Abstract
OBJECTIVE
Stereotactic radiosurgery makes brain arteriovenous malformations (AVM) more manageable during their microsurgical resection. To better characterize these effects, we compared results of microsurgical resection of radiated (RS+) and non-radiated (RS−) AVMs to demonstrate that prior radiosurgery facilitates surgery and decreases operative morbidity.
METHODS
From of series of 344 patients who had AVM resections at the University of California, San Francisco (1997–2007), 21 RS+ patients were matched with 21 RS− patients based on pre-treatment clinical and AVM characteristics. Matching was blinded to outcomes, which were assessed with the modified Rankin Scale (mRS).
RESULTS
Mean AVM volume was reduced by 78% (P<0.01) and Spetzler-Martin grades were reduced in 52% of RS+ patients (P < 0.001). Preoperative embolization was used less in RS+ than in RS− patients (P< 0.001). Mean operative time (P< 0.01), blood loss (P < 0.05), and length of hospital stay (P< 0.05) were lower in the RS+ group. Surgical morbidity was 14% higher in RS− patients, and they had significant worsening in mRS scores after surgery while RS+ patients did not (P<0.01). RS+ patients deteriorated between AVM diagnosis and surgery due to hemorrhages during the latency period (P< 0.05).
CONCLUSION
Prior radiosurgery facilitates AVM microsurgery and decreases operative morbidity. Radiosurgery is recommended for unruptured AVMs that are not favorable for microsurgical resection. Microsurgical resection is recommended for radiated AVMs that are not completely obliterated after the 3-year latency period, but altered favorably for surgery, even in asymptomatic patients. Prompt resection of persistent AVMs should be considered to avoid the risk of post-latency hemorrhage and to optimize patient outcomes.
Keywords: Arteriovenous malformation, microsurgical resection, stereotactic radiosurgery, endovascular embolization, multimodality treatment
INTRODUCTION
Brain arteriovenous malformations (AVM) are managed with endovascular embolization, microsurgical resection, stereotactic radiosurgery (RS), or combinations that vary widely(2, 4, 12, 18). Radiosurgery is typically recommended for AVMs that are small (< 2–3 cm diameter), unruptured, and surgically inaccessible, but may also be recommended to reduce the size of large AVMs otherwise associated with significant surgical morbidity(1). Radiation induces intimal hyperplasia and medial hyalinization in arteries that progressively thickens their walls and narrows their lumen, which can obliterate some small-volume AVMs and shrink some high-volume AVMs to sizes more favorable for surgical resection. These biological changes in radiated AVMs make them more manageable during surgery. Radiated arteries are thickened and sclerotic, making them more responsive to cautery and easier to occlude. Blood flow through the nidus is often decreased significantly. Radiation-induced gliosis adjacent to the AVM creates easier planes of dissection. Therefore, radiated AVMs offer clear surgical advantages, but these advantages have not been quantified, nor has their impact on patient outcomes.
Using a post-hoc analysis of matched patients, we compared management data and surgical outcomes in patients with AVMs treated previously with radiosurgery (RS+) and patients with AVMs not treated with radiosurgery (RS−). Ease of microsurgical resection was evaluated by a variety of measures including pre-operative embolization, intraoperative blood loss, surgical staging, completeness of resection, and length of hospital stay. We hypothesized that radiation-induced changes in AVMs facilitate microsurgical resection and improve neurological outcomes in patients with persistent AVMs after radiosurgery.
PATIENTS AND METHODS
Patients
This study was approved by the Committee on Human Research at the University of California, San Francisco. From a surgical series of 344 patients treated microsurgically between 1997 and 2007 by the senior author (MTL), 23 patients had previously been treated with radiosurgery. Twenty-one of these patients had sufficient clinical data for inclusion. Data was obtained from an ongoing registry of AVM patients treated at our institution, maintained prospectively as part of the UCSF Brain Arteriovenous Malformation Study Project.
The 21 RS+ patients were individually matched with 21 RS− patients using criteria known to affect patient outcomes after AVM resection, including AVM size, location, deep venous drainage, Spetzler-Martin grade, patient age, and hemorrhagic presentation (Table 1) (7, 8, 14). In addition to these characteristics, RS+ and RS− patients were matched by modified Rankin Scale (mRS) determined at the time of diagnosis before any treatment (Table 2). The matching was performed independently by an observer blinded to final outcomes.
TABLE 1.
Patient and brain arteriovenous malformation characteristics before treatmenta
| RS+ |
RS− |
Total | P value | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Sex | ||||||
| Male | 7 | 33% | 10 | 48% | 17 | 0.346 |
| Female | 14 | 67% | 11 | 52% | 25 | |
| Total | 21 | 21 | 42 | |||
| Ethnicity | ||||||
| Caucasian | 1 | 5% | 3 | 14% | 4 | 0.589 |
| African American | 13 | 62% | 10 | 48% | 23 | |
| Hispanic | 2 | 10% | 1 | 5% | 3 | |
| Asian | 5 | 24% | 6 | 29% | 11 | |
| Other/unknown | 0 | 0% | 1 | 5% | 1 | |
| Total | 21 | 21 | 42 | |||
| AVM size (cm) | ||||||
| <3 | 4 | 19% | 5 | 24% | 9 | 0.474 |
| 3–6 | 17 | 81% | 16 | 76% | 33 | |
| >6 | 0 | 0% | 0 | 0% | 0 | |
| Total | 21 | 21 | 42 | |||
| Venous drainage | ||||||
| Superficial only | 8 | 38% | 7 | 33% | 15 | 0.747 |
| Deep | 13 | 62% | 14 | 67% | 27 | |
| Total | 21 | 21 | 42 | |||
| Eloquence | ||||||
| Noneloquent | 4 | 19% | 4 | 19% | 10 | >0.999 |
| Eloquent | 17 | 81% | 17 | 81% | 34 | |
| Total | 21 | 21 | 42 | |||
| Spetzler-Martin grade | ||||||
| I | 1 | 5% | 1 | 5% | 2 | >0.999 |
| II | 2 | 10% | 2 | 10% | 4 | |
| III | 8 | 38% | 8 | 38% | 16 | |
| IV | 10 | 48% | 10 | 48% | 20 | |
| V | 0 | 0% | 0 | 0% | 0 | |
| Total | 21 | 21 | 42 | |||
| Pretreatment ICH | ||||||
| + | 8 | 38% | 8 | 38% | 16 | >0.999 |
| − | 13 | 62% | 13 | 62% | 26 | |
| Total | 21 | 21 | 42 | |||
RS+, treated with radiosurgery; RS−, not treated with radiosurgery; AVM, arteriovenous malformation; ICH intracerebral hemorrhage.
TABLE 2.
Pretreatment neurological functiona
| Pretreatment mRS score | Preoperative radiosurgery |
Total | P value | |||
|---|---|---|---|---|---|---|
| Yes |
No |
|||||
| No. | % | No. | % | |||
| 0 | 3 | 14% | 3 | 14% | 6 | >0.999 |
| 1 | 9 | 43% | 9 | 43% | 18 | |
| 2 | 4 | 19% | 4 | 19% | 8 | |
| 3 | 2 | 10% | 2 | 10% | 4 | |
| 4 | 3 | 14% | 3 | 14% | 6 | |
| 5 | 0 | 0% | 0 | 0% | 0 | |
| Total | 21 | 21 | 42 | |||
mRS, modified Rankin Scale.
Treatment
Patients were treated with radiosurgery after considering all therapeutic options with a multidisciplinary team consisting of neurosurgeons, neurologists, interventional neuroradiologists, and radiation oncologists. Three patients underwent radiosurgery treatment at outside institutions. All patients treated at UCSF were treated with the Leksell Gamma Knife (models U, B, C) using existing planning software. Dose prescription followed the dose volume relationship predicting a 3% risk of permanent radiation injury as described by Flickinger et al.(3). While most AVMs were treated covering the entire nidus volume in a single session, some of the largest targets were treated using a volume staged method that incorporated implanted fiducials or anatomic landmarks for re-registration and transformation of plans between stages to minimize overlap of fields in normal tissue(17). In our first 10 years of experience treating large AVMs, volume staged methods were used when isodose volume covering the entire nidus was over 15cc. Due to limited success with this approach, we reduced the volume per session in staged patients to approximately 8 cc and increased the marginal dose from 15 Gy to 16 Gy. Patients were followed clinically at 3, 6, 12, 18, and 24 months, then at annual intervals to assess treatment response and side effects. MR imaging was done at 12, 24, and 36 months to assess the radiographic response to treatment.
Radiosurgery was administered in a single session in 14 patients, in two sessions in 6 patients, and in three sessions in one patient, for an average of 1.4 radiosurgical treatments per patient before resection. The average time between radiosurgery and resection was 56.2 months. The mean maximum and minimum target doses were 32.7 and 17.0 Gy, respectively, for single treatments, and 31.5 and 16.5 Gy for repeat treatments. The mean isodose line (IDL) volume was 10.2 cm3 for those treated with a single session and 7.1 cm3 for repeat treatments.
All radiosurgical treatments were performed by a single neurosurgeon (MWM) except in 3 patients who had radiosurgical treatment performed at another institution. All microsurgical procedures were performed by a single neurosurgeon (MTL). Microsurgical AVM resection in RS+ patients was performed in patients with a persistent AVM on catheter angiography, and either hemorrhage or symptoms related to the AVM.
Outcome Analysis
Neurological outcome was assessed using the modified Rankin Scale (mRS)(5, 20). A single clinical nurse, under the supervision of a neurologist, performed all clinical assessments before any treatment, preoperatively, at 6 months postoperatively, and during the follow-up period. All patients had follow-up data within 6 months of analysis. Patients with AVM-related seizures that were controlled with medications, and without any other neurological symptom, were assigned a mRS score of 0. Alternatively, neurologically intact patients with refractory AVM-related seizures that interfered with daily activities (working, school, driving, etc.) were assigned a score of 2. Surgical morbidity was assessed by comparing mRS 6 months postoperatively with mRS immediately before surgery.
Statistical Analysis
Data analysis was performed using the Statistical Package for Social Sciences (version 13.0; SPSS, Inc. Chicago, IL). Nominal data were analyzed using matched or unmatched chisquare tests of association between the two treatment groups. Ordinal data were analyzed by nonparametric testing. Other data were tested using the student's t-test. Statistical significance was considered if P < 0.05.
RESULTS
Reduction of AVM Size and Grade after Radiosurgery
Comparing AVM volumes before radiosurgery with volumes before microsurgical resection, radiosurgery reduced mean AVM volumes by 78% (P< 0.01) (Figure 1A). This reduction in AVM volume downgraded Spetzler-Martin scores in 11 patients: nine patients had a decrease in grade by one point, and two patients had a decrease in grade by two points. These changes in Spetzler-Martin grade were due primarily to reductions in size, but deep draining veins occluded in two patients to lose a point in this category. Overall, radiosurgery shifted the distribution of AVM grades from high grades to more intermediate grades (P< 0.001) (Figure 1B).
Figure 1.
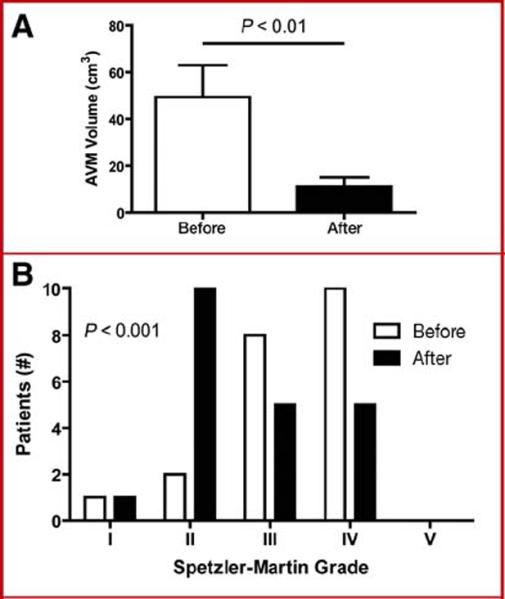
Radiosurgery decreases AVM volume and Spetzler-Martin grades. Calculated AVM volumes were lower after treatment in RS+ patients (A). Distribution of BAVM Spetzler-Martin grades before and after radiosurgical treatment were significantly different (B).
Reduction of Preoperative AVM Embolization after Radiosurgery
Radiosurgery reduced the use of preoperative embolization by 56% (P< 0.001) (Figure 2A). Only 8 RS+ patients underwent embolization, whereas 18 RS− patients underwent embolization. This reduction in preoperative embolization was due to smaller AVM sizes in patients treated with radiosurgery and, in some cases, difficulty catheterizing arteries with radiation changes. AVM embolization was generally performed immediately before surgery. It was avoided before stereotactic radiosurgery because embolized AVM can be missed during radiosurgical targeting, can then recanalize during the latency period, and can also recruit new feeding arteries to enlarge the nidus.
Figure 2.
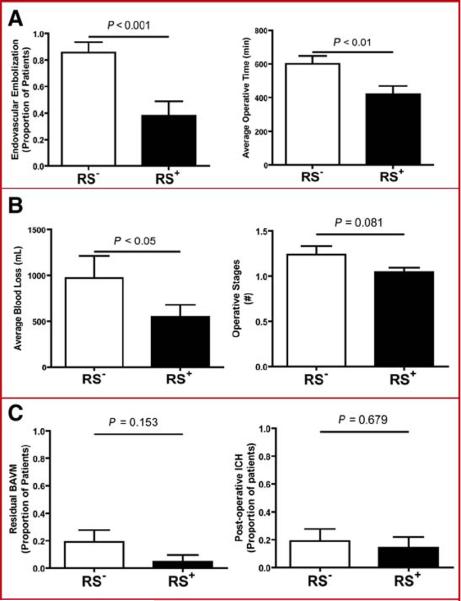
Radiosurgery decreases the need for preoperative embolization and facilitates AVM resection. RS+ patients were less likely than RS− patients to undergo embolization prior to microsurgical resection (A). Operative time was less in RS+ patients compared to RS− patients (B). Blood loss was less in the RS+ group when compared to the RS− group (C). The number of operative stages tended to be lower for RS+ patients (D). The incidence of residual AVM was lower in RS+ patients (E). The incidence of post-operative hemorrhage was slightly lower in RS+ patients (F).
Radiosurgery Facilitates AVM Resection
Average operative times were lower in RS+ patients compared to RS− patients, with a 30% reduction in the length of surgery (P< 0.01) (Figure 2B). Blood loss during AVM resection was also 45% lower in RS+ patients compared to RS− patients (P< 0.05) (Figure 2C). There were trends towards fewer operative stages (Figure 2D), more complete AVM resections (Figure 2E), and less post-operative intracerebral hemorrhages (Figure 2F) in RS+ patients compared to RS− patients, but these differences were not statistically significant. Although these measures are indirect, together they indicate greater ease of AVM resection after radiosurgery.
Reduction of Surgical Morbidity after Radiosurgery
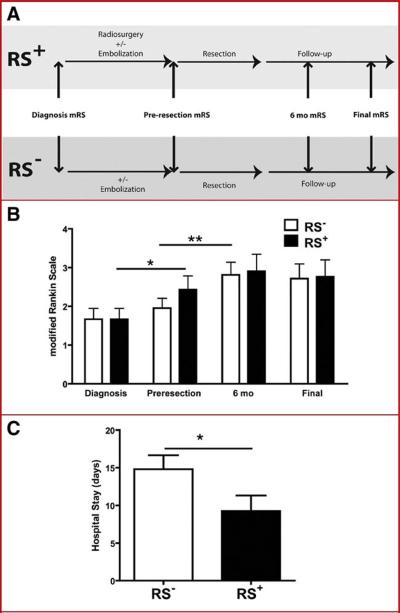
Comparisons of surgical morbidity were made by analyzing mRS scores immediately before surgery (pre-resection mRS) and 6 months after surgery in RS+ patients and RS− patients (Figure 3A). A statistically significant increase in mRS scores was observed in RS− patients at 6 months, but not in RS+ patients (P< 0.01) (Figure 3B). In the group treated with radiosurgery, 5 patients were neurologically worse after surgery (24%); in the group not treated with radiosurgery, 8 patients were neurologically worse (38%). These differences indicate that resection of AVMs treated previously with radiosurgery is associated with reduced morbidity.
Figure 3.
Radiosurgery reduces surgical morbidity but does improve final outcome. Schematic demonstrating the time course for neurological assessments using the modified Rankin Scale (A). RS+ patients had a significant worsening in neurological function between the time of diagnosis and time of resection (diagnosis to pre-resection, * P< 0.05), whereas RS− patients had a significant worsening in mRS associated with BAVM resection (pre-resection to 6 months, ** P< 0.01) (B). Outcomes at 6 months and last follow-up did not differ between the two groups (C). Hospital stay after BAVM resection was lower in RS+ patients compared to RS− patients (D).
During the interval from AVM diagnosis to surgery, patients in the control group not treated with radiosurgery experienced minor, statistically insignificant changes in mRS scores, due largely to morbidity associated with embolization preoperatively. In contrast, RS+ patients experienced a significant increase in their average mRS score (P< 0.05) (Figure 3B). This change was due to intracerebral hemorrhages in 7 patients (33%), of which 5 resulted in measurable increases in mRS scores. Therefore, RS+ patients went to surgery in worse neurological condition than the RS− patients. Post-radiosurgical hemorrhages occurred at an average of 4.8 years after treatment (range, 4 months – 9 years), with 5 hemorrhages occurring well beyond the latency period
At short-term (6 month) and long-term follow-up (mean duration, 7.1 years), there were no significant differences in mean mRS scores between patient groups (Figure 3B). Thus, although RS+ patients went to surgery in worse neurological condition than the RS− patients, they achieved similar neurological outcomes later because radiosurgery reduced their surgical morbidity. In other words, patients treated with radiosurgery benefited from decreased surgical morbidity, but some of this benefit was offset by new preoperative neurological deficits resulting from AVM hemorrhages during the latency period.
The average hospital stay after AVM resection was lower for RS+ patients than for RS− patients (P< 0.05), reflecting lower surgical morbidity (Figure 3C).
Illustrative Cases
Case 1
This 53 year-old woman presented with headaches (mRS 1) and a left parietal AVM centered in the somatosensory cortex and abutting the motor strip. Angiography demonstrated a Spetzler-Martin grade III AVM (S2E0V1). She underwent stereotactic radiosurgical treatment leading to a decrease in AVM volume and Spetzler-Martin grade (S1E0V1 after radiosurgery). The patient continued to be asymptomatic with an angiogram demonstrating minimal AVM residual two years after treatment (Figure 4). Nearly 9 years after radiosurgical treatment, she presented with sudden headache which progressed to right hemiparesis and aphasia (mRS 4). CT scan demonstrated an intraparenchymal hemorrhage with mass effect, and angiography showed persistent AVM. The patient underwent emergency AVM resection but had no improvement in mRS at 6 months or last follow-up. While the patient had no surgical morbidity, the damage from the intraparenchymal hemorrhage was permanent. This case demonstrates that the microsurgical benefits of radiosurgery were negated by AVM hemorrhage during the latency period.
Figure 4.
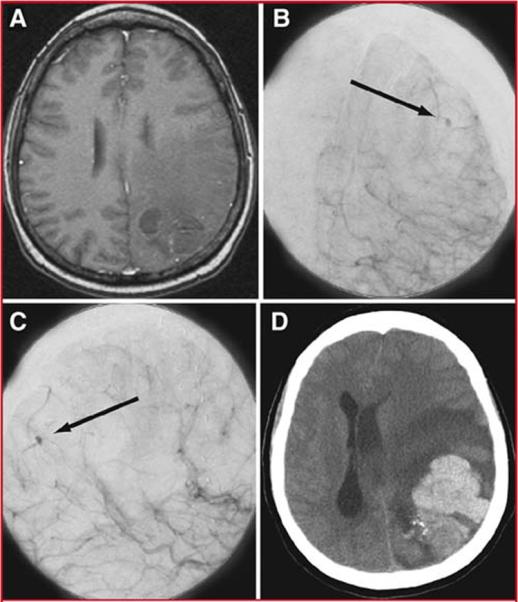
Illustrative Case 1. Axial T1-weighted MRI with gadolinium (A), and anteroposterior (B) and lateral (C) projections of a cerebral angiogram after left internal carotid injection demonstrated minimal persistent AVM after radiosurgical treatment. Small early pooling of contrast is seen during the arterial phase (arrow) (B-C). A non-contrast head CT (D) demonstrated a large intraparenchymal hemorrhage with severe midline shift and transtentorial herniation.
Case 2
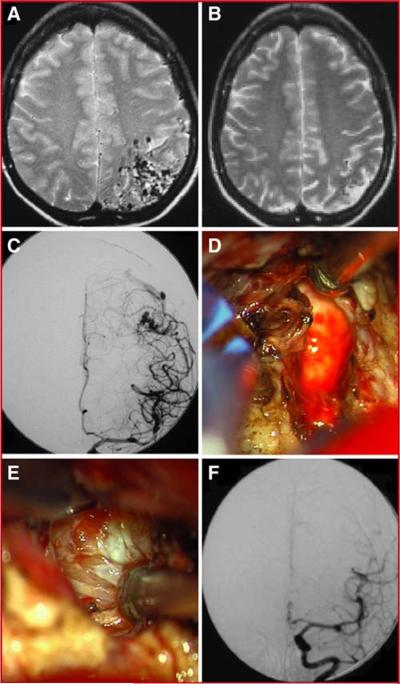
This 38 year-old woman presented with seizures (mRS 1) and a left parietal AVM centered in the somatosensory cortex and abutting the motor strip (Figure 5). Angiography demonstrated a Spetzler-Martin grade IV AVM (S2E1V1). Radiosurgical treatment significantly reduced the AVM size and nearly 3 years after radiosurgery her Spetzler-Martin grade had decreased by two points (S1E1V0). She continued to have seizures when her anti-epileptic medications were weaned, and her desire to discontinue anti-epileptic medications led her to microsurgical resection. The AVM resection was uncomplicated and her mRS score remained 1 at 6 months and last follow-up. She was successfully weaned off anti-epileptic medications. This case demonstrates that the microsurgical benefits of radiosurgery are significant in the absence AVM hemorrhage during the latency period.
Figure 5.
Illustrative Case 2. Corresponding T2-weighted MRIs before (A) and after (B) radiosurgical treatment demonstrate a significant decrease in AVM size. An anteroposterior projection of a left internal carotid injection demonstrates the residual AVM after radiosurgical treatment (C). Intraoperative images demonstrate the gliotic margin (D) and sclerotic arterial feeders (E) that are frequently encountered after radiosurgical treatment. A post-operative angiogram demonstrates complete resection of the AVM (F).
DISCUSSION
This study compared results of microsurgical resection of radiated and non-radiated AVMs to demonstrate that prior radiosurgery facilitates surgery and decreases operative morbidity. Radiosurgery reduces AVM volume and Spetzler-Martin grade, and induces biological changes that improve tissue handling during surgery. In addition to these advantages, radiosurgery is associated with a serious disadvantage: the risk of a debilitating hemorrhage during the latency period that can compromise final neurological outcomes, eliminating the benefit of lower surgical morbidity.
Radiosurgical Changes in Brain AVMs
Radiosurgery initiates an occlusive arteriopathy that resembles atherosclerosis. Radiation is particularly damaging to endothelial cells, degrading their DNA and preventing their repair of intima within a hemodynamically stressed environment. Smooth muscle cells in the media respond to a depleted endothelium by proliferating and secreting collagen and other lipoproteins, which thickens arteries and progressively occludes the AVM. This slow obliterative process accounts for decreases in AVM volume after radiosurgery. Volume reduction clearly influences Spetzler-Martin grades; more than half of our radiated AVMs lost points in the grading scheme.
Other biological factors besides decreased AVM size and grade are responsible for decreased surgical morbidity with radiated AVMs. Radiation arteriopathy decreases the caliber and overall number of arteries feeding an AVM, which in turn diminishes AVM flow. Intraoperatively, sclerotic arteries are easy to cauterize and occlude. Perforating arteries that typically supply the deep borders of AVMs near eloquent white matter tracts are transformed from thin and friable to thick and coagulable, which decreases bleeding intraoperatively. Ragged margins around a diffuse AVM are often obliterated by radiosurgery to compact the nidus. Gliosis, scarring, and sometimes cystic degeneration in adjacent brain facilitates the dissection because this brain tissue is less vascular than normal brain and planes of separation are more distinct. Hemosiderin deposition and occasionally hematomas are encountered during the dissection, indicating subclinical hemorrhage during the latency period and also creating new planes of separation between nidus and brain. These biological changes contribute to easier and safer surgery, which we measured as shorter operating times, reduced blood loss, less staging, and decreased lengths of hospital stay.
Timing of AVM Surgery after Radiosurgery
Not all biological changes induced by radiosurgery are beneficial. Deep draining veins occluded in two of our patients, indicating that radiation can directly compromise venous outflow or indirectly promote venous thrombosis from altered intranidal hemodynamics. Changes in AVM drainage may be responsible for the higher than expected hemorrhage rate after radiosurgery. Seven patients had post-radiosurgical hemorrhages and only 3 had hemorrhaged before treatment, suggesting that radiosurgery might have changed the bleeding tendencies of their AVMs. Hemorrhage resulted in neurological deterioration in 5 of these patients (71%). In addition, post-radiosurgical hemorrhages occurred at an average of 4.8 years after treatment (range, 4 months – 9 years), with 5 hemorrhages occurring well beyond the latency period.
Our study suggests that early surgery for AVMs that persist after radiosurgery capitalizes on favorable biological changes and lower operative morbidity while avoiding devastating post-radiation hemorrhage. Surgery should be timed to allow the AVM to respond fully to radiation, but should not incur the hemorrhage risk from delay beyond the latency period when the AVM tissue is no longer responding to the radiation. Our clinical experience with AVM radiosurgery matches published experiences reporting that 3-year latency periods maximize an AVM's biological response(9). The average interval from radiosurgery to surgery in our study was 4.5 years. Perhaps earlier surgery near or at the end of the latency period might have spared five patients from AVM rupture and associated neurological deficits, while still taking advantage of the facilitated resections and lower operative morbidity that follow radiosurgery.
An aggressive surgical approach to patients with persistent AVMs seems justified. While conservatism is natural in patients with unruptured, large or high-grade AVMs, radiation appears to alter their bleeding disposition and surgical delays can be costly. Decreases in Spetzler-Martin grades after radiosurgery can change a high-grade AVM best managed non-operatively to one that can be resected with acceptable risk. It may be more appropriate to maintain the initial Spetzler-Martin grade at diagnosis throughout the course of treatment rather than to reassign grades after radiosurgery. However, dramatic changes in the AVM and its grade after radiosurgery can inform the sometimes difficult decision to proceed with surgery.
Analysis of persistent AVMs and management decisions should be individualized. This experience demonstrates that radiosurgery does convert difficult AVMs and poor surgical candidates into easier AVMs and good surgical candidates. In addition, radiosurgery converts good surgical candidates, who nonetheless opted for radiosurgery, into better surgical candidates after incomplete radiosurgical response. Radiosurgery can therefore be applied as a surgical adjunct with an unpredictable and variable effect. It should not be applied palliatively. When patients and treatment teams embark on a therapeutic course that begins with radiosurgery, they should remain committed to working towards complete and expeditious obliteration, progressing aggressively with preoperative embolization and microsurgical resection with persistent AVMs.
Limitations
In order to demonstrate that radiation-induced changes in AVMs facilitate microsurgical resection, we relied on indirect measures that were quantifiable, like intraoperative blood loss, surgical staging, completeness of resection, and length of hospital stay. Ease of microsurgical resection was difficult to quantify directly because there are no measures of an AVM's coagulability or gliosis in the dissection planes. Nonetheless, these advantages are real and obvious to the neurosurgeon. It was difficult to demonstrate that radiation-induced changes in AVMs reduce operative morbidity when analyzing such a small cohort of patients with so many variables that influence surgical outcomes (6–8, 14). Methodologically, we carefully matched radiosurgical patients with non-radiosurgical patients based on variables critically affecting outcome (AVM size, location, venous drainage, Spetzler-Martin grade, patient age, and hemorrhagic presentation), while remaining blinded to treatment outcomes. We acknowledge that case matching is susceptible to selection bias. Extensive regression analysis of a larger, more inclusive cohort of patients has appeal, but case-control matching with this small, highly variable cohort is statistically more appropriate and yielded robust differences. The decrease in surgical morbidity would have been more obvious if patients were matched immediately before surgery, but this methodology would miss important hemorrhagic complications related to radiosurgery. Finally, selection biases undoubtedly influenced our results. Institutionally, we have a strong bias for microsurgery initially, and not all of our radiosurgical patients with persistent AVMs were selected for surgery. Many AVM patients treated radiosurgically were managed nonoperatively because the radiation response was mild and surgical risks remained high. Others were re-treated with additional radiosurgery. A review of outcomes with either repeated radiosurgery or observation was outside the scope of this article.
CONCLUSIONS
AVM microsurgery after radiosurgery is associated with reduced use of preoperative embolization, easier resection, improved tissue handling during surgery, and lower operative morbidity. Nevertheless, final outcomes did not differ between radiosurgical and non-radiosurgical patients because the surgical benefits were negated by hemorrhages during the latency period. We recommend radiosurgery for unruptured AVMs that are not favorable for microsurgical resection. We recommend microsurgical resection for radiated AVMs that are not completely obliterated after the 3-year latency period, but altered favorably for surgery, even in asymptomatic patients.
Acknowledgements
We thank the members of the University of California, San Francisco Brain Arteriovenous Malformation study project (http://avm.ucsf.edu) who contributed to the data collection and management (Mary Nelson Tran, Kwun Yee T. Poon, Achal S. Achrol, Charles E. McCulloch, S. Claiborne Johnston, Nancy J. Quinnine, Christopher F. Dowd, Van V. Halbach, Randall T. Higashida) and to patient treatment (Lisa T. Hannegan and Wade S. Smith).
Footnotes
Financial Disclosure The authors have no financial disclosure or other disclaimer.
REFERENCES
- 1.Chang SD, Marcellus ML, Marks MP, Levy RP, Do HM, Steinberg GK. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1–11. doi: 10.1227/01.neu.0000068700.68238.84. discussion 11–13. [DOI] [PubMed] [Google Scholar]
- 2.Fleetwood IG, Steinberg GK. Arteriovenous malformations. Lancet. 2002;359:863–873. doi: 10.1016/S0140-6736(02)07946-1. [DOI] [PubMed] [Google Scholar]
- 3.Flickinger JC, Kondziolka D, Lunsford LD, Pollock BE, Yamamoto M, Gorman DA, Schomberg PJ, Sneed P, Larson D, Smith V, McDermott MW, Miyawaki L, Chilton J, Morantz RA, Young B, Jokura H, Liscak R. A multi-institutional analysis of complication outcomes after arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys. 1999;44:67–74. doi: 10.1016/s0360-3016(98)00518-5. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander RM. Arteriovenous Malformations of the Brain. N Engl J Med. 2007;356:2704–2712. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann A, Stapf C, Hofmeister C, Mohr JP, Sciacca RR, Stein BM, Faulstich A, Mast H. Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke. 2000;31:2361–2364. doi: 10.1161/01.str.31.10.2361. [DOI] [PubMed] [Google Scholar]
- 6.Kim EJ, Halim AX, Dowd CF, Lawton MT, Singh V, Bennett J, Young WL. The relationship of coexisting extranidal aneurysms to intracranial hemorrhage in patients harboring brain arteriovenous malformations. Neurosurgery. 2004;54:1349–1357. doi: 10.1227/01.neu.0000124483.73001.12. discussion 1357–1348. [DOI] [PubMed] [Google Scholar]
- 7.Lawton MT. Spetzler-Martin Grade III arteriovenous malformations: surgical results and a modification of the grading scale. Neurosurgery. 2003;52:740–748. doi: 10.1227/01.neu.0000053220.02268.9c. discussion 748–749. [DOI] [PubMed] [Google Scholar]
- 8.Lawton MT, Du R, Tran MN, Achrol AS, McCulloch CE, Johnston SC, Quinnine NJ, Young WL. Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery. 2005;56:485–493. doi: 10.1227/01.neu.0000153924.67360.ea. discussion 485–493. [DOI] [PubMed] [Google Scholar]
- 9.Liscak R, Vladyka V, Simonova G, Urgosik D, Novotny J, Jr., Janouskova L, Vymazal J. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005–1014. doi: 10.1227/01.NEU.0000255474.60505.4A. discussion 1015–1006. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama K, Kawahara N, Shin M, Tago M, Kishimoto J, Kurita H, Kawamoto S, Morita A, Kirino T. The Risk of Hemorrhage after Radiosurgery for Cerebral Arteriovenous Malformations. 2005. pp. 146–153. [DOI] [PubMed] [Google Scholar]
- 11.Ming K, Rosenbaum PR. Substantial gains in bias reduction from matching with a variable number of controls. Biometrics. 2000;56:118–124. doi: 10.1111/j.0006-341x.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 12.Pollock BE. Stereotactic radiosurgery for arteriovenous malformations. Neurosurg Clin N Am. 1999;10:281–290. [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Silber JH. Matching and thick description in an observational study of mortality after surgery. Biostatistics. 2001;2:217–232. doi: 10.1093/biostatistics/2.2.217. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Mejia RO, Chennupati SK, Gupta N, Fullerton H, Young WL, Lawton MT. Superior outcomes in children compared with adults after microsurgical resection of brain arteriovenous malformations. J Neurosurg. 2006;105:82–87. doi: 10.3171/ped.2006.105.2.82. [DOI] [PubMed] [Google Scholar]
- 15.Schneider BF, Eberhard DA, Steiner LE. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87:352–357. doi: 10.3171/jns.1997.87.3.0352. [DOI] [PubMed] [Google Scholar]
- 16.Silber JH, Rosenbaum PR, Trudeau ME, Even-Shoshan O, Chen W, Zhang X, Mosher RE. Multivariate matching and bias reduction in the surgical outcomes study. Med Care. 2001;39:1048–1064. doi: 10.1097/00005650-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Smyth MD, Sneed PK, Ciricillo SF, Edwards MS, Wara WM, Larson DA, Lawton MT, Gutin PH, McDermott MW. Stereotactic radiosurgery for pediatric intracranial arteriovenous malformations: the University of California at San Francisco experience. J Neurosurg. 2002;97:48–55. doi: 10.3171/jns.2002.97.1.0048. [DOI] [PubMed] [Google Scholar]
- 18.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg GK, Chang SD, Levy RP, Marks MP, Frankel K, Marcellus M. Surgical resection of large incompletely treated intracranial arteriovenous malformations following stereotactic radiosurgery. J Neurosurg. 1996;84:920–928. doi: 10.3171/jns.1996.84.6.0920. [DOI] [PubMed] [Google Scholar]
- 20.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]