Abstract
We investigated intra-arterially administered autologous bone marrow mononuclear cells (MNCs) in rats with acute ischemic stroke. Long Evans rats (2 to 3 months or 12 months old) underwent tandem reversible common carotid artery (CCA)/middle cerebral artery (MCA) occlusion (CCAo/MCAo) for 3 h and then 24 h later underwent tibial bone marrow harvest. Ten million or 4 million cells were re-injected by an intra-carotid infusion. Control animals underwent marrow needle insertion and then saline injection into the carotid artery. Animals were assessed on a battery of neurological tests. MNCs in the ischemic brain were tracked using Q-dot nanocrystal labeling. Infarct volume and cytokines in the ischemia-affected brain were analyzed. Cell-treated animals in the younger and older groups showed improvement from 7 to 30 days after stroke compared with vehicle-treated animals. MNCs significantly reduced infarct volume compared with saline. There was a significant reduction in tumor necrosis factor-α, interleukin-1α (IL-1α), IL-β, IL-6, and a significant increase in IL-10 in injured brains harvested from the cell-treated groups compared with saline controls. Labeled MNCs were found in the peri-infarcted area at 1 h and exponentially decreased over the ensuing week after injection. Autologous bone marrow MNCs can be safely harvested from rodents after stroke, migrate to the peri-infarct area, enhance recovery, and modulate the post-ischemic inflammatory response.
Keywords: bone marrow, cell therapy, stroke
Introduction
Cellular therapy has emerged as a potential novel neurorestorative approach for the treatment of ischemic and hemorrhagic stroke. Cells originating in the bone marrow are one of many different cellular sources that have been shown to improve outcome in animal models of middle cerebral artery occlusion (MCAo). For example, bone marrow stromal cells (BMSCs), which exhibit the properties of multipotential stem cells, reduce neurological deficits in rodent models of stroke (Chen et al, 2001). BMSCs are easily isolated from the bone marrow because they adhere to plastic, can be grown and scaled to sufficient quantities in culture, and rodents injected with BMSCs show no evidence of adverse events up to one year after injury (Shen et al, 2007). The safety of BMSCs has even been investigated in five stroke patients in Korea. In that study, these cells were harvested from the stroke patient's own bone marrow and required 30 days to grow to a pure population of sufficient quantity for autologous intravenous administration(Bang et al, 2005). A pure population of autologous BMSCs at the present time is, therefore, not a practical therapeutic option for acute or sub-acute stroke.
An alternative bone marrow population is the mononuclear cell (MNCs) fraction from which MSCs are purified. MNCs contain a population of mesenchymal and hematopoietic stem cells, which secrete a host of cytokines and growth factors involved in natural repair processes (Takahashi et al, 2006). They can be rapidly derived from the bone marrow of animals and patients, separated and isolated within 3 h in our experience and then returned back to the animal or patient. MNCs have been shown to improve outcome in animal models of cerebral ischemia (Iihoshi et al, 2004; Kamiya et al, 2008). Preliminary studies even suggest that autologous MNCs can improve cardiac function in end-stage heart failure by possibly upregulating endogenous repair responses (Perin et al, 2003), and they have been shown to improve outcome with variable results in patients with acute myocardial infarction (Kamihata et al, 2001; Strauer et al, 2002). Autologous MNCs are, therefore, a potentially attractive candidate to promote stroke recovery.
No studies have examined whether MNCs can be safely extracted from the bone marrow of animals after ischemic stroke and whether autologous intra-arterial (IA) delivery at 24 h after stroke in a clinically relevant manner leads to enhanced recovery. Herein, we present an animal model of focal ischemic stroke in which MNCs were extracted from the bone marrow of the tibia of young and middle-aged Long Evans rats at 24 h after MCAo and then were autologously administered via carotid injection within 2 h after bone marrow harvest. We hypothesized that bone marrow could be safely removed from young and older rats after ischemic stroke, MNCs could then be rapidly isolated from this bone marrow, re-injected autologously, and would reduce infarct volume and neurological deficits in young and older animals. We also quantified the presence of labeled MNCs in the injured brain and present another mechanism underlying the potential benefits of MNCs in rodent stroke by assessing their effects on the post-ischemic inflammatory response.
Materials and methods
MCA Occlusion
Focal ischemia of 180-min duration in male Long Evans rats that were 350 g (3 months old) or 700 g (12 months old) was induced by tandem left middle cerebral artery (MCA) and left common carotid artery (CCA) occlusion (CCAo/MCAo), as described previously (Aronowski et al, 1997). For these procedures, animals were anesthetized with chloral hydrate (0.45 g/kg). In brief, the femoral artery was cannulated for blood pressure (BP) and blood gas recording. The temperature of the temporalis muscle was monitored/controlled using a feed-forward temperature controller. The CCA was isolated through a midline incision and tagged with a suture. Two burr holes were made in the skull: a 1 × 2.5-mm rectangular burr hole to expose the left MCA and a 1-mm-diameter burr hole to facilitate local cerebral perfusion (CP) measurement. CP in rat can be measured directly through the drill-thinned skull. A 0.005-inch diameter stainless steel wire was placed underneath to occlude the left MCA rostral to the rhinal fissure, proximal to the major bifurcation of the MCA, and distal to the lenticulostriate arteries. The left CCA was then be occluded using Heifetz aneurysm clips. Interruption of local blood flow through the MCA was inspected under the microscope and verified with a laser Doppler flowmeter (LDF) placed over the ischemic area at 2 mm posterior and 6 mm lateral to the bregma (Aronowski et al, 1997). At 24 h after stroke, local CP using LDF was measured over the penumbra area (3.8 mm posterior and 4 mm lateral from the bregma) in a subset of animals that underwent either MNC or saline injection (Aronowski et al, 1997) (N=4 per group).
Bone Marrow Harvest
We were able to perform bone marrow harvest in rats the day after they have undergone CCAo/MCAo. The rats were anesthetized with an intraperitoneal (IP) injection of chloral hydrate, 0.4 g/kg. An incision was made through the skin to the medial aspect of the tibia. The periosteum was removed and the surgeon drilled a 1.25 × 2.5-mm burr hole extending into the medullary cavity. A 20-gauge hypodermic needle was inserted into the medullary cavity and connected to a heparinized syringe. Bone marrow (1 to 1.5 ml) was aspirated while rotating and moving the needle back and forth. The medullary cavity was flushed with saline and the content aspirated. The burr hole was sealed with bone wax and the skin closed with a nylon suture.
Animal Groups
All animals underwent CCAo/MCAo and then 22 h later bone marrow procedure. Animals were then randomly assigned to MNC treatment or control. Those animals assigned to the MNC group underwent harvest as described above and then received MNCs via carotid injection. Young animals received 10 million cells while older animals received 4 million cells. A lower dose of cells was chosen for older animals based on calculations of MNC yield from bone marrow harvested in older patients with congestive heart failure who are currently undergoing MNC administration in a clinical trial at our center. Four million cells was, therefore, selected to choose a dose of cells that we believe would be feasible to obtain from a bone marrow harvest in older patients with vascular disorders. Those animals assigned to the control group underwent sham bone marrow procedure consisting of needle injection and marrow flushing, but marrow was not harvested; they then received saline via carotid injection at 24 h after MCA occlusion. This control group was designed based on the idea that if cells were removed from the bone marrow, they would not be returned; therefore, instead, we elected to insert the needle into the bone marrow without harvest. In preliminary experiments, we compared animals that underwent CCA/MCAo and bone marrow harvest with animals that only underwent CCA/MCAo and found no differences in behavior on a battery of neurological tests.
Bone Marrow Cell Processing
The cells from the bone marrow aspirate were triturated, centrifuged, and washed in phosphate-buffered saline+0.5% bovine serum albumin. Cells were then suspended in Media 199 and counted using a hemocytometer and Coulter counter. The cell suspension was added on top of 20 mL Ficoll–Paque PLUS in a 50 mL conical vial and then centrifuged. The MNCs were collected, washed with phosphate-buffered saline+0.5% bovine serum albumin (or media 199), and counted. Cells were suspended in sterile, cold phosphate-buffered saline at the desired concentration. The overall procedure took 2 h to complete.
Characterization of the MNC Population
We performed flow cytometry to characterize the MNC population using a previously published protocol (Harting et al, 2008).
Delivery Route
For IA delivery, under a surgical microscope the left CCA, left external carotid artery (ECA) and the left internal carotid artery (ICA) were isolated via a midline incision. An aneurysm clip ligation of the CCA and ICA was performed. The ECA was ligated with a nylon suture and nicked at a 45-degree angle. A modified PE10 catheter was inserted into the ECA and advanced so that the tip sat into the ICA. The ligature was tightened around the stump of the ECA until hemostasis was achieved. The ICA was unclipped and marrow cells or saline infused slowly over 5 mins. The catheter was then flushed with saline. The catheter was withdrawn and the ligature was tightened around the stump of the ECA. The skin incision was closed with a nylon suture.
Analysis of Long-Term Motor Dysfunction
All sensorimotor testing was performed during the light cycle. Animals were pre-tested and then tested on days 1, 7, 14, 21, and 28 post-ischemia. We used a battery of sensorimotor tests sensitive to cortical damage produced by our rodent ischemia model and include the following:
Asymmetry in the Use of Forelimbs for Postural Support (Cylinder Test): Animals were placed into a plexiglas cylinder and their behavior observed for forelimb-use asymmetry during vertical movements along the wall of the cylinder. The final score was calculated as (non-impaired forelimb movement−impaired forelimb movement)/(non-impaired forelimb movement+impaired forelimb movement+both movement), as previously described in the rat (Schallert et al, 2000). A total of 20 movements were recorded during the 10-min test.
Asymmetry-Corner Test
In the home cage, an animal was placed between two angled boards. When entering deep into the corner, both sides of the vibrissae are stimulated together. The animal then rears forward and upward, then turning back to face the open end. Twenty trials were performed for each rat and the percentage of left turns versus right turns was calculated. Only turns involving full rearing along either board were recorded.
Infarct Volume
Young animals (n=5 per group) underwent CCA/MCA occlusion and then either received autologous MNCs or saline as described above. At 7 days after stroke, animals were killed and decapitated. The brains were quickly removed, cut into 2-mm sections, and stained with 2,3-5-triphenyltetrazolium chloride. The infarct volume was calculated from the difference between the volume of contralateral cortex and the volume of the 2,3-5-triphenyltetrazolium chloride -stained portion (non-ischemic) of ipsilateral cortex of each rat. This indirect measure corrects the total infarct volume for edema.
Cytokine Analysis
A separate group of young animals (n=8) underwent CCA/MCA occlusion and then either received autologous MNCs or saline as described above. At 48 h after stroke, animals were killed and ischemic brain tissue was homogenized, centrifuged, and then the supernatant was analyzed for cytokine levels. Cytokine levels in the brain supernatant were determined with a commercially available multiplex bead-based suspension immunoassay (Bio-Rad Laboratories, Hercules, CA, USA) following the manufacturer's protocol. Briefly, fluorescently dyed microspheres (beads) were conjugated with antibodies for targeted cytokines. The antibody-coupled beads were washed with serum containing the cytokine. A series of washes were performed to remove unbound protein and the beads were exposed to a detection antibody specific for a separate epitope on the cytokine, resulting in a sandwich immunoassay format. Streptavidin–phycoerythrin was added, which bound to the detection antibody. Beads were drawn into a flow cytometer, which identified and quantitated each cytokine based on bead fluorescence. Concentrations were calculated using a standard curve derived from a recombinant cytokine standard (Bioplex manual).
Assessment of Transplanted MNCs
To track the transplanted cells in the brain, Q-dot nanocrystals, a red fluorescent maker (655 nm), was used. MNCs were harvested and labeled with Q-dot as described previously (Rosen et al, 2007). Transient CCAo/MCAo and cell administration were performed as described above (n=4 per time point). At 1 h, 3 h, 6 h, 12 h, 24 h, 72 h, and 7 days after CCAo/MCAo, rats were anesthetized deeply with chloral hydrate, perfused with saline, and perfusion-fixed with 4% paraformaldehyde in phosphate-buffered saline. The brains were removed carefully and cut into 20-μm-thick coronal sections at the level of the infarct in the frontal and parietal cortex. Sections were counterstained with 4,6-diamidino-2-phenylindole or fluorescein isothiocyanate and then visualized by fluorescence microscopy to detect Q-dot-labeled cells. Total number of labeled cells was then counted within rectangular regions placed on estimated ischemic core and peri-infarct areas of the cortex in the ischemic hemispheres in a fixed manner for each rat (Kamiya et al, 2008).
TUNEL Analysis
The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) method was performed to assess cell death by using the ApoTag Fluorescein In-Situ Apoptosis Detection Assay kit (Chemicon International, Billerica, MA, USA). Cryostat sections were fixed, incubated with terminal deoxynucleotidyltransferase enzyme and nucleotide mix, followed by TUNEL blocking reagents according the manufacturer's instructions. Anti-digoxigenin-fluorescein isothiocyanate conjugate (65 μL/5 cm2) was applied.
Statistical Analysis
Data are presented as mean±s.d. Cytokine analysis was analyzed using Student's t-test and corrected for multiple comparisons. For statistical analysis, repeated-measures, two-way analysis of variance and Bonferroni posttest were used for comparison among groups at different days after stroke in the behavioral tests. Statistical significance was set at the P<0.05 level.
Results
Bone Marrow Harvest and MNC Yield
Bone marrow harvest after stroke did not cause hemodynamic compromise based on blood pressure and heart rate (data not shown), and there was no observed increase in mortality for the study period. We were able to conduct autologous MNC treatment in all animals randomized to receive cells. Needle aspiration from one tibia 22 h after stroke consistently without exception yielded over 10 million MNCs from one rat (range 11 to 30 million). The procedure did not cause limb impairment that we could observe in any of the animals that underwent a bone marrow procedure. All animals bore their own weight on the ipsilateral limb after harvest; thus, animals fully participated in our behavioral studies.
Phenotypic Characterization
The MNC fraction of bone marrow is a mixture of different cellular populations. We provide immunophenotypic characterization of MNCs in Table 1, which gives the breakdown of cell-surface markers of mononuclear cells derived from the bone marrow of young Long Evans rats. The mesenchymal stem cell (MSC) population in the MNC fraction was approximated by assessing the percentage of cells that were CD29+/90+/CD45−.
Table 1. Immunophenotype of mononuclear cells.
| Marker | Rat 1 (%) | Rat 2 (%) | Rat 3 (%) | Average (%) | s.e.m. |
|---|---|---|---|---|---|
| CD3 | 8.7 | 6.7 | 6.2 | 7.20 | 0.76 |
| CD4 | 6.9 | 7.6 | 8.5 | 7.67 | 0.46 |
| CD8 | 8.9 | 8.2 | 7.6 | 8.23 | 0.38 |
| CD34 | 18.2 | 20.1 | 22.3 | 20.20 | 1.19 |
| CD11b | 20.1 | 23.4 | 18.3 | 20.60 | 1.50 |
| CD45 | 83.2 | 86 | 88.6 | 85.93 | 1.56 |
| CD90 | 56.7 | 61 | 68.6 | 62.10 | 3.48 |
| CD29 | 95.2 | 96.9 | 97.8 | 96.63 | 0.76 |
| 29+/90+/45− | 0.77 | 0.4 | 0.3 | 0.49 | 0.14 |
Intra-arterial Delivery Enhances Recovery in Young Rats
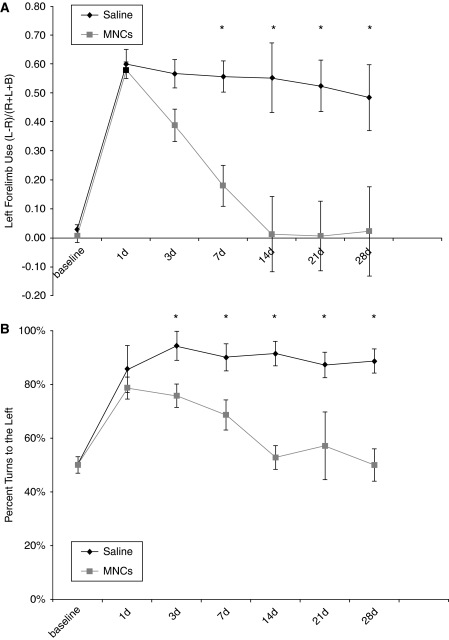
Long Evans rats underwent left CCA/MCA occlusion for 3 h, which consistently led to right-hemibody deficits. MNCs (10 million cells) or vehicle were injected at 24 h after CCAo/MCAo into the ipsilateral carotid artery. All animals survived to the study endpoint and underwent serial neurological testing throughout the study period. Neurological testing demonstrated that both groups of animals were impaired before injection of cells or saline. In the vehicle-treated group, deficits persisted in both the cylinder (0.48±0.11) and corner tests (90%±5% turns to the left) at 30 days. The cell-treated young animals began to show improvement by 3 to 7 days after stroke and their deficits resolved on both tests by 30 days (cylinder, 0.02±0.15 and corner, 50%±6% (P<0.05 for either test compared with vehicle controls)) (Figures 1A and 1B).
Figure 1.
Young Long Evans rats treated with MNCs have improved recovery after cortical stroke. Animals underwent CCAo/MCAo, and 24 h later either received an IA injection of autologous MNCs or saline followed by neurological testing for 30 days. (A) The cylinder test demonstrates preferential left forearm placement in animals that have undergone left CCAo/MCAo and IA saline injection at 24 h after stroke. Deficits persist up to 28 days after stroke. Animals that receive MNCs, however, at 24 h after stroke show resolution of deficits over time (P<0.05 for days 7, 14, 21, and 28; n=7 per group). (B) The corner test demonstrates preferential turning to the left in animals that have undergone left CCAo/MCAo and IA saline injection at 24 h after stroke. Deficits persist up to 28 days after stroke. Animals that receive MNCs, however, at 24 h after stroke show resolution of deficits over time (P<0.05 for days 14, 21, and 28; n=7 per group).
Intra-arterial Delivery Enhances Recovery in Older Rats
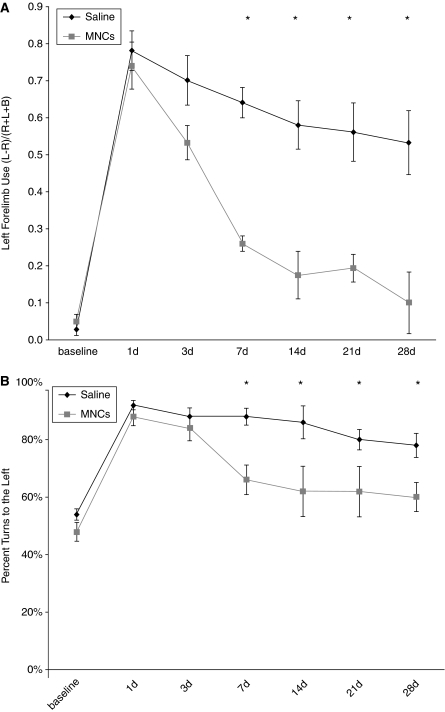
Retired breeder Long Evans rats underwent left CCA/MCA occlusion for 3 h, which consistently caused right-hemibody deficits. MNCs (4 million cells) or saline vehicle were injected at 24 h after focal ischemia. Among the older animals, cell-treated rats (n=5) showed significantly reduced deficits (cylinder, 0.26±0.05 and corner, 66%±13%) compared with vehicle-treated rats (cylinder, 0.64±0.11 and corner, 88%±8%) starting day 7 (P<0.05 for either test compared with vehicle-treated controls). There continued to be significant improvement in deficits over time in MNC-treated animals. Vehicle-treated animals had the following scores at 30 days: cylinder, 0.53±0.09 and corner, 78%±4%. Cell-treated animals had the following scores at 30 days: cylinder, 0.1±0.08 and corner, 60%±5% (P<0.05 for either test compared with vehicle controls) (Figures 2A and 2B).
Figure 2.
Twelve-month-old, Long Evans rats treated with MNCs show improved recovery after cortical stroke. Retired breeders underwent CCAo/MCAo, and 24 h later either received an IA injection of autologous MNCs or saline. Saline-treated rats show persistent severe deficits lasting for at least 28 days as determined using cylinder (A) and corner (B) tests. Cell-treated animals show significant reductions in neurological deficits on the cylinder (A) and corner (B) tests starting at 7 days after stroke (n=5 per group).
Physiological Monitoring During Cell Infusion
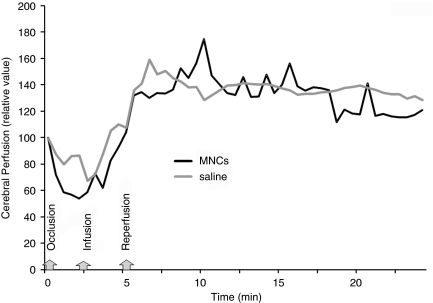
We monitored retired breeder animals (n=4 per group) that underwent CCAo/MCAo and then either received saline or MNCs. No significant changes in local cerebral perfusion were observed between the two groups after infusion (Figure 3). No animals experienced a decrease in cerebral perfusion after cell injection. During infusion we also monitored blood pressure, heart rate, arterial blood gases, and temperature, all of which were unaffected by MNCs administration. There were no differences in these variables during cell or saline infusion (data not shown).
Figure 3.
Cerebral perfusion during IA infusion of MNCs or saline. At 24 h after acute stroke, the external carotid and common artery were ligated, which corresponded with a decline in cerebral perfusion as usual in this model (labeled occlusion). Then a catheter was inserted to the ICA to infuse saline or MNCs in a final volume of 1 ml (labeled infusion). In response to the infusion of MNCs or saline, the cerebral perfusion increased in all animals tested beyond baseline. The CCA was unclamped (labeled reperfusion) and then there was a return of cerebral perfusion to mildly elevated levels in both saline- and MNC-treated groups. Data represent the mean of four animals per group.
MNCs in the Brain After IA Delivery
We found labeled MNCs in the per-infarct regions as early as 1 h after IA injection. Figure 4 shows representative photomicrographs of brain sections demonstrating fluorescence-labeled MNCs in the peri-ischemic areas of the brain 1 h to 7 days after IA administration. There was an exponential decrease in the number of labeled MNCs in the peri-infarct area (Figure 4E). No labeled cells were observed in the contralateral hemisphere or cerebellum (data not shown). Immunohistochemistry with TUNEL showed that some Q-dot-labeled MNCs were TUNEL positive (Figure 4F to 4H) at 3 h after injection.
Figure 4.
Twenty-four hours after CCAo/MCAo, Long Evans rats received an intra-carotid injection of MNCs. The MNCs were derived from the bone marrow and labeled with Q tracker nanocrystals before infusion. Fluorescence microscopic images illustrate time-dependent elimination of MNCs in per-infarcted rat brain. One hour (A), 6 h (B), 24 h (C), and 7 days (D) after intra-carotid injection. Green: Fluorescein isothiocyanate (to unspecifically label the brain) and red: Q-Tracker. Magnification, × 40. (E) A histogram showing a reduction in the number of labeled MNCs in the peri-infarcted zone as a function of time (n=4 per group). (F to H) Fluorescence microscopic images of two different areas in the peri-infarct region illustrating Q-dot-labeled MNCs (left panel), TUNEL-stained cells in the same region (middle panel), and colocalization of Q-MNCs with TUNEL-positive immunoreactivity. Colocalization of Q-MNCs with TUNEL is represented by yellow fluorescence due to merging of red fluorescence with the green fluorescence (right panel). Magnification, × 40. (I) A diagram to show the borderzone area of the infarct (peri-infarct) where samples were obtained for histological analysis.
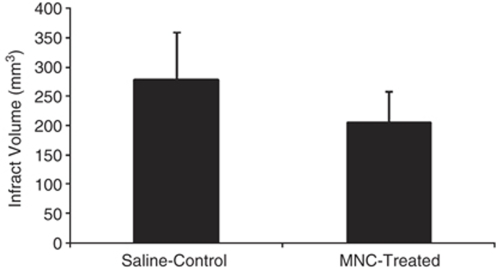
Infarct Volume
Infarction size was corrected for edema. The rats treated with MNCs showed significant reduction in infarction size when compared with saline control animals (P<0.03) (Figure 5).
Figure 5.
A bar graph exhibiting significantly (P<0.03) reduced infarct volume in MNC-treated rats as compared with saline-treated control animals. Data are mean±s.d. (n=5 per group).
Cytokine Analysis
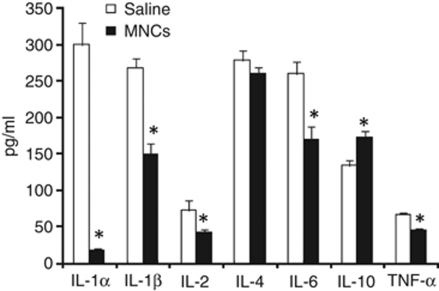
At 48 h after stroke in young animals, tissue harvested from the ischemic hemisphere had significantly lower levels of IL-1α, IL-1β, IL-2, tumor necrosis factor-α, and IL-6 in cell-treated animals compared with saline-treated animals (P<0.05) (Figure 6). IL-10 level was significantly increased in injured brains from cell-treated animals compared with that in saline-treated ischemic controls (P<0.05).
Figure 6.
Ischemic brain tissue after stroke treated with MNCs or saline was homogenized, centrifuged, and then the supernatant was analyzed using a multiplex bead-based enzyme-linked immunosorbant assay for the presence of selective cytokines. As shown, levels of several proinflammatory cytokines were significantly lower (n=5, P<0.05) in tissue samples taken from cell-treated animals as compared with that in saline-treated controls, whereas that of the anti-inflammatory cytokine, IL-10, was significantly increased (n=5, P<0.05).
Discussion
Acute IA infusion of autologous MNCs after stroke reduced neurological deficits in young and older rats. MNCs were found in the peri-infarct area within 1 h after injection, but decreased within hours and were nearly undetectable by 7 days afterwards. MNCs modulated post-ischemic inflammatory cytokines within the brain and reduced infarct volume.
In this clinically relevant rodent model, we were able to extract over 10 million MNCs from the tibia of rats after they had a stroke without causing mortality or limb impairment. Injection of MNCs within hours of harvest did not alter cardiovascular hemodynamics (data not shown) or cerebral perfusion (Figure 3). MNC-treated animals showed significant improvement in neurological deficits on two different behavioral tests. Impairment was reduced by 7 days after injection in the young animals and recovery was sustained over a 30-day period. Among older animals administered a lower number of cells, recovery was also enhanced in the cell-treated animals. Normally, 12-month-old, Long Evans rats are close to a period of time in their lifespan when they begin to develop comorbidities such as cardiomyopathy; mortality begins to increase around 14 months of age, according to our experience. Therefore, our results may suggest the possibility that both younger and older animals with stroke derived benefit from autologous MNC therapy.
Previous studies have investigated the therapeutic effects of MNCs in focal cerebral ischemia (Baker et al, 2007; Iihoshi et al, 2004; Kamiya et al, 2008), but differ from the present report in several ways. First, we show that older animals with stroke also derive benefit from autologous MNCs. Second, in the prior studies, MNCs were extracted several days before the stroke and re-injected 6 h after MCA occlusion (Baker et al, 2007) or extracted 1 h before stroke and re-injected at reperfusion (Kamiya et al, 2008). To our knowledge, this is the first clinically relevant study to show the feasibility of harvesting MNCs from the bone marrow at 22 h after ischemic stroke, rapidly isolating them, and then immediately re-injecting the cells with an IA delivery at 24 h after stroke. We chose 24 h in contrast to these prior studies because this is a more clinically practical time point to harvest bone marrow from patients when they are out of the window for thrombolytic or neuroprotective therapies. We also chose saline as a control given that some clinical studies testing mononuclear cells have used saline in their control group (Yao et al, 2008); however, we have also tested the effects of autologous dead MNCs, which do not reduce neurological deficits in our stroke model (data not shown).
Despite the concern for edema formation, brain inflammation, and emboli, our study supports the feasibility and safety of performing IA cell delivery in the acute setting of ischemic stroke. A recent report, however, found that intra-carotid delivery of MSCs in some animals led to cerebral blood flow reduction and mortality (Walczak et al, 2008). There are several differences that distinguish our work from this study. First, MSCs grown in culture for days to weeks likely adhere to each other and, therefore, can predispose to emboli. Injection of these cells could, therefore, lead to microembolization within the cerebrovasculature. MNCs, conversely, are not grown in culture, do not adhere to each other, and they did not lead to a decrease in cerebral blood flow in the animals that were studied (Figure 3). Second, MSCs have an average cell size of at least 12 to 18 μm whereas MNCs have an average cell size of 7 μm (Fischer et al, 2008). Third, the prior study used an endovascular suture to occlude the arterial lumen, thereby potentially injuring the arterial wall, causing the cells to adhere to a damaged endothelial surface and obstruct the lumen. Our ischemia model involves a focal occlusion of the MCA with a wire through a small craniotomy; however, any manipulation of the vasculature can induce vessel damage.
We chose an IA approach because it is less invasive than an intracranial approach and is clinically relevant to acute ischemic stroke with the intention to focus cell delivery to the area of pathology. Intravenous delivery has been shown to deposit bone marrow cells in the liver, spleen, and lungs (Chen et al, 2001). In addition, our previous work has shown that more purified cells such as MSCs, when administered intravenously, can be trapped in the lung (Fischer et al, 2008). We, therefore, bypassed the first pass filter of the lung with an intra-carotid injection. This delivery method led to immediate deposition of cells in the peri-infarct area, but labeled cells exponentially decreased to minimal levels in the peri-infarct region over the ensuing week after injection. This is the first report to provide a kinetic analysis of labeled MNCs in the injured brain over time, and we did see evidence that labeled MNCs were TUNEL positive at 3 h after injection, indicating that MNCs are dying after they migrate to the area of injury. One explanation may be that the injured brain poses a toxic environment to MNCs.
The mechanisms underlying cell-therapy-induced recovery are unclear. Prior studies suggest that MNCs decrease infarct volume (Baker et al, 2007; Iihoshi et al, 2004; Kamiya et al, 2008) and increase angiogenesis and vessel density in the peri-infarct areas of the brain (Baker et al, 2007). Other studies suggest that some MNCs show neural markers in and around the ischemic lesion after intravenous injection in the rodent stroke model (Iihoshi et al, 2004). The early recovery seen in our study argues against angiogenesis or neurogenesis as the predominant mechanisms underlying the efficacy of MNCs in reducing neurological deficits. We found a significant reduction in the levels of several proinflammatory cytokines in the ischemic brains harvested from rodents treated with MNCs as compared with saline controls. In addition, IL-10 level was significantly increased in cell-treated animals as compared with saline controls. Immunomodulation may, therefore, be another important mechanism underlying the effects of intra-arterially administered MNCs in the post-ischemic brain, similar to what has been reported for other types of cellular therapeutics (Vendrame et al, 2005). However, we also found that MNCs reduce infarct volume when administered at 24 h after stroke. It is, therefore, possible that the reduction in inflammatory cytokines may be secondary to reduced tissue injury. The few cells observed in the peri-infarcted areas by the time improvement in neurological deficits was seen suggest that these cells may release factors to exert cytoprotective and immunomodulatory effects and then die off rapidly in the peri-infarcted regions. Other studies have also shown that tissue protection is still possible to achieve even when cellular treatments are administered within the first 1 to 2 days after stroke. It has been shown that there is ongoing injury in the peri-infarct areas through such mechanisms as apoptosis, which contribute to infarct maturation over the ensuing days after stroke (Vendrame et al, 2004). Further work is, therefore, needed to determine a therapeutic time window and dose response of MNCs in ischemic stroke.
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
References
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Baker AH, Sica V, Work LM, Williams-Ignarro S, de Nigris F, Lerman LO, Casamassimi A, Lanza A, Schiano C, Rienzo M, Ignarro LJ, Napoli C. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc Natl Acad Sci USA. 2007;104:3597–3602. doi: 10.1073/pnas.0611112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first pass effect. Stem Cells Dev. 2008;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting M, Jimenez F, Pati S, Baumgartner J, Cox C., Jr Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10:243–253. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Ueda M, Igarashi H, Nishiyama Y, Suda S, Inaba T, Katayama Y. Intra-arterial transplantation of bone marrow mononuclear cells immediately after reperfusion decreases brain injury after focal ischemia in rats. Life Sci. 2008;83:433–437. doi: 10.1016/j.lfs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronicischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, Robichaud KJ, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25:2128–2138. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, Parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged femalerats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, de Mesquita D, Collier L, Bickford PC, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Huang R, Qian J, Cui J, Ge L, Li Y, Zhang F, Shi H, Huang D, Zhang S, Sun A, Zou Y, Ge J. Administration of intracoronary bone marrow mononuclear cells on chronicmyocardial infarction improves diastolic function. Heart. 2008;94:1147–1153. doi: 10.1136/hrt.2007.137919. [DOI] [PubMed] [Google Scholar]