Abstract
The Drosophila midgut epithelium undergoes continuous regeneration by multipotent intestinal stem cells (ISCs). Notch signaling has dual functions to control the ISCs behavior: it slows down the ISCs proliferation and drives the activated ISCs into differentiation pathways in a dose-dependent manner. Here we identified a molecular mechanism that unites these two contradictory functions. We found JAK-STAT signaling controls ISC proliferation and this ability is negatively regulated by Notch at least through a transcriptional control of the JAK-STAT signaling ligand, unpaired (upd). Our work reveals a novel mechanism of how stem cells, under steady conditions, balance the proliferation and differentiation to maintain the stable cellular composition of a healthy tissue.
Keywords: JAK-STAT, STEM CELL, DROSOPHILA, INTESTINE
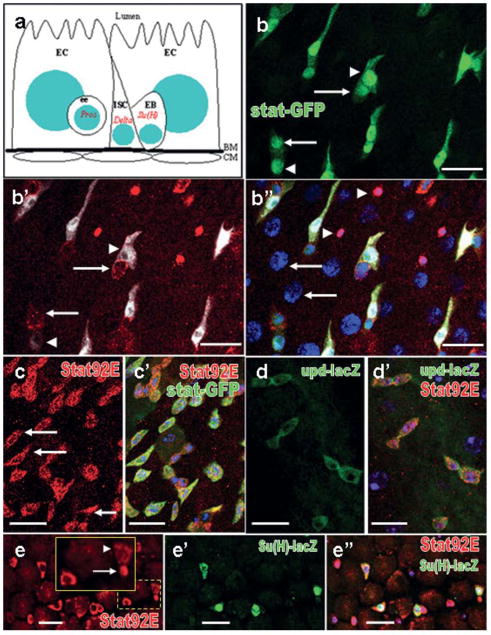
The Drosophila intestinal stem cell (ISC) is emerging as an excellent system to investigate stem cell behaviors due to its simple and well-characterized lineage (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). ISCs are aligned on the basement membrane enclosing the digestive duct. When an ISC divides, it produces two daughter cells, with one retaining stem cell properties and the other becomes an immature daughter cell, enteroblast (EB), which will eventually differentiate into an enterocyte (EC) or an enteroendocrine (ee) cell (Fig. 1a). ISCs are characterized by expression of high levels of cytoplasmic Delta-rich vesicles, which triggers Notch signaling in neighboring EBs (Ohlstein and Spradling, 2007). Su(H)GBE-lacZ, a transcriptional reporter of Notch signaling has been used as EB cell marker (Micchelli and Perrimon, 2006). The two differentiated cell types, EC and ee-cells, are more apically localized toward the lumen. The ee-cells express the homeodomain transcription factor Prospero (Pros) in the nucleus and the mature ECs can be unambiguously distinguished from other cell types by their polyploid nuclei and large cell bodies as well as by expression of ferritin 1 heavy chain homologue (Fer1HCH) at high levels specifically in ECs of young intestines (Biteau et al. 2008).
Figure 1.
Notch (N) is known to promote the tissue homeostasis by initiating ISC differentiation and specifying the terminal cell fates of EBs (Ohlstein and Spradling, 2007). It was also noticed that Notch suppresses the default proliferation state of ISC to slow down its turnover speed (Micchelli and Perrimon, 2006). Thus, an interesting question arises of how N is able to exert these two seemingly contradictory functions within a single stem cell system.
The Drosophila JAK-STAT signal transduction pathway regulates cell proliferation in several different stem cell systems (Decotto and Spradling, 2005; Singh et al. 2007; Sheng et al., 2009). The signaling is initiated by the glycosylated Unpaired proteins (upd, upd2, upd3) binding to a transmembrane receptor, domeless (dome), signals through the only Drosophila JAK kinase homologue, hopscotch (hop), and activates the only Drosophila STAT homologue, stat92E. The activated Stat92Es dimerize and enter into the nucleus to turn on the transcription of the target genes, including stat92E itself (Arbouzova and Zeidler, 2006).
We reported here that the canonical JAK-STAT signaling promotes ISCs proliferation, allowing activated ISCs to go through either self-renewal or differentiation. Under normal conditions, this function is suppressed by Notch at least through a transcriptional repression of the signaling ligand, unpaired (upd). Our work revealed that Notch, working as a differentiation signal, has a negative feedback to the ISC activation process. As a result, a stable cellular architecture of the gut epithelium is maintained, which is important for its proper physiological functions.
MATERIALS AND METHODS
FLY STOCKS
Fly stocks used in this study, described either in FlyBase or as otherwise specified, were as follows: esg-Gal4 (a kind gift from S. Hyashi); FRT82B Stat92E06346; FRT82B Stat92Ej6C8; hopC111 FRT19A; Notch55e11 FRT19A (kind gifts from K. Irvine); Su(H)1B115 FRT40A, UAS-Nact and UAS-NDN (kind gifts from M. Fortini); FRT82B neur11 (a kind gift from B. Ohlstein and A. Spradling); stat-GFP (a kind gift from G. Baeg); upd-lacZ (a kind gift from H. Sun); Su(H)GBE-lacZ (a kind gift from S. Bray); UAS-dTCFΔN, and tub-GAL80ts. Homologous recombination was used to generate double mutants, including N55e11hopC111 FRT19A; and FRT82B neur11Stat92E06346.
All flies were cultured on standard medium in either a 25°C incubator or at room temperature (about 23°C) unless otherwise indicated.
MARCM CLONE ASSAY
To induce MARCM clones of genes on the X-chromosome, including the mutations hopC111, N55e11, and double mutants of hopC111 and N55e11, we generated the following flies: (1) tub-Gal80 FRT19A/mutant- FRT19A; SM6B, hsflp/+; act>Gal4, UAS-GFP/+. To induce MARCM clones of Su(H), we generated the following flies: hsflp122/+; tub-Gal80 FRT40A/Su(H)1B115 FRT40A; act>Gal4, UAS-GFP/+. To induce MARCM clones of Stat92E06346, neur11 and their double mutant, we generated the following flies: hsflp122/+; act>Gal4, UAS-GFP/+; FRT82B tub-Gal80/FRT82B mutant-. One- or two-day-old adult female flies were heat-shocked at 37 °C for 60 min twice a day with an interval of 8 hours. The flies were transferred to fresh food daily after the final heat shock, and midguts were processed for analysis at the indicated times.
TEMPERATURE-SHIFT EXPERIMENT
Flies carrying transgenes of esg-Gal4, UAS-GFP; tub-Gal80ts alone or together with the respective UAS-line were raised at 18°C and shifted to 30°C (restrictive temperature) to turn on the Gal4 transcriptional activity. The following UAS-lines were used: UAS-NDN, UAS-dTCFΔN, UAS-upd.
JAK2 INHIBITOR TREATMENT
The JAK2 inhibitor of AG490 (T-9142, LC Laboratories) was dissolved into DMSO (25mg/ml) and 100 ul (or 200 ul) was directly added on the surface of fly vial food to reach a final concentration of 250ng/ml (or 500ng/ml). 2-day-old adult flies (UAS-NDN/+; esg-Gal4, UAS-GFP/+; tub-Gal80ts/+) were used for experiments: they were transferred from 18° C to 30° C and fed with normal or AG490 added food. 12 days later, guts were processed for analysis. Fly food was replaced every 2 days.
APOPTOSIS ASSAY
Apoptosis was analyzed using the ApopTag Red in situ apoptosis detection kit (Millipore, Cat# S7165)
HISTOLOGY AND IMAGE CAPTURE
The fly intestines were dissected in PBS and fixed in PBS containing 4% formaldehyde for 30 minutes. After four times 15-min rinses with PBT (PBS + 0.1% Triton X-100), the samples were incubated with primary antibody at room temperature for 2 hours or at 4°C overnight. The tissues were then incubated with the fluorescence-conjugated secondary antibody for 2 hours at room temperature. Samples were mounted in 90% Glycerol. We used the following antibodies: rabbit polyclonal anti-Stat92E (1:500; Hou et al., 1996); rabbit polyclonal anti-β-Gal (1:1000; Cappel); mouse anti-β-Gal (1:100, Promega); mouse anti-Dl (1:50; DSHB); mouse monoclonal anti-Pros (1:50; DSHB); rabbit polyclonal anti-GFP (1:500; Molecular Probes); mouse monoclonal anti-GFP (1:500; Molecular Probes); and rabbit anti-phospho-Histone H3 (Upstate Biotechnology, 1:1000). Secondary antibodies were goat anti-mouse and goat anti-rabbit IgG conjugated to Alexa 488 or Alexa 568 (1:400; Molecular Probes). DAPI (Sigma) was used to stain DNA. Images were captured with the Zeiss LSM 510 confocal system and processed with LSM Image Browser and Adobe Photoshop.
RESULTS
JAK-STAT IS EXPRESSED IN THE PROGENITORS OF THE DROSOPHILA MIDGUT
In an effort to dissect signalings controlling ISC behavior, we found a broad JAK-STAT expression in the adult Drosophila midgut. First, a JAK-STAT reporter line (stat-GFP; Bach et al., 2007) revealed the signaling is in both ISCs (Dl+, arrows in Fig. 1b′) and EBs [Su(H)GBE-lacZ+, arrowheads in Fig. 1b′], but is mostly absent from ECs (polyploid nuclei, arrows in Fig. 1b″) and ee-cells (pros+ in nucleus, arrowheads in Fig. 1b″) (Fig. 1b-b″). Consistent with this discovery, the Stat92E protein (red in Fig. 1c) completely co-localizes with the GFP reporter (Fig. 1c′). Moreover, a transcriptional reporter of the signaling ligand (upd-lacZ, Sun, et al., 1995, Green in Fig. 1d) indicates upd is produced in the same stat92E-expressing cells (Fig. 1d′). Taken together, we confirmed the signaling ligand, the nuclear effector, and the signaling output in the two undifferentiated cell types of the Drosophila midgut epithelium.
Interestingly, we also noticed that the Stat92E protein was mainly concentrated in the cytoplasm of most ISCs and EBs (Fig. 1e-e″), but a few of ISCs [Stat92E+, Su(H)GBE-lacZ−, Fig. 1e-e″] coupled with EBs [Su(H)GBE-lacZ+, Fig. 1e′] had strong Stat92E in the nucleus (arrow in Fig. 1e). It is known that the translocation of STATs into nucleus is a hallmark of strong JAK-STAT signaling (reviewed in Arbouzova and Zeidler, 2006). We speculate that these cells with nuclear accumulation of Stat92E represent a group of activated ISCs and a strong JAK-STAT signaling might function in the ISCs.
JAK-STAT IS REQUIRED FOR ISC PROLIFERATION
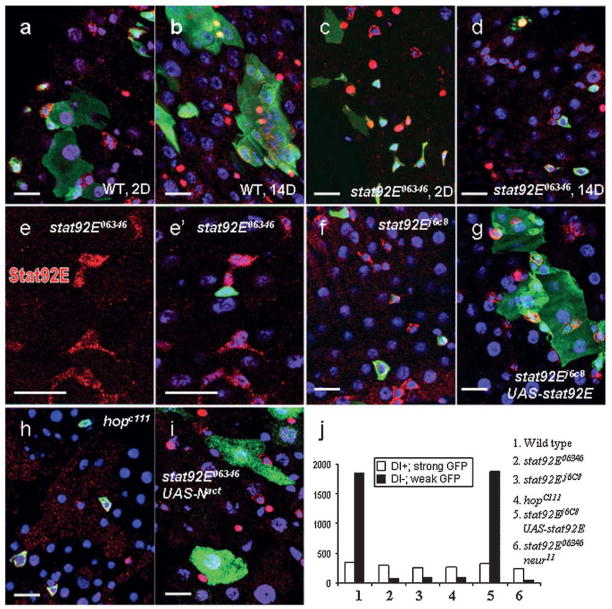
To examine if and how JAK-STAT functions in the homeostasis of the midgut, we generated JAK-STAT mutant clones using a repressible cell marker technique (MARCM, Lee and Luo, 1999). stat92E06346 represents a loss of function allele (Hou et al., 1996). Two days after clone induction (ACI), we could detect similar number of clones in both wild type and stat92E mutant samples (Fig 2a, b), indicating comparable clone induction efficiency. Both samples contained several types of GFP-positive cells, including ECs (marked by their large nuclei and cell bodies), ee-cells (pros+) and ISCs (Dl+) (Fig. 2a, c). Due to the relative short clone-chasing time, the stat92E− ECs and ee-cells probably originated from transient clones (Ohlstein and Spradling, 2006). We speculate that either the Stat92E protein has not been completely turned over yet or it indicates JAK-STAT plays little roles to specify the ISC daughter cell fates.
Figure 2.
It takes about one week for transient clones to disappear because of cell turnover in the midgut (Ohlstein and Spradling, 2006). Two weeks ACI, we found most wild type ISCs had finished at least one cell cycle and stayed with their progenies in big clusters (Fig. 2b). In contrast, most stat92E06346 clones were composed of ISC-like cells (Dl+, esg/GFP) or a small number of isolated EC- and ee-like cells (weak GFP, big nuclei or pros+, Fig. 2d). Because of the dramatically decreased differentiated cells in stat92E mutants, the ISC-like cells occupy a large portion of the total GFP positive clones (Fig. 2j). We confirmed the phenotype was associated with loss of stat92E by staining Stat92E protein (Fig. 2e, e′).
Similar phenotypes were obtained using a different stat92E allele (stat92Ej6C8, Fig. 2f, j), which could be rescued by supplying wild type Stat92E proteins (UAS-stat92E, Fig. 2g). We also checked hopC111, a loss of function alleles of Drosophila JAK (Binari and Perrimon, 1994), and observed the same results (Fig. 2h).
The significant loss of differentiated cells in the JAK-STAT mutant clones could be explained by two mechanisms: excess cell death or poor ISC proliferation. Four days ACI, there were still abundance of ECs and ee-cells in JAK-STAT mutant clones. In addition, we did not find induced apoptosis (Apoptag analysis, data not shown), thus cell death could not account for the reduction of differentiated cells in old clones. We also counted the ISC-like cells of 30-day-old mutant clones, and only found a slight decrease compared with 14-day-old samples, reflecting a slower ISC proliferation (22/gut vs. 29/gut). We concluded that without JAK-STAT, ISCs stay at quiescent states and could not go through cell cycle to generate new differentiated daughter cells or make self-renewal. The small number of remaining ECs and ee-cells in old JAK-STAT mutant clones might come from transient clones, either it represent some slow turning-over cells, or it is due to the leaky of FLP recombinase production (hsp-flp). Interestingly, forced expression of a constitutive form of N (Nact), is still able to transform the quiescent ISC-like cells in JAK-STAT mutant clones into the EC-like cells (Fig. 2i, Ohlstein and Spradling, 2007) suggesting JAK-STAT does not interfere with the normal differentiation pathway specified by Notch.
ELEVATED JAK-STAT ACCELERATES ISC PROLIFERATION
Since loss of JAK-STAT leads to poor ISC proliferation, we wonder if elevated signaling is sufficient to accelerate this process. We increased JAK-STAT signaling by expressing high levels of Upd using the following flies: esg-Gal4, UAS-GFP/UAS-upd; tub-Gal80ts/+. esg-Gal4 drives the expression of GFP and upd in both ISCs and EBs (Micchelli and Perrimon, 2006, Fig. 3a), which can be blocked by Gal80ts at 18°C. Two days after shifting to the restrictive temperature at 30°C, we found an increased number of both ISC-like cells (Dl+, strong esg/GFP, Fig. 3b, c) and young ECs underneath (arrows in Fig. 3b and insert, marked with big cell bodies and weak GFP due to the stable GFP protein). We stained for the mitotic marker phospho-Histone H3 (PH3) and found a significant increase of the mitotic index (Ph3+ cells/gut; Fig 3, numbers under each genotype). Interestingly, there are also many paired ISCs (inserts of Fig. 3b′ and 3c′). We believe they represent the newly formed ISCs right after self-renewal and have not started differentiation yet. This result suggests that increased elevated JAK-STAT accelerates the general ISC proliferation process. 5 days after shifting to 30°C, flies became inactive and started to die. We dissected these flies and found all of them developed gut hyperplasia (n=12, Fig. 3c). The gut wall was much thicker than controls (composing more than 3 cell layers vs. 1–2 in controls) and it was mixed with excessive ISC-like cells (Fig. 3c′) and a large amount of young daughter cells (Dl−, weak GFP, large nuclei, toward the lumen). As a result, the percentage of mature ECs (counted as cells with nuclei at least 4 times bigger than the diploid cells: 8n vs. 2n) within total cell pool was significantly decreased (about 20% vs. 70% in controls).
Figure 3.
To further confirm both the self-renewal and differentiation were accelerated, we tried to block one of the two pathways. Wingless (wg)/APC signaling was known to promote ISC self-renewal without interfering its differentiation pathway (Lin et al, 2008; Lee et al., 2009). We expressed a dominant-negative form of dTCF (esg-Gal4/UAS-dTCFΔN) to block wg and ISC self-renewal. 5 days after shifting to 30°C, there was a significant reduction of ISCs in dTCFΔN-overexpressed samples (Fig. 3d, d′; esg/GFP, Dl+), indicating the exhausted ISCs could not be replenished. The remaining ISCs represented the quiescent stem cells that had not been activated during the experiment. Interestingly, when we co-expressed dTCFΔN and upd, the number of ISC decreased much more quickly (compare Fig. 3d and e). Most esg/GFP+ progenitors were only weakly labeled with GFP and showed big nuclei and body size, representing young ECs (Fig. 3e). Thus, the ISC self-renewal and differentiation pathways were not coupled when the overall ISC proliferation was accelerated by JAK-STAT; the over-expression of dTCFΔN specifically impaired ISC self-renewal but not its differentiation. We believe that elevated JAK-STAT pushed most (if not all) ISCs into activated state. Once the self-renewal pathway was blocked, they had to go only through the differentiation pathway that eventually results in fast ISC exhaustion. Taken together, we concluded that JAK-STAT controls ISC proliferation, a prerequisite for the downstream self-renewal and differentiation pathways.
NOTCH SUPPRESSES JAK-STAT THROUGH A TRANSCRIPTIONAL CONTROL OF UPD
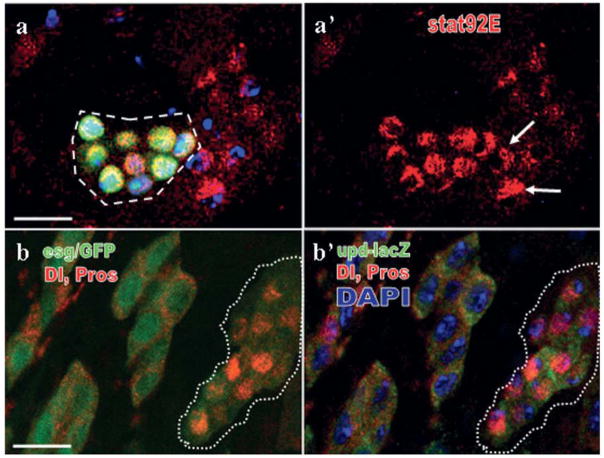
The over-proliferation phenotype upon elevated JAK-STAT reminds us the similar consequences in N mutant background. It has been shown that loss of N is sufficient to block ISCs differentiation and induce cell cycle at the same time, causing ISC-like tumors (Ohlstein and Spradling, 2006 and 2007; Micchelli and Perrimon, 2006, Fig. 4a). To investigate if JAK-STAT and N have any crosstalk, we checked the expression of stat92E in N mutant clones. It turned out that Stat92E was predominantly localized within the nuclei of N- cells (Fig. 4a, a′), suggesting a strong induction of JAK-STAT signaling. Similar results were obtained when we used a loss of function allele of Su(H), a signal transducer of N in the nucleus (data not shown). We propose a default function of Notch is to suppress JAK-STAT in the Drosophila midgut epithelium. Interestingly, we also noticed some cells next to the N– clones had also nuclear concentrated Stat92E, implying a non-cell autonomous induction (arrow in Fig. 4b′). In Drosophila, JAK-STAT is triggered by Upd proteins, which can secrete into the cell matrix to work on adjacent cells (Arbouzova and Zeidler, 2006). We examined upd-lacZ to monitor the transcription of upd in cells that expressed a dominant-negative form of N (upd-lacZ/UAS-NDN; esg-Gal4, UAS-GFP/+; tub-Gal80ts/+). After shifted to 30°C for 5 days, all these flies developed ISC- and ee-like tumors, a typical loss of Notch phenotype (Fig. 4b, b′). upd-lacZ is normally expressed only in ISCs and EBs (Fig. 1d′), but it could be induced cell-autonomously in both the ISC- and ee-like clusters where Notch was down-regulated (green in Fig. 4b′). These data suggest that Notch could directly suppress the transcription of upd to inhibit JAK-STAT in the Drosophila midgut.
Figure 4.
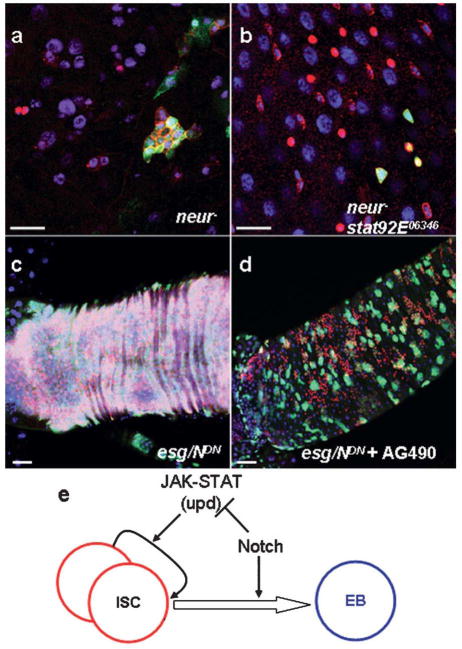
Next, we asked if the elevated JAK-STAT signaling is a cause of the N- tumors. neuralized (neur) positively regulates Notch by stabilizing Dl on cell surface and loss of neur developed the same N− phenotype, including ISC- and ee-like tumors (Ohlstein and Spradling, 2007, Fig. 5a). Interestingly, the double mutant clones of neur and stat92E gave rise to a similar phenotype of stat92E− alone (Fig. 4b). Moreover, we blocked Notch by expressing NDN and fed these flies with a mammalian JAK2/STAT3 inhibitor (AG490, Beckles et al., 2006). After cultured at 30°C for 12 days, all the flies fed with normal food developed strong loss of Notch phenotype (Fig. 5c). In contrast, such phenotype could be mildly suppressed (8 out of 10 samples; Fig. 5d). And the suppression was more evident as the inhibitor dose was increased (250 and 500ng/ml of fly food were tested). These results further support that Notch functions through JAK-STAT to negatively regulate ISC proliferation and JAK-STAT seems to be an important signaling to mediate the tumor phenotype in Notch mutant background.
Figure 5.
DISCUSSION
In this work, we characterized JAK-STAT as an important signaling to control ISC proliferation. First, we found differential Sat92E sub-cellular localizations: a small number of ISCs (often coupled with newly formed EBs) have strong JAK-STAT signaling manifested by nuclear accumulated Stat92E (Fig. 1e), while most other ISCs and EBs has cytoplasmic concentration. Second, we observed a slight reduction of the ISC-like cells (Dl+) and an obvious loss of differentiated cells in JAK-STAT mutant clones (Fig. 1j). These results suggested a compromised ISC proliferation when JAK-STAT was blocked and we speculate the small number of ISCs with strong JAK-STAT represents the activated stem cells undergoing proliferating (Fig. 1e).
Recently, two groups reported the insulin receptor (InR) and EGFR signaling pathways control the ISC proliferation (Amcheslavsky, 2009; Jiang and Edgar, 2009a). Mutant analysis of either pathway revealed the missing of big ISC lineages, which is similar with the JAK-STAT mutant phenotype (Fig. 2). We speculate that different cell growth factors and cytokines might work coordinately to regulate ISC proliferation. It would be worth to investigate if missing of two or more of these growth signals would bring even faster ISC loss or cell death. Interestingly, several groups have characterized that the proliferation of ISCs could be stimulated upon various damage treatments, and the compensatory reaction was mediated through the induced JAK-STAT signaling (Amcheslavsky et al., 2009; Buchon et al., 2009; Cronin et al., 2009; Jiang et al., 2009b). This is quite consistent with our conclusion, although we only focused on its role under normal conditions. In particular, during the preparation of this manuscript, one of these works (Jiang et al., 2009b) demonstrated that there were transient EB cells formed in JAK-STAT mutants and hence they concluded that JAK-STAT might not interfere with the basal ISCs proliferation otherwise. The different observations regarding the EBs could be explained by the low JAK-STAT requirement in normal tissue homeostasis. We found a rather weak JAK-STAT signaling in the epithelium (revealed by weak upd-lacZ and cytoplasmic Stat92E in most cells) is sufficient to maintain the normal tissue homeostasis. The RNA interference experiment in their work and the available JAK-STAT mutants may not be sufficient to completely block the signaling. We speculate a longer chasing time after JAK-STAT mutant clone induction might help to reveal the defects (14 days or 30 days in our work vs. 8 days in their report).
It has been observed that Notch is able to promote ISCs differentiation by restricting its proliferation (Ohlstein and Spradling, 2006), but the molecular mechanism remains unknown. We demonstrated here that N has at least two functions to control the ISC behavior. First, it promotes the differentiation of ISC. Second, it down-regulates JAK-STAT to slowdown ISC proliferation at least through a transcriptional control of upd. It should be pointed out that N was normally turned on in EB cells, but we could not detect a clear difference of the upd-lacZ pattern between ISCs and EBs due to its general weak expression (Fig. 1d.) Thus, it is possible that Notch also has post-transcriptional controls of upd or genes other than N are also involved in the above process.
To perform their physiological functions properly, many adult tissues need to maintain a stable cellular architecture, which is maintained by a fixed ratio of progenitors vs. mature daughter cells. Using the adult Drosophila intestinal stem cells as a model, we uncovered a novel mechanism in which a differentiation signal negatively feeds back to the stem cell proliferation pathway to stabilize the mature daughter cell numbers (Fig. 5e). The Notch activity oscillates within a narrow threshold to control the ISC behavior. High Notch promotes ISC differentiation to generate more daughter cells; on the other hand, it also slows down the ISC proliferation speed. In turn, low Notch tends to reduce the differentiated cells; but the repression of stem cell proliferation channel is also released. Eventually, the numbers of stem cells and mature differentiated cells are kept at stable levels.
Acknowledgments
We thank T. Xie, H. Sun, B. Ohlstein, A. Spradling, K. Irvin, M. Fortini, S. Bray, G. Baeg, the Bloomington Stock Center for fly stocks, and S. Lockett for help with the confocal microscope. This research was supported by the Intramural Research Program of NIH, National Cancer Institute.
Footnotes
Author Information The authors declare no competing financial interests.
References
- Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009a;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbouzova NI, Zeidler MP. JAK/STAT signaling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:1605–1616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Beckles DL, Mascareno E, Siddiqui MAQ. Inhibition of Jak2 phosphorylation attenuates pressure overload cardiac hypertrophy. Vascular Pharmacology. 2006;45:350–357. doi: 10.1016/j.vph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994;8:300–312. doi: 10.1101/gad.8.3.300. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host and Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, IPYT Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009b;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. marelle acts downstream of the Drosophila hop/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996;84:411–419. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:225–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signaling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]