing improved outcomes for patients receiving adjuvant chemotherapy regardless of age, only 50% of patients age 75 and older initiated this treatment. Starting doses were lower than in the standard regimens tested in trials for 18% of patients, but such dose attenuation did not vary by age. Older patients were much less likely to receive oxaliplatin-containing regimens, which have been shown in clinical trials of patients <75 years to be more effective, but also more toxic, than standard regimens.4, 7 In contrast to trial-based recommendations for a six-month course of adjuvant chemotherapy,27-29 only two-thirds of patients were still using chemotherapy at six months, with higher discontinuation rates with increasing age. Among patients receiving adjuvant chemotherapy, older patients did not experience more adverse events than younger patients in either unadjusted analysis or after controlling for comorbidity and treatment characteristics.
Older patients receiving adjuvant chemotherapy in our study had less burden of illness than age-matched patients not receiving chemotherapy. Selection of less vulnerable patients might be one reason that older patients tolerated adjuvant chemotherapy better than younger patients. It is reassuring that adjustment for six dimensions of burden of illness beyond measures of demographics and chemotherapy initiation, regimen type, and duration, did not alter the finding that older patients were no more likely to have late adverse events than younger patients. We also confirmed our results with propensity score methods as a means to reduce selection bias by equating treated and untreated patients based upon observable characteristics.30 Nevertheless, residual confounding remains possible and some part of our results may reflect selection of healthier elderly patients for chemotherapy use. In this regard, our population-based findings are consistent with those of published clinical trials and observational studies demonstrating that older patients with stage III colon cancer receiving chemotherapy do not experience more adverse events than younger patients.1-5, 8, 10, 12, 31 This consistency of findings should reassure clinicians and patients who are concerned that toxicities may outweigh benefits, especially for older patients.
Adjuvant chemotherapy is generally intended to prevent disease recurrence and prolong survival for patients expected to live at least five years; it is not usually indicated for patients with more limited life expectancy, regardless of age. Of note, women and men who reach age 70 have an additional median life expectancy of 16.2 and 13.7 years respectively, and those who survive to age 80 have an additional life expectancy of 9.8 and 8.2 years,8, 32-34 suggesting that adjuvant chemotherapy should be considered for many older patients. However, the dearth of clinical trial data for older patients should be noted.
Older patients who received chemotherapy, including 41 patients >=80 years old, did not suffer higher rates of adverse events than younger patients, but the duration of follow-up of our cohort is not yet sufficient to know whether survival benefits expected from adjuvant chemotherapy were preserved for older patients using lower doses and shorter durations of treatment.5, 35 We plan to follow-up this cohort for measures of clinical benefit to learn whether the lower doses and shorter courses of treatment represent a clinical advance for older patients, or whether these modified regimens affect cancer recurrence and disease-free survival.
Our findings underscore that practical clinical trials of adjuvant chemotherapy for older patients with stage III colon cancer are needed, including patients with comorbidities and functional impairment.36 This would be consistent with the nation's commitment to comparative effectiveness research. Such trials should include patients across diverse community practice settings regardless of whether they have comorbidity.37 Our data suggest that physicians would be more comfortable enrolling their patients in such a trial if the treatment arm involved standard 5FU-based chemotherapy without the addition of oxaliplatin. Recent data appear to support this position. An analysis of aggregate trial data, published in abstract form, reported that among patients over 70, newer adjuvant regimens were no more effective than standard 5-FU/leucovorin.35
Our study documents the safety of adjuvant chemotherapy for older patients across diverse community settings, while also noting only half of older patients receive adjuvant chemotherapy and those who do, receive shorter than recommended duration. Strategies to help clinicians uncertain about the safety of adjuvant chemotherapy for older patients with comorbidity could increase the likelihood that evidence-based chemotherapy benefits are realized in population-based settings. Using decision support tools built upon published trials and population based analyses such as these, can help clinicians to predict effectiveness of chemotherapy, even for patients with comorbid conditions and advanced age.37, 38 Systematic monitoring of symptoms and signs among chemotherapy users, combined with interventions to evaluate and treat these clues, could help clinicians to support patients achieve the goal of completing evidence-based treatment dosage and duration goals. While it may not be possible to fully avoid diarrhea with 5-flourouracil or neuropathy with oxaliplatin, clinicians who identify symptoms and signs early and take steps to avoid these early signals from cascading into serious adverse outcomes may enable their patients to complete recommended treatment courses, while also improving quality of life for patients. In aggregate, these results can help clinicians and patients to estimate, anticipate, and optimize the safety of adjuvant chemotherapy for elderly patients with stage III colon cancer in diverse practice settings, increasing the likelihood that evidence-based chemotherapy benefits are realized in population-based settings.
Our study has several strengths. Patients were identified in representative populations or health systems with relatively few exclusion criteria and so are likely to broadly represent care in the community. The sample size was large enough to yield relatively stable estimates of rates in subgroups defined by treatment and age (including 109 patients >=80 years), and to support modeling for other covariates. We included a rich set of adverse events that are likely to affect patient's quality and quantity of life, and that occurred during the time window that corresponds with adjuvant chemotherapy use. Adverse events were identified from the various physicians caring for these cancer patients using inpatient and ambulatory records from oncologists, surgeons, other specialists and primary care physicians.
Our study also has limitations. Follow-up is not yet complete for analyses of recurrence rates and longer-term survival. Although we adjusted for a rich set of covariates using rigorous statistical methods, patients might have been selected for treatment according to unmeasured characteristics and these might have also been related to adverse events. We collected clinically important adverse events rather than using the Common Toxicity Criteria (CTC) grading scheme developed for clinical trials.39 Of note, CTC's were designed to be collected prospectively by trial staff, so that its classification scheme is not well-suited for abstraction from medical records alone. Nevertheless, our abstracted rate of neuropathy (11%) among patients <75 years is consistent with the prevalence of Grade III neuropathy noted in the MOSAIC trial.4 The use of different measures and data sources means we cannot directly compare the event rates we observed with clinical trials involving the same agents. However, because we focused on events that were clinically important rather than transient symptoms or isolated abnormal laboratory values, our data may be even more appropriate for informing clinical decision-making with future patients.
Our results suggest that based upon age alone, adjuvant chemotherapy need not be avoided for patients with stage III colon cancer who are expected to survive at least five years. Understandably, clinicians and patients are concerned about how the substantial burden of illness typically associated with older age could influence outcomes associated with chemotherapy. However, our results indicate that older patients receiving adjuvant chemotherapy in diverse settings appear to tolerate the treatment without an increased risk of adverse events compared with younger patients. Among older patients selected by clinicians for adjuvant chemotherapy, usually the less toxic non-oxaliplatin based regimens, this analysis shows older patients tolerate adjuvant chemotherapy, although more often with earlier discontinuation of treatment. However, this empirical analysis leaves open the question of whether other older patients who were untreated might also tolerate and benefit from the use of adjuvant chemotherapy.
Based upon randomized trials and now corroborated in a multisite community sample, use of adjuvant chemotherapy among selected older patients appears safe. However, avoiding chemotherapy and delivering doses and durations lower than recommended based on the definitive clinical trials may prevent older patients from achieving the full expected benefits of adjuvant chemotherapy.
Acknowledgements
The authors are grateful to Afshin Rastegar, MS, Research Programmer, RAND, Santa Monica, CA for conducting statistical analyses using salary support from the Cancer Care Outcomes Research and Surveillance (CanCORS Consortium grant from the National Cancer Institute (U01 CA093348) for this work at RAND.
Funding/Support and Role of Sponsor
The Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center at the Dana-Farber Cancer Institute (U01 CA093344) and the Primary Data Collection and Research Centers at RAND and University of California, Los Angeles (U01 CA093348), Harvard Medical School and Northern California Cancer Center (U01 CA093324), Dana-Farber Cancer Institute and Cancer Research Network (U01 CA093332), University of Alabama at Birmingham (U01 CA093329), University of Iowa (U01 CA01013), University of North Carolina (U01 CA093326) and by a Department of Veteran's Affairs grant to the Durham VA Medical Center (U01 CDA093344 (MOU) and HARQ 03-438MO-03). The funding organizations had no other role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Appendix A.
Late1 Clinical Adverse Events Day 31 Post Surgical Resection Through 15 Months Post Diagnosis for Patients with Stage 3 Colon Cancer (Unadjusted)
 |
 |
Appendix B.
Poisson Models Predicting Late Adverse Event Rates According to Specification of Adjuvant Chemotherapy
| Model defines chemotherapy with a dummy for Any Adjuvant Chemotherapy (vs. none) | Model defines chemotherapy as Oxaliplatin, Non-Oxaliplatin, or Missing Regimen type (vs. none) | |||
|---|---|---|---|---|
| Coefficient1 | p-value | Coefficient1 | p-value | |
| Male gender | 0.3024 | 0.049 | 0.3360 | 0.03 |
| Married or living together | -0.2022 | 0.192 | -0.2191 | 0.155 |
| Hispanic | -0.3476 | 0.199 | -0.3229 | 0.241 |
| African American | -0.2417 | 0.241 | -0.1858 | 0.371 |
| Asian | -0.3824 | 0.237 | -0.3460 | 0.291 |
| Brief survey | 0.0061 | 0.978 | 0.0133 | 0.953 |
| Surrogate survey | 1.1587 | 0.000 | 0.9276 | 0.001 |
| Age <55 years | 0.2769 | 0.268 | 0.0294 | 0.91 |
| Age 55-64 years | 0.6660 | 0.002 | 0.5101 | 0.023 |
| Age 65-74 years | 0.4623 | 0.023 | 0.3469 | 0.094 |
| Income <$20,000 | -0.2127 | 0.323 | -0.2468 | 0.253 |
| Income $20-40,000 | 0.1622 | 0.334 | 0.2223 | 0.185 |
| Pre-diagnosis health status | 0.0048 | 0.418 | 0.0031 | 0.598 |
| No comorbidity | 0.1037 | 0.68 | 0.0323 | 0.899 |
| Mild comorbidity | 0.0137 | 0.954 | -0.0510 | 0.83 |
| Moderate comorbidity | 0.4361 | 0.082 | 0.4131 | 0.102 |
| Early adverse outcome score (<=30 days post resection) | 0.0236 | 0.761 | 0.0435 | 0.566 |
| History of prior cancer | -0.1081 | 0.63 | -0.0837 | 0.71 |
| Early do-not-resuscitate order | 1.9675 | 0.000 | 1.9225 | 0.000 |
| Site 1 | 0.0096 | 0.976 | 0.0170 | 0.957 |
| Site 2 | -0.1521 | 0.678 | -0.3179 | 0.389 |
| Site 3 | -0.4737 | 0.175 | -0.5085 | 0.153 |
| Site 4 | 0.4752 | 0.139 | 0.2613 | 0.419 |
| Site 6 | 0.0982 | 0.752 | -0.1031 | 0.745 |
| Calendar date for colon cancer diagnosis | 0.0311 | 0.392 | -0.0363 | 0.363 |
| Adjuvant chemotherapy | 1.0021 | 0.016 | ||
| Oxaliplatin-based regimen | 1.5078 | 0.001 | ||
| Non-oxaliplatin-based regimen | 0.6485 | 0.13 | ||
| <55years*no adjuvant chemotherapy use | 1.1025 | 0.097 | 1.2772 | 0.054 |
| Non-oxaliplatin-regimen*surrogate survey | 0.3611 | 0.264 | 0.9230 | 0.008 |
| Chemotherapy initiation date (# days after diagnosis) | -0.0022 | 0.455 | -0.0029 | 0.344 |
| Missing adjuvant chemotherapy regimen | 1.2150 | 0.023 | ||
| Reduced dose adjuvant chemotherapy | -0.2942 | 0.145 | -0.3815 | 0.061 |
| Chemotherapy duration >=6 months | 0.1614 | 0.422 | 0.1800 | 0.371 |
| Count of # of days from 1st to last chemotherapy dose | 0.0003 | 0.738 | 0.0001 | 0.891 |
| Intercept | -9.2339 | 0 | -8.6081 | 0 |
Coefficients are in the log scale. Positive signs indicate an increased rate of adverse events for the higher value of the independent variable and negative signs indicate a decreased rate of adverse events for the higher value of the independent variable.
Footnotes
Conflicts of Interest and Financial Disclosures
None of the authors have any potential conflicts of interest or financial disclosures to report.
Previous Presentations of the Information
An abstract of this study was presented at the AcademyHealth Annual Research Meeting on June 8, 2008, in Washington, D.C, and at the ASCO Annual Meeting on June 2, 2008, in Chicago, IL.
Data Access and Responsibility
Dr. Kahn had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990 Feb 8;322(6):352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 2.Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol. 1989 Oct;7(10):1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 3.Zaniboni A, Labianca R, Marsoni S, et al. GIVIO-SITAC 01: A randomized trial of adjuvant 5-fluorouracil and folinic acid administered to patients with colon carcinoma--long term results and evaluation of the indicators of health-related quality of life. Gruppo Italiano Valutazione Interventi in Oncologia. Studio Italiano Terapia Adiuvante Colon. Cancer. 1998 Jun 1;82(11):2135–2144. doi: 10.1002/(sici)1097-0142(19980601)82:11<2135::aid-cncr7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004 Jun 3;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 5.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001 Oct 11;345(15):1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007 Jun 1;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 7.Andre T, Boni C, Navarro M, et al. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J Clin Oncol. 2009 July 1;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. 2009. [DOI] [PubMed] [Google Scholar]
- 8.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001 Jun 6;93(11):850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 9.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002 Mar 1;20(5):1192–1202. doi: 10.1200/JCO.2002.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 10.Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002 Mar 5;136(5):349–357. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 11.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003 Apr 1;21(7):1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 12.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005 Dec 7;294(21):2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 13.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 14.Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol. 2004 Sep;15(9):1330–1338. doi: 10.1093/annonc/mdh344. [DOI] [PubMed] [Google Scholar]
- 15.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant Chemotherapy for Stage III Colon Cancer: Do Physicians Agree About the Importance of Patient Age and Comorbidity? J Clin Oncol. 2008 May 20;26(15):2532–2537. doi: 10.1200/JCO.2007.15.9434. 2008. [DOI] [PubMed] [Google Scholar]
- 16.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004 Aug 1;22(15):2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients’ experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006 Aug;14(8):837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 18.CanCORS website [September 30, 2009]; Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium http://healthservices.cancer.gov/cancors/.
- 19.Keating N, Landrum M, Rogers S, et al. Physicians factors associated with discussions about end-of-life care. Cancer. 2009 doi: 10.1002/cncr.24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Joint Committee on Cancer . AJCC Cancer Staging Atlas. Springerlink; Chicago, IL: 2006. [Google Scholar]
- 21.Piccirillo JF, Costas I, Grove L. A critical appraisal of the use of ICD-9_CM codes to classify comorbidity and complications. Journal of Registry Management. 2003 November 1;30(4):117–122. 2003. [Google Scholar]
- 22.Piccirillo JF, Costas I. The impact of comorbidity on outcomes. ORL J Otorhinolaryngol Relat Spec. 2004 Jul-Aug;66(4):180–185. doi: 10.1159/000079875. [DOI] [PubMed] [Google Scholar]
- 23.StataCorp . Stata Statistical Software: Releast 9. StataCorp LP; College Station, TX: 2005. [Google Scholar]
- 24.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999 Jun;55(2):652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 25.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000 Sep;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000 Sep;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.O'Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998 Jan;16(1):295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 28.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005 Dec 1;23(34):8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 29.Dencausse Y, Hartung G, Sturm J, et al. Adjuvant chemotherapy in stage III colon cancer with 5-fluorouracil and levamisole versus 5-fluorouracil and leucovorin. Onkologie. 2002 Oct;25(5):426–430. doi: 10.1159/000067436. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 31.Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006 May 20;24(15):2368–2375. doi: 10.1200/JCO.2005.04.5005. [DOI] [PubMed] [Google Scholar]
- 32.Arias E. Natl Vital Stat Rep. Vol. 56. National Center for Health Statistics; Hyattsville, MD: 2007. United States Life Tables, 2004. p. 30. [PubMed] [Google Scholar]
- 33.Minino AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep. 2007 Aug 21;55(19):1–119. [PubMed] [Google Scholar]
- 34.Kohne CH, Folprecht G, Goldberg RM, Mitry E, Rougier P. Chemotherapy in elderly patients with colorectal cancer. Oncologist. 2008 Apr;13(4):390–402. doi: 10.1634/theoncologist.2007-0043. [DOI] [PubMed] [Google Scholar]
- 35.Sargent D, Sobrero A, Grothey A, et al. Evidence for Cure by Adjuvant Therapy in Colon Cancer: Observations Based on Individual Patient Data From 20,898 Patients on 18 Randomized Trials. J Clin Oncol. 2009 January 5; doi: 10.1200/JCO.2008.19.5362. 2009:JCO.2008.2019.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tunis SR, Stryer DB, Clancy CM. Pratical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 37.Kahn KL. Moving research from bench to bedside to community: there is still more to do. J Clin Oncol. 2008 Feb 1;26(4):523–526. doi: 10.1200/JCO.2007.13.1870. [DOI] [PubMed] [Google Scholar]
- 38.Adjuvant Online! [February 4, 2010];Decision Making Tools for Healthcare Professionals. http://www.adjuvantonline.com/index.jsp.
- 39.National Cancer Institute [February 7, 2010];Cancer Therapy Evaluation Program: Common Toxicity Criteria. http://ctep.cancer.gov/reporting/ctc.html.