Abstract
This study involves the interactions of proteins with Langmuir monolayers of a metal-chelating lipid, where adsorption is driven by a strong specific interaction between histidines on the proteins and divalent metal ions loaded into the lipid headgroups. A comparison of the structural rearrangement of the lipid film upon adsorption of myoglobin and a synthetic peptide, each of which have multiple histidines, with that upon the adsorption of lysozyme, which has only one histidine, suggests that the lipid rearrangement in the former case is due to the multiplicity of binding sites. The kinetics and manner of rearrangement change with the binding energy and film pressure.
Knowledge of the dynamical processes involved when proteins associate with lipid membranes is important for understanding many biological processes such as cell signaling [1] and toxin assault on cells [2,3] as well as for the development of novel biomaterials. A deeper understanding of protein interactions with lipid membranes could aid in the development of new drugs, as well as biosensors and other synthetic architectures. Many biophysical aspects of this interaction have yet to be understood in full detail. For example, the effects of single versus multiple-site binding, the conditions leading to insertion of segments into the lipid membrane, and understanding when conformational changes of the protein occur are all important but unresolved issues. This Letter focuses on the effects on lipid monolayers of the binding of proteins through multiple versus single specific binding sites. For the present model system, we show that irreversible binding through multiple sites on the protein leads to alteration of lipid in-plane order, and that the kinetics and manner of rearrangement change with the binding energy and film pressure.
Several lipid membrane platforms exist for biomimetic studies, including vesicles, supported lipid bilayers, and lipid monolayers at the air-water interface. Since lipid bilayer membranes in physiological conditions can expand and contract as they interact with proteins and other analytes, it is desirable to understand the interaction with proteins under controlled pressure (variable area per molecule). Langmuir monolayers offer a model system where the surface area and surface pressure are not only controlled but can be monitored to give additional insight into the protein-membrane interaction.
In this study, the effect of protein adsorption on the phase behavior of lipid monolayers was examined using time-resolved grazing incidence x-ray diffraction (GIXD), x-ray reflection (XR), and neutron reflection (NR). GIXD yields information on the laterally ordered, diffracting portion of the lipid monolayer [4]. By monitoring the diffraction peak arising from the ordered packing of the lipid tails, the effect of protein binding on the structure and phase behavior of the lipid film can be examined. XR and NR provide information about the adsorbed amount as well as the thickness of the adsorbed protein layer [5]. XR also provides the in-plane averaged electron density distribution within the lipid monolayer [6].
Previous studies involving film balance, fluorescence microscopy, and atomic force microscopy have revealed that adsorption of proteins can alter lipid phase behavior [7–10]. A handful of studies have examined detailed local changes in the ordered packing structure of lipids in gel phase upon protein adsorption by GIXD [11–15]. Several of these investigated the effect of electrostatic interactions on the extent of segmental insertion by varying the charge on the lipid head group for a given adsorbing protein or peptide [11,13,15]. In the present study we examined the effects of multiple versus single specific binding sites on the protein, and we followed the dynamics of the lipid rearrangements with time-resolved GIXD.
We examined structural changes within Langmuir monolayers of the metal-ion chelating lipid 1,2-distearyl-rac-glycero-3-triethyleneoxide iminodiacetatic acid (DSIDA) [16] upon interaction with the globular proteins horse myoglobin (Sigma) and chicken egg white lysozyme (Sigma) at a constant surface pressure (Π) of 40 mN/m. When loaded with a divalent metal ion-like Cu2+ or Ni2+, DSIDA targets histidine residues on the proteins. The use of metal-ion coordination to target the adsorption of proteins to lipid membranes has been studied extensively by others [16,17]. With this convenient model system, the energy of the specific interaction can be adjusted by the choice of the metal ion loaded into the DSIDA headgroup. Cu2+-IDA and Ni2+-IDA have interaction energies with histidine of 8.1 and 6.8 kT, respectively [18]. Horse myoglobin has a total of 11 histidines, five of which are exposed on the surface in the native state. Lysozyme has only one histidine, located on the exposed surface. Our studies also included a synthetic alanine- and glutamine-rich α-helical peptide containing two central histidines spaced ~6 Å apart along the backbone. The helicity of the 34-amino-acid-long peptide was confirmed via circular dichroism (data not shown), and the positioning of the histidines was chosen to ensure that both histidines could readily bind to the monolayer. The process of binding and rearrangement is relatively slow for the present systems, requiring many hours to reach a final time-independent state. This allowed a detailed study of the evolution of the structure within the surface layers by GIXD, NR, and XR.
XR and GIXD were performed using the liquid surface spectrometers at the Advanced Photon Source (CMC-CAT, Argonne National Laboratory) and at HASYLAB (BW1, HASYLAB, Germany). NR was performed using the NG7 (National Institutes of Standards and Technology) and SPEAR (Los Alamos National Laboratory) reflectometers. The experiments were performed using a Teflon trough with one movable barrier containing a phosphate buffered subphase at pH 7.3. Feedback control allowed measurements to be performed while maintaining a constant Π. Addition and circulation of metal-ion and protein solutions were accomplished using a peristaltic pump with inlet and outlet tubes placed at opposite ends of the trough. DSIDA was spread onto the subphase from a chloroform solution to a pressure of ~10–15 mN/m and then slowly compressed to ~40 mN/m. Upon addition of CuCl2 or NiCl2 to the subphase and circulation to give a final concentration of 10 μM, a drop in Π of ≥ 10 mN/m was observed. This was due to charge neutralization of the iminodiacetate (IDA) headgroup upon metal-ion binding [16,19]. Subsequent to this drop in Π the monolayer was compressed to a value of 40 mN/m, and maintained at that value for the remainder of the experiment. The protein was then added to the subphase behind the barrier and circulated underneath the monolayer. XR, GIXD, or NR data collection was then initiated.
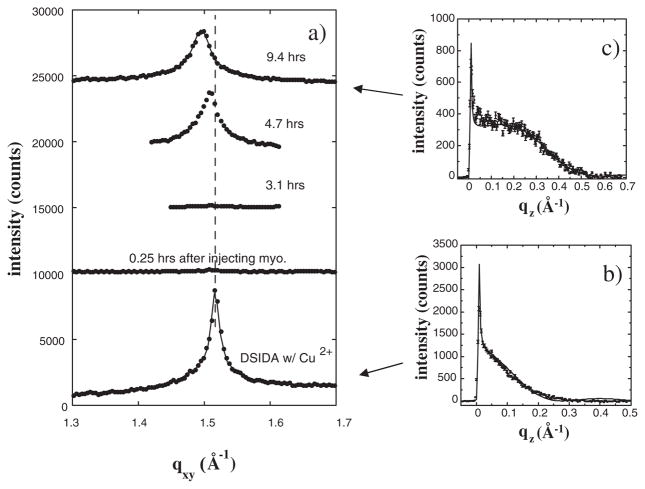
GIXD data for a DSIDA monolayer loaded with Cu2+ ions and also after injection of myoglobin (10 μM) are shown in Fig. 1. One principle in-plane Bragg peak is observed at the horizontal scattering vector component qxy = 1.52 Å−1 for the Cu2+-loaded DSIDA monolayer, indicating the existence of a 2D ordered lipid phase with a hexagonal unit cell of dimension ah = 4.77 Å, γ = 120°, and area per DSIDA molecule = 39.8 Å2. We cannot rule out a small distortion of the hexagonal cell suggested by a slight shoulder on the lower qxy side of the peak. Shortly after injecting myoglobin into the subphase the Bragg peak almost completely disappeared (Fig. 1). Disruption of the ordered packing occurred within minutes after first contact with myoglobin. We note that no change in the Bragg peak occurred when the experiment was repeated without Cu2+ ions in the DSIDA headgroups (data not shown). Thus, binding through the specific interaction of histidine with the bound Cu2+ ions is essential to the process that destabilizes the lipid ordered phase.
FIG. 1.
(a) Bragg peaks from GIXD for films of DSIDA/Cu2+ prior to injecting myoglobin, and at various times after injection for a surface pressure of 40 mN/m. For clarity, the data have been offset vertically. The solid lines through the data corresponds to fits using two Lorenzians, where one Lorenzian was a very minor component suggesting a possible slight distortion of hexagonal packing of alkyl tails. (b) Bragg rod profile for the Bragg peak for DSIDA/Cu2+. The rod was fitted (solid line) by approximating the coherently scattering part of the acyl chain by a cylinder of constant electron density. (c) Bragg rod profile for the Bragg peak for DSIDA/Cu2+/myoglobin (9.4 h).
An altered Bragg peak slowly emerged after several hours. Both the shape and position of the recovered Bragg peak differed from those of the original peak. By 9.4 h the peak had shifted to lower qxy (1:48 Å−1) and the integrated intensity was reduced by a factor of 0.72 relative to that prior to injecting myoglobin. The lower integrated intensity of the Bragg peak of DSIDA/Cu2+/myoglobin indicates a smaller fraction of the surface area occupied by the ordered domains. The shifted peak position indicates an increase in the size of the hexagonal unit cell to ah = 4.85 Å and in the area per DSIDA molecule to 46.2 Å2. The shift in the peak position was accompanied by a dramatic increase in the full width at half maximum (FWHM) of the Bragg peak and a corresponding decrease in the in-plane coherence length (the average size of the ordered lipid regions) Lxy calculated using the Scherrer formula [20] from 340 Å for DSIDA/Cu2+ to 170 Å measured 9.4 h after myoglobin injection. These results all reflect the perturbative effects of adsorbed myoglobin.
Similar trends were also seen in the out-of-plane scattering. The Bragg rod profiles integrated over the qxy region corresponding to the Bragg peak are also shown in Fig. 1 for the DSIDA monolayer loaded with Cu2+ [Fig. 1(b)] and 9.4 h after injecting myoglobin [Fig. 1(c)]. Prior to injecting myoglobin, the lipid molecules in the monolayer had almost no tilt from the surface normal. In that case the coherently scattering length of the DSIDA tails measured along the molecular backbone, Lc, was 22.5 ± 0.5 Å which is in good agreement with the calculated length of the hydrocarbon tails in all-trans configuration. However, the analysis of the Bragg rod after adsorption of myoglobin gave a shorter Lc of 20.1±0.5 Å and a tilt of the tails of 10.3° from the surface normal [21], further indication of alteration in the packing of the DSIDA.
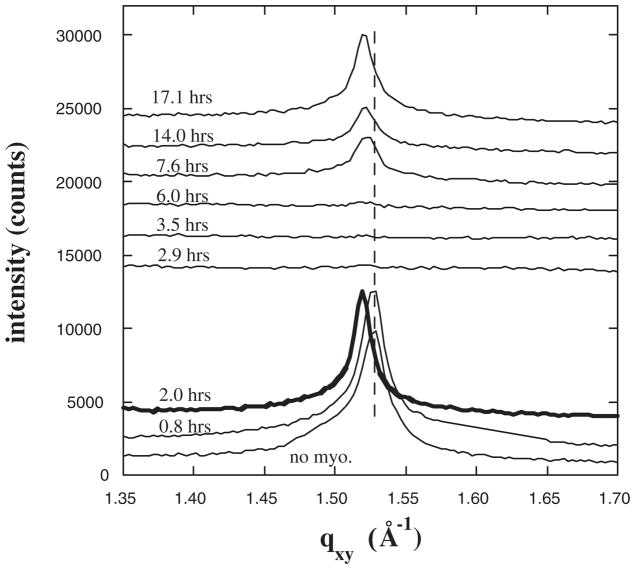
Analogous GIXD data for myoglobin adsorbing to Ni2+-DSIDA are shown in Fig. 2. As with Cu2+-loaded DSIDA, the principle diffraction peak disappears upon initial interaction with myoglobin and then returns at a slightly lower qxy. However, for the more weakly interacting Ni2+-DSIDA, the time scale is slower and the shift in the peak upon return is not as great (Δqxy = 0.008 Å−1) as with Cu2+-DSIDA (Δqxy = 0.018 Å−1). NR and XR show that myoglobin adsorbs to full monolayer coverage for both Ni2+-DSIDA and Cu2+-DSIDA at constant area [22] and at a constant π of 40 mN/m (data not shown).
FIG. 2.
Bragg peaks from GIXD for films of DSIDA/Ni2+ prior to injecting myoglobin, and at various times after injection for a surface pressure of 40 mN/m. For clarity the data have been offset vertically.
An analogous study was performed with lysozyme injected into the subphase (60 μM). In that case, no change in the Bragg peak was detected despite the fact that the surface density of adsorbed protein measured by XR and NR also reached full monolayer coverage (data not shown). Additional insight into the interaction of these two proteins with Cu2+-DSIDA and Ni2+-DSIDA was obtained by diluting the subphase following adsorption. For both lysozyme and myoglobin, no decrease in the adsorbed amount was detected by XR or NR over a period of 10–15 h following dilution by a factor of 10 or more, regardless of which metal ion was loaded into the headgroup. Furthermore, this result was obtained whether subphase dilution was performed at early stages of adsorption or after attaining full coverage. These results indicate that the interaction of a single protein histidyl with either Cu2+-DSIDA or Ni2+-DSIDA is irreversible on our experimental time scale.
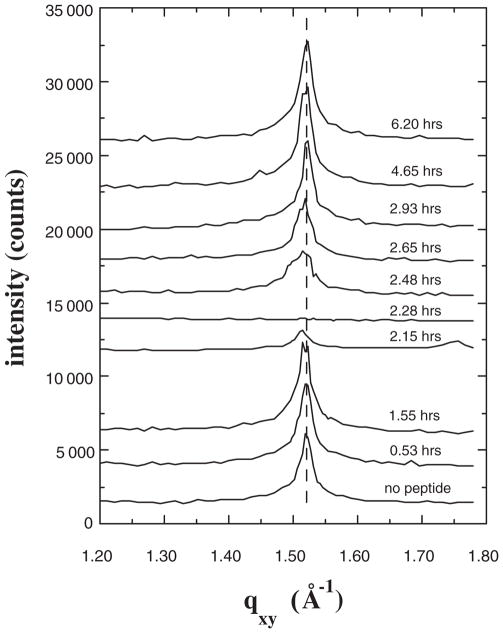
Further insight into the importance of multiple-site binding in disrupting lipid packing was provided in an analogous study performed with the synthetic α-helical peptide with two central histidines mentioned earlier. As observed for myoglobin and lysozyme, the peptide adsorbed to nearly a complete monolayer underneath the lipid layer, whereas only slight adsorption was detected under the same conditions for a control peptide lacking any histidines. The GIXD data are shown in Fig. 3. Again the Bragg peak nearly completely disappeared upon peptide binding, but then reappeared on a more rapid time scale than was observed for myoglobin. Importantly, in this case the recovered peak was unshifted from the original. These results further support the conclusion that multiple-site binding is responsible for the disappearance of the peak. In addition, combined with the data for myoglobin, the results indicate that a separate phenomenon is responsible for alterations in the recovered ordered phase indicated by the shift and broadening of the peak.
FIG. 3.
Bragg peaks from GIXD for films of DSIDA/Cu2+ prior to injecting the α-helical synthetic peptide with two central histidines (sequence Ac-QAAQAAAAQAAAAHAAAHAAAAAQAAAAQAAQGGW-NH2), and at various times after injection for a surface pressure of 40 mN/m.
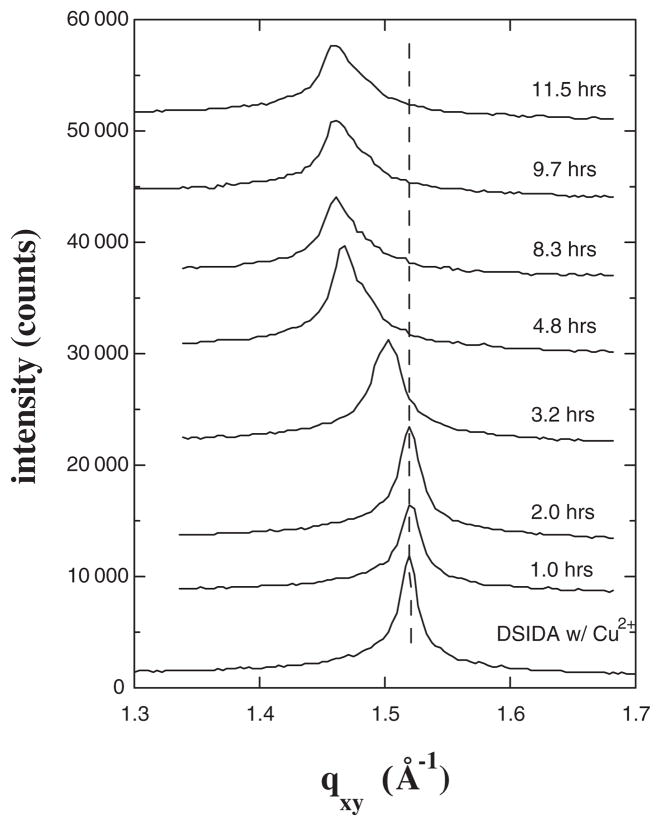
Finally, an analogous study was performed with myoglobin at a film pressure of 35 mN/m rather than 40 mN/m. These data are shown in Fig. 4. In this case the system appeared to pass smoothly from the initial lipid ordered state to a perturbed ordered state upon protein binding, without passing through a state absent in-plane order. However, we cannot rule out the possibility that the peak disappeared and reappeared very quickly. Comparison of the results at 40 mN/m and 35 mN/m shows that the greater mobility of the lipids at 35 mN/m resulted in a reduced time scale for alteration of the ordered phase. In addition, the shift in the peak position was greater (Δqxy = 0.059 Å−1) at 35 mN/m than was observed at 40 mN/m.
FIG. 4.
Bragg peaks from GIXD for films of DSIDA/Cu2+ prior to injecting myoglobin, and at various times after injection for a surface pressure of 35 mN/m.
We propose that the disappearance of the Bragg peak is due to distance constraints imposed on the lipid monolayer when a protein or peptide binds by multiple sites through strong irreversible interactions. The system subsequently rearranges into a new ordered phase that accommodates the lipids with bound protein. Several effects could explain the alteration of the packing structure in the new ordered phase, such as misregistry between the original lipid ordered phase and the binding sites on the protein, crowding of the proteins underneath the lipid film, or insertion of protein segments into some portion of the lipid monolayer. The present data support the latter interpretation, as different shifts were observed upon myoglobin binding depending upon both the interaction energy and the lipid film pressure. If the distance between binding sites on the protein or the protein dimensions dictated the structure of the altered lipid phase, we would expect a fixed position for the shifted peak independent of film pressure or binding energy. Apparently segmental insertion occurs to a greater extent for myoglobin adsorbing to Cu2+-loaded DSIDA than for myoglobin adsorbing to Ni2+-loaded DSIDA, and also at lower film pressure. The position of the histidines on myoglobin may be such that some insertion of segments into the headgroups is required for multiple histidines to bind. Alternatively, there is some evidence that myoglobin unfolds to some extent upon adsorption and this may play a role in segmental insertion [22]. This reasoning then implies that no insertion occurs upon adsorption of the peptide to Cu2+-loaded DSIDA, as no shift was observed in the recovered Bragg peak. The lack of insertion likely results from the accessibility of the two histidines such that no segmental insertion is required for both histidines to bind.
The binding domains of the bacterial toxins botulinum, tetanus, and cholera all contain multiple binding sites [2,3]. The disruption of lipid packing through multiple-site binding may play a role in facilitating insertion of the catalytic domains for these systems. The present work shows that time-dependent GIXD is a powerful method to examine subtle alterations in local packing in such systems.
Acknowledgments
Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States Department of Energy under Contract No. DE-AC04-94AL85000. Use of the Advanced Photon Source (CMC-CAT) was supported by the U. S. Department of Energy, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38. We would like to thank Dr. Kristian Kjaer from the Riso National Laboratory in Denmark and Dr. Thomas Gog and Dr. I. Kuzmencko from the Advanced Photon Source at Argonne National Laboratory for collaboration on the GIXD and XR measurements.
Footnotes
PACS numbers: 87.15.Kg, 61.10.Kw, 61.12.Ha, 87.16.Dg
References
- 1.Bray D. Annu Rev Biophys Biomol Struct. 1998;27:59. doi: 10.1146/annurev.biophys.27.1.59. [DOI] [PubMed] [Google Scholar]
- 2.Lalli G, Bohnert S, Deinhardt K, Verastegui C, Schiavo G. Trends in Microbiol. 2003;11:431. doi: 10.1016/s0966-842x(03)00210-5. [DOI] [PubMed] [Google Scholar]
- 3.Miller CE, Majewski J, Faller R, Satija S, Kuhl TL. Biophys J. 2004;86:3700. doi: 10.1529/biophysj.103.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Als-Nielsen J, Jacquemain D, Kjaer K, Leveiller F, Lahav M, Leiserowitz L. Phys Rep. 1994;246:251. doi: 10.1126/science.252.5012.1532. [DOI] [PubMed] [Google Scholar]
- 5.Russell TP, Mater TP. Sci Rep. 1990;5:171. [Google Scholar]
- 6.Als-Nielsen J, Kjaer K. The Proceedings of the NATO Advanced Study Institute, Phase Transitions in Soft Condensed Matter; Geilo, Norway. 1989; New York: Plenum Publishing Corp; 1989. pp. 113–137. [Google Scholar]
- 7.Bakowsky U, Rettig W, Bendas G, Vogel J, Bakowsky H, Harnagea C, Rothe U. Phys Chem Chem Phys. 2000;2:4609. [Google Scholar]
- 8.Peschke J, Mohwald H. Colloids Surf. 1987;27:305. [Google Scholar]
- 9.Heckl WM, Zaba BN, Mohwald H. Biochim Biophys Acta, Mol Basis Dis. 1987;903:166. doi: 10.1016/0005-2736(87)90166-0. [DOI] [PubMed] [Google Scholar]
- 10.Bondurant B, Last JA, Waggoner TA, Slade A, Sasaki DY. Langmuir. 2003;19:1829. [Google Scholar]
- 11.Konovalov O, Myagkov I, Struth B, Lohner K. Eur Biophys J. 2002;31:428. doi: 10.1007/s00249-002-0233-3. [DOI] [PubMed] [Google Scholar]
- 12.Weygrand M, Kjaer K, Howes PB, Wetzer B, Pum D, Sleytr UB, Losche M. J Phys Chem B. 2002;106:5793. [Google Scholar]
- 13.Gidalevitz D, Ishitsuka Y, Muresan AS, Konovalov O, Waring AJ, Lehrer RL, Lee KYC. Proc Natl Acad Sci USA. 2003;100:6302. doi: 10.1073/pnas.0934731100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G, Majewski J, Ege C, Kjaer K, Weygrand MJ, Lee KYC. Phys Rev Lett. 2004;93:028101. doi: 10.1103/PhysRevLett.93.028101. [DOI] [PubMed] [Google Scholar]
- 15.Ege C, Majewski J, Wu G, Kjaer K, Lee KYC. Chem Phys Chem. 2005;6:226. doi: 10.1002/cphc.200400468. [DOI] [PubMed] [Google Scholar]
- 16.Shnek DR, Pack DW, Sasaki DY, Arnold FH. Langmuir. 1994;10:2382. [Google Scholar]
- 17.Ng K, Pack DW, Sasaki DY, Arnold FH. Langmuir. 1995;11:4048. [Google Scholar]
- 18.Sinha PC, Saxena VK, Nigam NB, Sriastava MN. Indian J Chem Sect A. 1989;28A:335. [Google Scholar]
- 19.Ishiguro R, Sasaki DY, Pacheco C, Kurihara K. Colloids Surfaces A. 1999;146:329. [Google Scholar]
- 20.Guinier A. X-Ray Diffraction. Freeman; San Francisco: 1963. [Google Scholar]
- 21.The shorter Lc indicates that a smaller fraction of the tail length is scattering coherently.
- 22.Kent MS, Yim H, Sasaki DY, Satija S, Seo YS, Majewski J. Langmuir. 2005;21:6815. doi: 10.1021/la047433q. [DOI] [PubMed] [Google Scholar]