Abstract
In this study, we examined the effect of CLA isomers in preventing age-associated muscle loss and the mechanisms underlying this effect, using 12 months old C57BL/6 mice fed 10% corn oil (CO) or a diet supplemented with 0.5% c9t11-CLA, t10c12-CLA or c9t11-CLA+t10c12-CLA (CLA-mix) for 6 months. Both t10c12-CLA and CLA-mix groups showed significantly higher muscle mass, as compared to CO and c9t11-CLA groups, measured by dual-energy-Xray-absorptiometry and muscle wet weight. Enhanced mitochondrial ATP production, with higher membrane potential, and elevated muscle antioxidant enzymes (catalase and glutathione peroxidase) production, accompanied by slight increase in H2O2 production was noted in t10c12-CLA and CLA-mix groups, as compared to that of CO and c9t11-CLA groups. Oxidative stress, as measured by serum malondialdehyde and inflammation, as measured by LPS-treated splenocyte IL-6 and TNF-alpha, were significantly less in CLA isomers groups. Thus, CLA may be a novel dietary supplement that will prevent sarcopenia by maintaining redox balance during aging.
Keywords: Lipid, sarcopenia, aging, redox balance, oxidative stress, inflammation
Introduction
With aging, a progressive loss of skeletal muscle mass and strength is observed, a condition termed “sarcopenia”. On average, aging individuals lose muscle mass at a rate of 1–2% per year, past the age of 50 [1; 2], resulting in a significant decrease of muscle strength [3]. Such age-related loss of muscle mass has far reaching consequences for the elderly, including impaired physical function, increased risk of falls, fractures, dependency, and death. Aging is also associated with an accretion of oxidative stress and increased incidence of oxidative injury in skeletal muscles [4; 5; 6]. Consequently, elevated oxidative stress has been thought to have a role in the development of sarcopenia [4; 7]. Although a number of mechanisms have been proposed as the underlying causes of sarcopenia, mitochondrial abnormalities, which generate excessive amount of reactive oxygen species (ROS), followed by elevated oxidative stress, have been suggested as the key factors in muscle alterations during aging [8]. Generation of free radicals and ROS is a normal continuous process in the life of aerobic living organisms. When production of ROS exceeds the endogenous antioxidant buffering capacity, oxidative stress is provoked. It has been suggested that mitochondrial dysfunction, resulting from an increase in oxidative stress, may be involved in sarcopenia [9; 10]. Altered mitochondrial electron transport chain activity in muscles is also known to be associated with sarcopenia during aging [11].
CLA refers to a mixture of positionally and geometrically conjugated dienoic isomers of linoleic acid (LA). The c9t11-CLA isomer represents approximately 80% of the total isomers in dairy and ruminant fats, whereas c9t11- and t10c12-CLA are equally abundant (usually 30–40% of each isomer) in commercial mixtures [12]. In recent decades, interest in CLA has increased due to its many bioactive properties related to health. The benefits seem to be very clear, especially in some experimental animal models [12; 13; 14; 15; 16]. In some animal models, dietary CLA reduce carcinogenesis, decrease body fat, increase lean body mass, enhance feed efficiency, protect against oxidative stress, modulate circulating lipids, and prevent impaired glucose tolerance in diabetes [17]. Several of these effects are controversial; in addition, some adverse results, for example, in some animal models, hepatomegaly [18], hepatic steatosis [19], have been noted. Very recently; however, CLA has received FDA approval as GRAS, (generally recognized as safe) for use in various food supplements.
One property that has been suggested to be responsible for CLA’s bioactivity is its ability to act as an antioxidant. CLA has been shown to control oxidative status [12]. We have already demonstrated in our previous studies that CLA can preserve age associated muscle loss in mice [16]. However, the mechanisms of action are yet to be determined. Moreover, isomer specific effects on the prevention of muscle loss are not known. In addition, it is unknown if CLA isomers have any impact on endogenous antioxidant concentrations or activity, mitochondrial ROS production and function. Therefore, the objective of this research was to determine if dietary CLA isomers can preserve age-associated skeletal muscle loss in a manner which would alter the oxidative stability of these tissues and to measure the comparative efficacy of two most common CLA isomers, c9t11-CLA and t10c12-CLA on prevention of age-associated muscle loss. The obtained results clearly indicate that t10c12-CLA isomer is the most effective CLA isomer. Further, a combination of two isomers, CLA-mix, is also found equally effective as of t10c12-CLA isomer.
Materials and Methods
Animals and experimental diets
Eleven months old female C57BL/6 (B6) mice, weighing 23–24 g, were purchased from Jackson Laboratories (Bar Harbor, Maine 04609 USA) and fed a standard lab chow diet. At 12 months of age, weight matched mice were divided into four groups. Each group consisted of 20 mice and were fed the American Institute of Nutrition (AIN)-93 diet [14; 16] containing corn oil (CO) with or without c9t11-CLA and t10c12-CLA, supplied by Lipid Nutrition (Channahon, IL), ad libitum for 6 months. The diets were supplemented with 10% CO as a high fat diet, with or without c9t11-CLA (0.5%), t10c12-CLA (0.5%) and a mixture of c9t11-CLA (0.25%) and t10c12-CLA (0.25%) (CLA-mix).
Measurement of lean mass and muscle mass
Total lean body mass was measured using dual-energy X-ray absorptiometry (DXA) Lunar PIXImus (GE, Madison, WI) and data was analyzed with PIXImus software as described previously [14; 15; 16]. Scanning was performed first at baseline (12 months of age) and again at the end of 6 months experimental diet (18 months of age). At the end of experimental diet mice were sacrificed and both the gastrocnemius and quadriceps muscles were carefully dissected and weighed to determine the muscle wet weights.
Isolation of skeletal-muscle mitochondria
Whole hind-limb skeletal-muscle mitochondria were isolated and purified following the method described by Muller et al [20]. H2O2 release, ATP synthesis and membrane potential experiments were conducted immediately after mitochondrial isolation.
Measurement of mitochondrial superoxide production, rates of ATP synthesis and membrane potential
Superoxide production was measured indirectly as H2O2 release from intact mitochondria. H2O2 was determined with Amplex™ Red (Molecular Probes, Eugene, OR), as described previously [20]. ATP synthesis was measured using the luciferin/luciferase assay kit from Roche, as described by Muller et al [20]. Membrane potential was monitored by fluorescence of the quench-dye Safranin O, as described by Muller et al [20].
Preparation of muscle cytosolic extract and measurement of catalase and glutathione peroxidase (GPX)
Quadriceps muscles were used to prepare cytosolic extracts using a Nuclear Extract Kit from Active Motive (Carlsbad, CA), following manufacturer’s instruction. 50 μg of cytosolic extracts were used to determine the activity of catalase and GPX using catalase and GPX Activity Assay Kits from Calbiochem (San Diego, CA), according to manufacturer’s instruction.
Measurement of serum malondialdehyde (MDA)
Serum MDA was analyzed using an OxiSelect™ MDA Adduct ELISA Kit from Cell Biolabs, Inc. (San Diego, CA), according to manufacturer’s instruction.
Measurement of cytokines
Splenocytes were isolated and cultured with/without LPS (5μg/ml) for 24 h, as described previously [21]. Culture supernatants were used to determine TNF-α and IL-6 by ELISA using BD OptEIA ELISA kits from BD Biosciences Pharmingen (San Diego, CA), as described previously [21].
Statistical analysis
Data are presented as mean values ± S.E.M. Differences among the groups (CO, c9t11-CLA, t10c12-CLA and CLA-mix) were tested by one-way analysis of variance (ANOVA), followed by Newman-Keuls post hoc test. A p value ≤ 0.05 was considered statistically significant. The analyses were performed using Graphpad prism for Windows (La Jolla, CA, USA).
Results
Effect of CLA isomers on muscle mass of aging mice
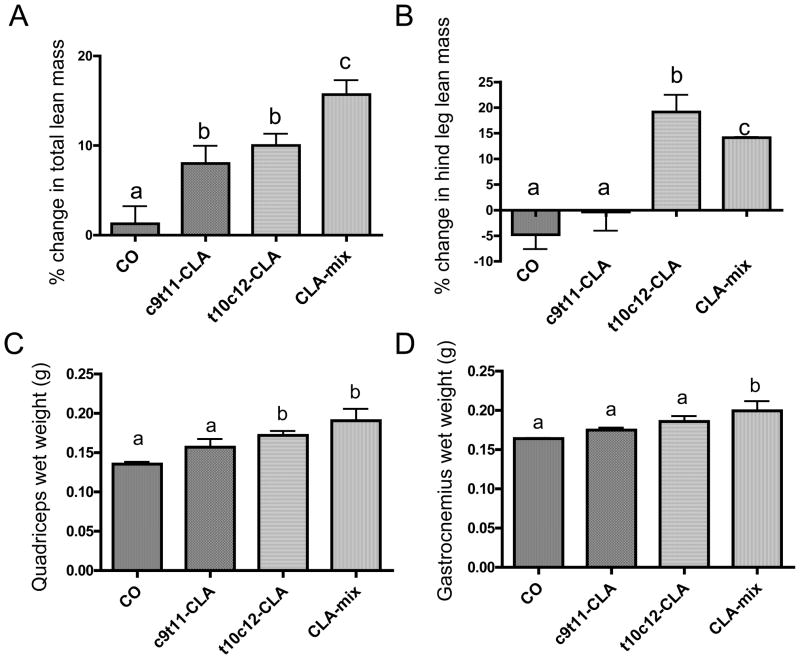
After feeding 12 months old B6 mice for 6 months with CO, with or without c9t11-CLA, t10c12-CLA and CLA-mix, total lean mass measured by DXA was significantly increased in c9t11-CLA, t10c12-CLA and CLA-mix fed mice, as compared to CO fed mice (Figure 1A). However, hind leg lean mass was decreased in CO and c9t11-CLA fed mice, whereas, t10c12-CLA and CLA-mix fed mice showed an increase in hind leg lean mass (Figure 1B). Moreover, quadriceps and gastrocnemius muscle wet weights were also higher in t10c12-CLA and CLA-mix fed mice, as compared to that of CO and c9t11-CLA fed mice (Figure 1C).
Figure 1. Effect of CLA isomers on muscle mass of B6 mice.
DXA scan was performed at 12 months of age (basal) and at 18 months of age (the end of experimental diets). A. Percent change in total lean mass. B. Percent change in hind leg lean mass. At the end of experimental diet wet weight of quadricep muscles (C) and gastrocnemius muscles (D) were also determined. Each bar represents the mean ± SEM (n=12–16 mice/group). Value with different superscripts are significantly different at P<0.05 by Newman-Keuls’ one way ANOVA.
Effect of CLA on muscle mitochondrial ROS production and muscle antioxidant capacity and overall oxidative stress level in mice
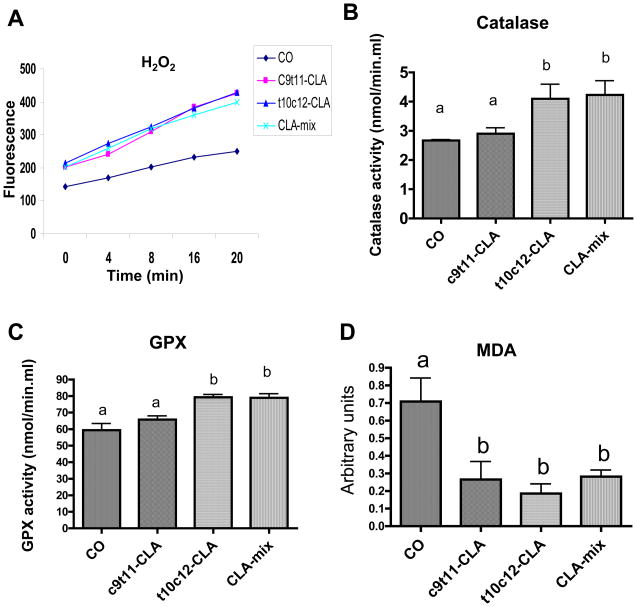
Oxidative stress plays a major role in age associated muscle loss and level of oxidative stress depends on the balance between ROS and antioxidant enzymes activity. Therefore, we first measured the level of H2O2, as ROS production, in muscle mitochondria and then, antioxidant enzymes (catalase and GPX) in the quadriceps muscles. We found that c9t11-CLA, t10c12-CLA and CLA-mix induce to the same extent of an increase in amplex red fluorescence, which represents H2O2 production, by the mitochondria (Figure 2A). Interestingly, we found an increased level of catalase and GPX activity in quadriceps muscle from CLA isomers fed mice, as compared to that of CO group (Figure 2B & C). However, the muscle of t10c12-CLA fed mice showed a higher antioxidant enzymes’ activity than that of c9t11-CLA fed mice. Finally, to compare the redox balance among different groups, we measured the level of serum MDA, one of the most common by-products of lipid peroxidation, as a marker of oxidative stress and found significantly less MDA level in CLA isomers fed mice (Figure 2D).
Figure 2. Effect of CLA on muscle mitochondrial ROS production and muscle antioxidant enzymes activity and serum MDA level of B6 mice.
At the end of experimental diet, mice were sacrificed and mitochondria from hind leg quadriceps and gastrocnemius were isolated and analyzed for H2O2 production with Amplex™ Red (A). This data represents 3 independent experiments of different day sacrifice. At the end of experimental diet, cytosolic proteins from quadriceps muscles were prepared and analyzed for catalase (B) and GPX (C) using catalase and GPX activity ELISA kits. At the end of experimental diet, blood serum was collected and analyzed for MDA level using OxiSelect™ MDA Adduct ELISA Kit. Each bar represents the mean ± SEM (n=8–10/group). Value with different superscripts are significantly different at P<0.05 by Newman-Keuls’ one way ANOVA.
Effect of CLA on muscle mitochondrial ATP production and membrane potential
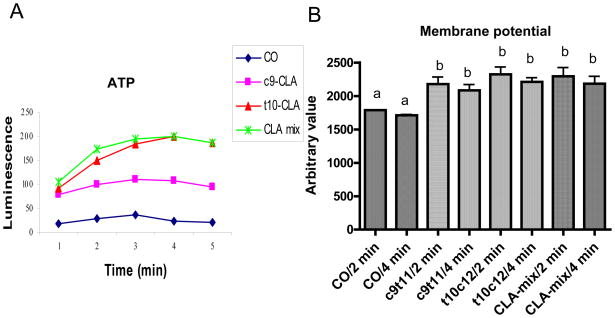
Declines in mitochondrial function in aging muscle are one of most pivotal factors leading to sarcopenia of old age. A high-fat-diet can deteriorate mitochondrial function. Therefore, we determined if CLA isomers supplementation can counteract the high-fat-diet induced-loss of mitochondrial function i.e. mitochondrial membrane potential and ATP production. Interestingly, we found an enhanced mitochondrial membrane potential and ATP production in CLA isomers groups than that of CO group (Figure 3A & B). Furthermore, t10c12-CLA isomer group showed much stronger effect than that of c9t11-CLA isomer group.
Figure 3. Effect of CLA on muscle mitochondrial ATP production and membrane potential.
At the end of experimental diet, mice were sacrificed and mitochondria from hind leg quadriceps and gastrocnemius were isolated and analyzed for ATP production in the presence of succinate (A) and membrane potential in the presence of safranin O (B). A. This data represents 3 independent experiments of different day sacrifice. B. Each bar represents the mean ± SEM (n=4/group). Value with different superscripts are significantly different at P<0.05 by Newman-Keuls’ one way ANOVA.
Effect of CLA on inflammatory response to immune cells
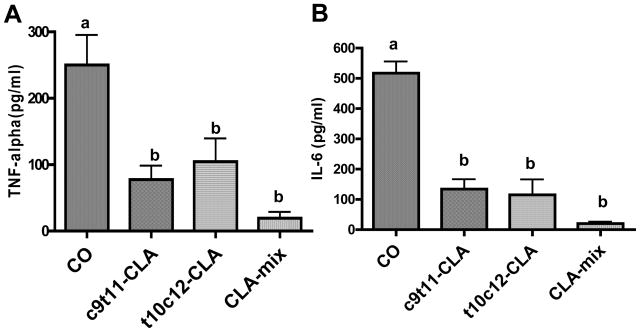
Inflammation is considered as one of the most important contributing factors in age associated skeletal muscle loss. Splenocytes, consist mainly of T cells and B cells, are one of the major cell types that respond to inflammation. Therefore, to see if CLA treatment can modulate the LPS-stimulated inflammation in splenocytes, we determined the level of TNF-α and IL-6 in LPS treated splenocytes cultures and found significantly less in CLA isomers supplemented mice (Figure 4).
Figure 4. Effect of CLA on LPS stimulated production of TNF-α and IL-6 in splenocytes.
At the end of experimental diet, mice were sacrificed and splenocytes were isolated and cultured for 24 h in the presence of LPS (5 μg/ml), culture supernatants were then analyzed for TNF-α and IL-6 using standard ELISA kits. Each bar represents the mean ± SEM of 4 independent triplicate cultures. Value with different superscripts are significantly different at P<0.05 by Newman-Keuls’ one way ANOVA.
Discussion
The progressive loss of skeletal muscle mass with advancing age is believed to play a major role in the pathogenesis of frailty and functional impairment that occurs with old age. Our hypothesis was that a high fat diet, which is pervasive to American people, when supplemented with CLA, counteracts mitochondrial dysfunction and enhances antioxidant capacity, and as a result, improves muscle mass and strength. Accordingly, we first confirmed the isomer specific effect of CLA on the prevention of age associated skeletal muscle loss in aging B6 mice. This is in accordance with previous data showing that CLA improves skeletal muscle mass [14; 16; 17; 22; 23]. However, these studies were conducted with an equal mixture of c9t11-CLA and t10c12-CLA isomers. This is the first study to show the effect of individual CLA isomers to attenuate/prevent/reverse age-related muscle wasting. We observed that inclusion of CLA isomers in the high-fat-diet (10% CO), improved fatty acid oxidation, mitochondrial function and antioxidant capacity of aging muscle.
Elevated oxidative stress is closely linked to age associated sarcopenia. Oxidative damage occurs due to imbalance between oxidants and antioxidants systems, in favor of the former. In this study, we observed a slight rise of mitochondrial ROS (expressed as H2O2) production in CLA isomers fed mice, compared to that of CO fed mice. However, mitochondrial ATP production and ROS neutralizing antioxidant enzymes production was also higher in CLA isomers supplemented groups than that of CO group. Since an unproductive side reaction of the respiratory chain is estimated to be responsible for 0.2 to 2% of the basal ROS level in cells [24 ], it seems reasonable to correlate this increase of ROS production to the increase in ATP production. Also, it may well be that we observed, in fact, an increase in lipid oxidation. Lines of evidence show that the most accepted mode of preventing age-associated sarcopenia, the resistance exercise which is known to increase ROS production, also protects against oxidative stress, due to the appropriate balance between oxidants and antioxidants. Similar to exercise, CLA isomers feeding also showed a better protection to oxidative stress, as observed by reduced MDA production in CLA isomers supplemented groups. Therefore, we hypothesize that the patho-physiological impact of the increased ROS, induced by the CLA isomers diet, is possibly highly counteracted by the increase of antioxidant enzymes (GPX and catalase). Such increase in antioxidant enzymes have been previously observed by CLA [25]. We propose here, this paradoxical effect is due to the ability of CLA feeding to increase the formation of ROS to a level that may induce a significant, but tolerable damage, which can in turn induce beneficial adaptations against oxidative stress.
Mitochondrion is the dominating target of oxidative damage in aged skeletal muscle [26; 27]. It appears that the significant declines in mitochondrial function in aging muscle may be important factors leading to sarcopenia of old age [1; 28]. Interference with mitochondrial ATP production and dysfunction can contribute to skeletal muscle sarcopenia [9; 29]. Drew, et al showed that ATP content and production decreased by >50% in rat gastrocnemius muscle mitochondria with age [9]. They also showed that an age-associated decline in ATP content and rate of ATP production is tissue specific, in that it occurs specifically in skeletal muscle [9]. We studied the effects of the high fat diet and CLA supplementation on mitochondrial function. Impairment of electron transport by the high fat diet might have increased formation of ROS in mitochondria, depleted antioxidants, and impaired the flow of electrons, propagating an increasing rate of oxidative stress. However, as compared to CO alone, CLA isomers supplementation could maintain a higher membrane potential and ATP production, resulting in a greater energy production capacity of mitochondria. A decreased loss of mitochondrial membrane potential, in CLA supplemented mice, indicates that there will be less mitochondrial damage under oxidative stress. Maintenance of muscle mitochondrial function by CLA isomers may explain, in part, the mechanism of muscle loss protection. Moreover, a much stronger positive effect on mitochondrial ATP production and membrane potential in t10c12-CLA group, as compared to c9t11-CLA group, indicates that t10c12-CLA isomer is the active component in CLA-mix.
Inflammation is likely another important contributing factor in sarcopenia and also contributes to erosion of muscle mass [30]. Inflammation and oxidative stress promote catabolic stimuli, such as, IL-6, IL-1, and TNF-α. Elevated levels of IL-6 carry a poor prognosis in older persons, and cellular IL-6 is a significant predictor of sarcopenia in women [30]. There are indications that cytokines, especially IL-1β, TNF-α, and IL-6, play a role in the pathogenesis of sarcopenia. CLA has been reported to preserve the gastrocnemius muscle mass, by reducing TNF receptors in muscle [23]. It was suggested that CLA may preserve muscle mass, by reducing the catabolic effects of TNF-α on skeletal muscle. Resistance exercise therapy also improves muscle mass and strength by reducing the inflammatory state. In this study, we also found a significant reduction of the LPS-stimulated inflammatory state (level of TNF-α and IL-6) of splenocytes from CLA isomers groups, as compared to CO group. The t10c12-CLA, but not the c9t11-CLA isomer, increased hind leg lean mass (Figure 1B). Yet both isomers decreased TNF-α and IL-6 levels (Figure 4), suggesting that decreased inflammation is not sufficient to account for the protective effects of t10c12-CLA on muscle loss. However, the t10c12-CLA, but not the c9t11-CLA isomer, significantly increased the activity of muscle antioxidant enzymes (Figure 2B and 2C) and production of mitochondrial ATP (Figure 3A), suggesting that the protective effects of t10c12-CLA on muscle loss may be due to the maintenance of proper redox balance and muscle function by providing sufficient energy to muscle during aging.
In conclusion, the t10c12-CLA isomer is the active component of the CLA-mix that exerts an anti-sarcopenic effect seen in this study. However, the t10c12-CLA isomer alone is known to have some adverse effects, such as, fatty liver formation, insulin resistance, etc. which can be corrected by combining with the c9t11-CLA isomer, which is known to improve insulin sensitivity as well as fatty liver formation [31]. As CLA-mix also showed an equal efficacy as of t10c12-CLA isomer alone, therefore, the CLA-mix could be an ideal dietary supplement to protect/delay age-associated skeletal muscle loss.
Acknowledgments
Supported by NIH-R21-AG027562. We acknowledge Paul Williams for critical review of this manuscript and Kazi Nishu for technical help.
References
- 1.Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S40–7. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- 3.Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, Gonzalez-Cadavid NF, Sinha-Hikim I. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13:822–32. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J Appl Physiol. 2008;105:1695–705. doi: 10.1152/japplphysiol.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic Biol Med. 1999;27:617–22. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 6.Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–400. doi: 10.1016/j.exger.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284:R474–80. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 10.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–27. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 11.Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol. 2002;92:2617–24. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- 12.Andreoli MF, Gonzalez MA, Martinelli MI, Mocchiutti NO, Bernal CA. Effects of dietary conjugated linoleic acid at high-fat levels on triacylglycerol regulation in mice. Nutrition. 2009;25:445–52. doi: 10.1016/j.nut.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Banu J, Bhattacharya A, Rahman M, O’Shea M, Fernandes G. Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis. 2006;5:7. doi: 10.1186/1476-511X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharya A, Rahman MM, Sun D, Lawrence R, Mejia W, McCarter R, O’Shea M, Fernandes G. The combination of dietary conjugated linoleic acid and treadmill exercise lowers gain in body fat mass and enhances lean body mass in high fat-fed male Balb/C mice. J Nutr. 2005;135:1124–30. doi: 10.1093/jn/135.5.1124. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya A, Rahman MM, McCarter R, O’Shea M, Fernandes G. Conjugated linoleic acid and chromium lower body weight and visceral fat mass in high-fat-diet-fed mice. Lipids. 2006;41:437–44. doi: 10.1007/s11745-006-5117-3. [DOI] [PubMed] [Google Scholar]
- 16.Rahman MM, Bhattacharya A, Banu J, Fernandes G. Conjugated linoleic acid protects against age-associated bone loss in C57BL/6 female mice. J Nutr Biochem. 2007;18:467–74. doi: 10.1016/j.jnutbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem. 2006;17:789–810. doi: 10.1016/j.jnutbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, Kim HJ, Tange T, Okuyama H, Kasai M, Ikemoto S, Ezaki O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 2000;49:1534–42. doi: 10.2337/diabetes.49.9.1534. [DOI] [PubMed] [Google Scholar]
- 19.Belury MA, Kempa-Steczko A. Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids. 1997;32:199–204. doi: 10.1007/s11745-997-0025-0. [DOI] [PubMed] [Google Scholar]
- 20.Muller FL, Liu Y, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J. 2008;409:491–9. doi: 10.1042/BJ20071162. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya A, Sun D, Rahman M, Fernandes G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. J Nutr Biochem. 2007;18:23–30. doi: 10.1016/j.jnutbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowska E, Muralitharan M, Cross RF, Bauman DE, Dunshea FR. Dietary conjugated linoleic acids increase lean tissue and decrease fat deposition in growing pigs. J Nutr. 1999;129:2037–42. doi: 10.1093/jn/129.11.2037. [DOI] [PubMed] [Google Scholar]
- 23.Graves E, Hitt A, Pariza MW, Cook ME, McCarthy DO. Conjugated linoleic acid preserves gastrocnemius muscle mass in mice bearing the colon-26 adenocarcinoma. Res Nurs Health. 2005;28:48–55. doi: 10.1002/nur.20052. [DOI] [PubMed] [Google Scholar]
- 24.Staniek K, Nohl H. Are mitochondria a permanent source of reactive oxygen species? Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2000;1460:268–275. doi: 10.1016/s0005-2728(00)00152-3. [DOI] [PubMed] [Google Scholar]
- 25.Arab K, Rossary A, Soulere L, Steghens JP. Conjugated linoleic acid, unlike other unsaturated fatty acids, strongly induces glutathione synthesis without any lipoperoxidation. Br J Nutr. 2006;96:811–9. doi: 10.1017/bjn20061910. [DOI] [PubMed] [Google Scholar]
- 26.Zangarelli A, Chanseaume E, Morio B, Brugere C, Mosoni L, Rousset P, Giraudet C, Patrac V, Gachon P, Boirie Y, Walrand S. Synergistic effects of caloric restriction with maintained protein intake on skeletal muscle performance in 21-month-old rats: a mitochondria-mediated pathway. Faseb J. 2006;20:2439–50. doi: 10.1096/fj.05-4544com. [DOI] [PubMed] [Google Scholar]
- 27.Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271:165–70. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- 28.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang JH, Hood DA. Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life. 2009;61:201–14. doi: 10.1002/iub.164. [DOI] [PubMed] [Google Scholar]
- 30.Payette H, Roubenoff R, Jacques PF, Dinarello CA, Wilson PW, Abad LW, Harris T. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51:1237–43. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- 31.Moloney F, Toomey S, Noone E, Nugent A, Allan B, Loscher CE, Roche HM. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes. 2007;56:574–82. doi: 10.2337/db06-0384. [DOI] [PubMed] [Google Scholar]