Abstract
Electrospray ionization (ESI) mass spectrometry (MS) is a powerful method for analyzing the active forms of macromolecular complexes of biomolecules. However, these solutions often contain high concentrations of salts and/or detergents that adversely effect ESI performance by making ion formation less reproducible, causing severe adduction or ion suppression. Many methods for separating complexes from nonvolatile additives are routinely used with ESI-MS, but these methods may not be appropriate for complexes that require such stabilizers for activity. Here, the effects of buffer loading using concentrations of ammonium acetate ranging from 0.22 to 1.41 M on the ESI mass spectra of a solution containing a domain truncation mutant of a σ54 activator from Aquifex aeolicus were studied. This 44.9 kDa protein requires the presence of millimolar concentrations of Mg2+, BeF3−, and ADP, (at ∼60 °C) to assemble into an active homo-hexamer. Addition of ammonium acetate can improve signal stability and reproducibility, and can significantly lower adduction and background signals. However, at higher concentrations, the relative ion abundance of the hexamer is diminished, while that of the constituent monomer is enhanced. These results are consistent with loss of enzymatic activity as measured by ATP hydrolysis and indicate that the high concentration of ammonium acetate interferes with assembly of the hexamer. This shows that buffer loading with ammonium acetate is effective for obtaining ESI signal for complexes that require high concentrations of essential salts, but can interfere with formation of, and/or destabilize complexes by disrupting crucial electrostatic interactions at high concentration.
Electrospray ionization mass spectrometry (ESI-MS) is an important technique for studying intact noncovalent complexes of biomolecules, owing to its capacity to provide information about stoichiometries [1], binding interfaces [2], relative [3, 4], and absolute [5] equilibrium constants, conformations [6], and assembly kinetics [7], with high sensitivity, speed, and specificity. An important factor in the ability to obtain high quality mass spectra is the compatibility of the analyte complex with the solvent systems typically employed in ESI-MS, i.e., aqueous ammonium acetate or ammonium bicarbonate solutions with minimal nonvolatile salts or detergents. Nonvolatile salts and detergents, often used to stabilize biomolecule complexes, are typically removed by buffer exchange into solutions of ammonium acetate or ammonium bicarbonate using microcentrifuge gel-filtration columns or dialysis before analysis [8]. Even low millimolar concentrations of metal ion salts, such as sodium chloride or phosphate, can cause severe ion suppression and peak broadening due to cluster and adduct formation. However, the roles of specific ions are well known for many noncovalent complexes of proteins with other proteins [9], peptides [10], nucleic acids [11], small molecules [12], cations [13], anions [14], nucleotides [15], etc., and the presence of these ions in the analyte solution can therefore be essential to obtain an accurate measurement of the native or native-like state of the complex. Other biophysical techniques used to study noncovalent complexes, such as native gel electrophoresis, small angle X-ray scattering, NMR, electron microscopy, and analytical ultracentrifugation are not compromised by the presence of metal ion salts.
Both desorption electrospray ionization [16] and matrix-assisted laser desorption ionization [17] are less affected by salts than ESI. Nonetheless, several approaches that make it possible to more readily electrospray from solutions containing metal ion salts and biomolecules have been developed. Konermann and coworkers used tartrate anions as weak chelators to minimize nonspecific adduction in ESI-MS studies of Ca2+ and Zn2+ binding proteins [18]. In an ESI-MS based enzymatic activity assay of cAMP-dependent protein kinase A, addition of methanol and acetic acid was shown to improve detection of phosphopeptides from solutions that required micromolar concentrations of cAMP, Mg2+, and ATP [19]. High concentrations of a volatile buffer can dramatically reduce the adverse effects of salts in ESI [20]. Addition of up to 7 M ammonium acetate to solutions containing 20 mM sodium chloride, and either cytochrome c or ubiquitin, resulted in a ∼7- and 11-fold improvement in signal-to-noise for these respective proteins [20]. This buffer loading technique [20] was shown to be effective for a noncovalent protein complex by Hernandez and Robinson [8], who demonstrated significantly improved resolution for the ∼150 kDa tetramer of alcohol dehydrogenase with 1 M ammonium acetate solutions that contained this protein and either 10 mM Tris-HCL or 10 mM HEPES. Buffer loading with 1 M ammonium acetate was used to obtain mass spectra of different oligomeric states of wild-type and mutant DnaB and DnaC proteins from Escherichia coli, which require up to 0.1 and 1.0 mM ATP and Mg(OAc)2, respectively, to assemble [21]. However, the stabilities of many complexes can depend on both ionic strength and specific ionic cofactors [22–27], so this buffer loading method is most effective when the salt tolerance of the complex is known.
Here, effects of ammonium acetate concentration on ESI mass spectral resolution and complex stability of a domain truncation mutant of a σ54 activator (NtrC4-RC) from the thermophilic bacterium Aquifex aeolicus, which requires millimolar concentrations of Mg2+, BeF3−, and ADP (at ∼60 °C) for assembly into a ∼270 kDa homohexamer [28, 29], are investigated. This truncated form of the full-length NtrC4 protein was chosen because it is somewhat more soluble than the full length protein and activation with the essential salts induces a change in the stoichiometries of the observed complexes from mixed homo-oligomers with no ATPase activity to a hexamer with similar ATPase activity to that of the full-length protein [29]. A spectrophotometric ATPase activity assay [30] of the hexamer was used to independently measure the effects of ammonium acetate concentration on the abundance of the functional form of this complex. At lower concentrations, ammonium acetate can increase the stability, reproducibility, and accuracy for measuring the mass and abundance of this noncovalent complex in solution, but at higher concentrations, it can interfere with assembly of the complex, presumably by displacement and/or interference of the critical electrostatic interactions with the essential salts.
Experimental
Protein Expression and Purification
Protein expression and purification are described in detail elsewhere [29]. Briefly, NtrC4-RC was subcloned into a PSKB3 plasmid with a His6 tag, expressed in E. coli. BL21 (DE3), harvested by sonication, and purified with a Ni-agarose column. The plasmid was sequenced at the University of California at Berkeley DNA sequencing facility.
Mass Spectrometry
Mass spectra were acquired using a quadrupole time-of-flight mass spectrometer equipped with a Z-spray ion source (Q-TOF Premier; Waters, Milford, MA, USA). Ions were formed using nanoelectrospray emitters prepared by pulling borosilicate capillaries (1.0 mm o.d./0.78 mm i.d.; Sutter Instruments, Novato, CA, USA) to a tip i.d. of ∼1 μm with a Flaming/Brown micropipette puller (model P-87, Sutter). A platinum wire (0.127 mm diameter, Sigma, St. Louis, MO, USA) was inserted through the capillary into the solution and electrospray was initiated and maintained by applying ∼1 kV to the wire relative to instrument ground. This voltage was adjusted to the lowest value at which ion current was stable. All other instrument parameters, including the source backing pressure (5.9 Torr), were the same for all experiments. The solutions were heated before and during data acquisition using a capillary heater described elsewhere [31]. The temperature of the capillary heater was monitored continuously with a thermocouple and temperature meter (Omega, Stamford, CT, USA). Raw data were smoothed three times using the Waters MassLynx software mean smoothing algorithm with a 75 unit window. Average molecular masses were obtained by deconvolution of the centroids of each peak in a given charge state distribution with a signal-to-noise >∼3, and those for the hexamer were corrected for non-specific adduction by the method of McKay et al. [32]. Relative abundances of proteins and complexes were estimated by summing the intensities of each charge state of a given protein or protein complex, and dividing that value by the sum of the intensities for each charge state in the distributions of monomer + dimer + hexamer. This does not take into account effects of mass dependent ion transfer or detection efficiency or ionization efficiency. Thus, the trends in these relative abundances as a function of ammonium acetate concentration are more meaningful than the actual absolute values. The instrument was calibrated with CsI clusters formed by nano-ESI of a 24 mg/mL solution of CsI in 70:30 Milli-Q:2-propanol before mass measurement.
ATPase Activity Assays
Individual 14.5 μL samples of NtrC4-RC in the presence of MgCl2, BeCl2, NaF, and various concentrations of ammonium acetate were incubated at 70 °C for 5 min. Then, 0.5 μL of 30 mM ATP was added to each sample, bringing the final reaction concentrations to 50 μM NtrC4-RC, 1 mM ATP, 1 mM MgCl2, 1 mM BeCl2, 9 mM NaF, and either 0.22, 0.34, 0.45, 0.68, 0.95, 1.14, or 1.41 M ammonium acetate. At various time points, 3 μL aliquots were quenched with 350 μL of 0.88 M HNO3. A color-developing solution (400 μL) consisting of 44 μBi(NO3)2, 31.1 μM (NH4)6Mo7O24, and 0.11% ascorbic acid was added to the quenched reactions. Exactly 4 min after the addition of color-developing solution, the amount of free phosphate in solution (a product of ATP hydrolysis) was measured using the absorbance of 700 nm light, which is proportional to phosphate concentration [30]. These absorbance measurements were obtained for multiple time points and used to calculate kcat, the moles of ATP hydrolyzed per mole of protein per minute. Measurement of kcat at each buffer concentration was performed in triplicate, and the averaged results, with error bars representing one standard deviation, are shown in Figure 1b.
Figure 1.
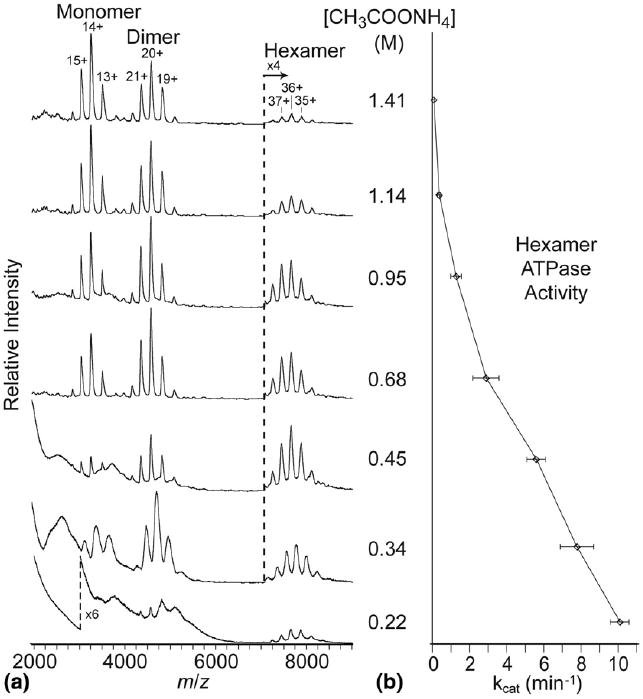
The effect of ammonium acetate concentration on the abundance of the hexameric oligomer of NtrC4-RC assembled in the presence of millimolar concentrations of MgCl2, NaF, BeCl2 and ADP measured at 63 °C by (a) nanoESI mass spectrometry and (b) ATPase activity, which is related to the concentration of the hexamer of NtrC4-RC in solution. kcat is calculated as moles of ATP hydrolyzed per mole of protein per minute.
Results and Discussion
ESI mass spectra obtained from solutions that contained 25 μM protein monomer, 1 mM ADP, 5 mM MgCl2, 1 mM BeCl2, 9 mM NaF, and seven different concentrations of ammonium acetate, ranging from 0.22 to 1.41 M, are shown in Figure 1a. BeF3−, which mimics phosphorylation, is formed from 1 mM BeCl2 as the limiting reagent and 9 mM NaF in excess. These solutions were heated to 63 °C before, and during, the experiments. The presence of the salts and nucleotide, and the elevated temperature, are required for a conformational change in the regulatory domain of the monomer that makes it possible for the protein to assemble into a hexamer [29]. Without the salts, nucleotide, and heat, this domain truncation mutant assembles into a heptamer and other mixed oligomers, whereas the stoichiometry of the complex composed of the native, full-length protein is a hexamer [29]. Three replicate spectra of each solution, each acquired using a different nanoelectrospray capillary, were measured and the spectra in Figure 1a were selected from the replicates as a typical spectrum with respect to peak centroids, widths, and relative abundances. Signal at the two lowest ammonium acetate concentrations was significantly less reproducible than at the higher concentrations.
The spectrum acquired with 0.22 M ammonium acetate (Figure 1a) has a low-intensity charge-state distribution consisting of broad peaks that correspond to the mass of the hexamer. The high baseline from m/z 2000 to ∼6000 may obscure a relatively low signal corresponding to the monomer, while the dimer is partially resolved with four charge states that have increasing peak widths with decreasing charge state. At 0.34 M ammonium acetate, charge-state distributions of both the monomer and dimer are clearly observed, albeit with very broad peaks (Table 1). The broad baseline below m/z 6000 decreases with increasing ammonium acetate concentration and is essentially flat at concentrations ≥0.68 M. In addition, there is a significant shift of the centroids of all of the peaks in the three charge state distributions to higher m/z at 0.34 M ammonium acetate concentration, presumably due to salt adduction, which leads to a significant increase in ion mass (Table 1). At ammonium acetate concentrations above 0.45 M, the relative abundance of the hexamer decreases dramatically with a concomitant increase in the relative abundance of the monomer. These trends continue with ammonium acetate concentration up to 1.41 M, where the relative abundance of the hexamer is only ∼2% and that of the monomer is ∼59% (Table 1). In comparison, the relative abundances of the hexamer and monomer at 0.45 M, the lowest concentration at which signal is reproducible, are ∼22% and ∼23%, respectively. At all concentrations of ammonium acetate, the experimental masses for monomer, dimer, and hexamer are higher than the theoretical molecular weights listed in Table 1, which were calculated from the known amino acid sequence of the NtrC4-RC protein. The mass of the unactivated monomer measured without essential salts or heat (44.9 kDa) is lower than that measured for the monomer from activated, heated solutions, indicating significant association with BeF3− and ADP in the ESI solution and non-specific adduction of Mg+ and/or Cl− [29]. No signal for other oligomers was observed. The dramatic increase in the relative abundance of monomer as a function of ammonium acetate concentration is consistent with interference of the hexamer assembly pathway rather than a decrease of hexamer due to nonspecific aggregation or other loss channels.
Table 1.
Experimental molecular weights (Exp. MW) and mean peak widths (full width at half-maximum) for the example spectra shown in Figure 1a, and mean relative abundances (Rel. abun.) from the three replicate measurements taken at each ammonium acetate concentration
| [Ammonium acetate] (M) | Monomer (44,929 Da) | Dimer (89,858 Da) | Hexamer* (269,574 Da) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. MW (Da) | Mean FWHM (m/z) | Rel. abun. (%) | Exp. MW (Da) | Mean FWHM (m/z) | Rel. abun. (%) | Exp. MW (Da) | Mean FWHM (m/z) | Rel. abun. (%) | |
| 1.41 | 45,623 ± 39 | 47 ± 6 | 59 ± 7 | 91,685 ± 15 | 60 ± 4 | 39 ± 6 | 272,227 ± 74 | 70 ± 6 | 2 ± 1 |
| 1.14 | 45,585 ± 8 | 45 ± 4 | 48 ± 1 | 91,566 ± 28 | 56 ± 4 | 48 ± 1 | 271,967 ± 104 | 71 ± 4 | 3 ± 2 |
| 0.95 | 45,552 ± 16 | 37 ± 2 | 43 ±4 | 91,469 ± 39 | 52 ± 3 | 51 ± 3 | 272,206 ± 92 | 64 ± 6 | 5 ± 2 |
| 0.68 | 45,614 ± 5 | 44 ± 5 | 43 ±8 | 91,560 ± 32 | 55 ± 1 | 51 ± 6 | 271,601 ± 59 | 75 ± 4 | 6 ± 2 |
| 0.45 | 45,587 ± 38 | 36 ± 3 | 23 ± 9 | 91,474 ± 38 | 53 ± 7 | 55 ± 6 | 271,817 ± 92 | 72 ± 6 | 22 ± 4 |
| 0.34 | 47,129 ± 382 | 136 ± 38 | 21 ± 7 | 93,885 ± 138 | 118 ± 7 | 66 ± 4 | 274,268 ± 214 | 98 ± 5 | 13 ± 4 |
| 0.22 | N/A | N/A | N/A | 91,406 ± 132 | 101 ± 62 | 15 ± 26 | 271,479 ± 158 | 71 ± 6 | 85 ± 26 |
Experimental molecular weights are corrected with the method of McKay et al. [32]. Errors for both experimental molecular weight and mean FWHM are reported as one standard deviation from the mass spectra shown in Figure 1. Errors in relative abundance correspond to one standard deviation in the three replicate measurements.
Ionic strength is well known as a factor in the stability of biomolecules, and high concentrations of ammonium acetate have been used to specifically reduce the binding affinities of protein complexes [3, 33]. To corroborate that the observed decrease in signal-to-noise of the hexamer from solutions with the highest concentrations of ammonium acetate occurs in solution before the ESI process, ATPase activity was measured in an independent assay [30] to determine the abundance of active hexamer in solution. The NtrC4 catalytic site for ATP hydrolysis is formed by interdomain contacts that exist only in the hexamer [28, 34, 35], so a change in the rate of ATP hydrolysis can be used to indicate the relative concentration of active hexamer in solution. The decrease in ATP hydrolysis with increasing ammonium acetate concentration (Figure 1b) is concomitant with the decrease in the relative abundance of the hexamer, and increase in the relative abundance of the monomer, measured by ESI-MS (Figure 1a) at the higher ammonium acetate concentrations. At the two lowest ammonium acetate concentrations, the low signal reproducibility and high baseline make it difficult to draw direct comparison with the activity assay. Nonetheless, these results are consistent with the ammonium acetate at high concentration displacing interactions between the protein and the essential salts/nucleotide, inhibiting the conformational change in the receiver domain that is required for oligomerization into the active form of the complex [29].
Conclusion
These results demonstrate that buffer loading with ammonium acetate can dramatically improve the resolution and the mass accuracy of nanoESI-MS measurements of noncovalent protein complexes that require low millimolar concentrations of nonvolatile salts and/or nucleotide to assemble into their physiologically relevant form. However, high concentrations of ammonium acetate can disrupt key interactions by displacement and/or interference of crucial electrostatic contacts that allow for assembly. Taken together, these effects provide additional evidence that within a range of suitable solution conditions, including temperature and ionic strength, ESI-MS can be invaluable for obtaining stoichiometric and thermodynamic information about macromolecular complexes.
Acknowledgments
The authors acknowledge generous financial support from the National Institutes of Health (E.R.W.: R01-GM064712-08) and (D.E.W.: R01-GM062163).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Sharon M, Robinson CV. The Role of Mass Spectrometry in Structure Elucidation of Dynamic Protein Complexes. Annu Rev Biochem. 2007;76:167–193. doi: 10.1146/annurev.biochem.76.061005.090816. [DOI] [PubMed] [Google Scholar]
- 2.Wang LT, Lane LC, Smith DL. Detecting Structural Changes in Viral Capsids by Hydrogen Exchange and Mass Spectrometry. Protein Sci. 2001;10:1234–1243. doi: 10.1110/ps.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapur A, Beck JL, Brown SE, Dixon NE, Sheil MM. Use of Electrospray Ionization Mass Spectrometry to Study Binding Interactions Between a Replication Terminator Protein and DNA. Protein Sci. 2002;11:147–157. doi: 10.1110/ps.27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnaswamy SR, Williams ER, Kirsch JF. Free Energies of Protein–Protein Association Determined by Electrospray Ionization Mass Spectrometry Correlate Accurately with Values Obtained by Solution Methods. Protein Sci. 2006;15:1465–1475. doi: 10.1110/ps.062083406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneshfar R, Kitova EN, Klassen JS. Determination of Protein– Ligand Association Thermochemistry Using Variable-Temperature Nanoelectrospray Mass Spectrometry. J Am Chem Soc. 2004;126:4786–4787. doi: 10.1021/ja0316972. [DOI] [PubMed] [Google Scholar]
- 6.van Duijn E, Simmons DA, van den Heuvel RHH, Bakkes PJ, van Heerikhuizen H, Heeren RMA, Robinson CV, van der Vies SM, Heck AJR. Tandem Mass Spectrometry of Intact GroEL-Substrate Complexes Reveals Substrate-Specific Conformational Changes in the Trans Ring. J Am Chem Soc. 2006;128:4694–4702. doi: 10.1021/ja056756l. [DOI] [PubMed] [Google Scholar]
- 7.Fandrich M, Tito MA, Leroux MR, Rostom AA, Hartl FU, Dobson CM, Robinson CV. Observation of the Noncovalent Assembly and Disassembly Pathways of the Chaperone Complex MtGimC by Mass Spectrometry. Proc Natl Acad Sci USA. 2000;97:14151–14155. doi: 10.1073/pnas.240326597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez H, Robinson CV. Determining the Stoichiometry and Interactions of Macromolecular Assemblies from Mass Spectrometry. Nat Protoc. 2007;2:715–726. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- 9.Bishop GR, Davidson VL. Catalytic Role of Monovalent Cations in the Mechanism of Proton Transfer which Gates an Interprotein Electron Transfer Reaction. Biochemistry. 1997;36:13586–13592. doi: 10.1021/bi970586a. [DOI] [PubMed] [Google Scholar]
- 10.Chi CN, Engstrom A, Gianni S, Larsson M, Jemth P. Two Conserved Residues Govern the Salt and pH Dependencies of the Binding Reaction of a PDZ Domain. J Biol Chem. 2006;281:36811–36818. doi: 10.1074/jbc.M607883200. [DOI] [PubMed] [Google Scholar]
- 11.Hiasa H, Shea ME, Richardson CM, Gwynn MN. Staphylococcus aureus Gyrase-Quinolone-DNA Ternary Complexes Fail to Arrest Replication Fork Progression In Vitro—Effects of Salt on the DNA Binding Mode and the Catalytic Activity of S. aureus Gyrase. J Biol Chem. 2003;278:8861–8868. doi: 10.1074/jbc.M209207200. [DOI] [PubMed] [Google Scholar]
- 12.Padua RA, Nagy JI, Geiger JD. Ionic-Strength Dependence of Calcium, Adenine-Nucleotide, Magnesium, and Caffeine Actions on Ryanodine Receptors in Rat Brain. J Neurochem. 1994;62:2340–2348. doi: 10.1046/j.1471-4159.1994.62062340.x. [DOI] [PubMed] [Google Scholar]
- 13.Waas WF, Dalby KN. Physiologic Concentrations of Divalent Magnesium Ion Activate the Serine/Threonine Specific Protein Kinase ERK2. Biochemistry. 2003;42:2960–2970. doi: 10.1021/bi027171w. [DOI] [PubMed] [Google Scholar]
- 14.Salhany JM, Sloan RL, Cordes KS. The Carboxyl Side Chain of Glutamate 681 Interacts with a Chloride Binding Modifier Site that Allosterically Modulates the Dimeric Conformational State of Band 3 (AE1). Implications for the Mechanism of Anion/Proton Cotransport. Biochemistry. 2003;42:1589–1602. doi: 10.1021/bi0205294. [DOI] [PubMed] [Google Scholar]
- 15.Fedosova NU, Esmann M. Nucleotide-Binding Kinetics of Na,K-ATPase: Cation Dependence. Biochemistry. 2004;43:4212–4218. doi: 10.1021/bi035707n. [DOI] [PubMed] [Google Scholar]
- 16.Jackson AU, Talaty N, Cooks RG, Van Berkel GJ. Salt Tolerance of Desorption Electrospray Ionization (DESI) J Am Soc Mass Spectrom. 2007;18:2218–2225. doi: 10.1016/j.jasms.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 17.McDonnell LA, Heeren RMA. Imaging Mass Spectrometry. Mass Spectrom Rev. 2007;26:606–643. doi: 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- 18.Pan JX, Xu K, Yang XD, Choy WY, Konermann L. Solution-Phase Chelators for Suppressing Nonspecific Protein-Metal Interactions in Electrospray Mass Spectrometry. Anal Chem. 2009;81:5008–5015. doi: 10.1021/ac900423x. [DOI] [PubMed] [Google Scholar]
- 19.de Boer AR, Letzel T, Lingeman H, Irth H. Systematic Development of an Enzymatic Phosphorylation Assay Compatible with Mass Spectrometric Detection. Anal Bioanal Chem. 2005;381:647–655. doi: 10.1007/s00216-005-3070-2. [DOI] [PubMed] [Google Scholar]
- 20.Iavarone AT, Udekwu OA, Williams ER. Buffer Loading for Counteracting Metal Salt-Induced Signal Suppression in Electrospray Ionization. Anal Chem. 2004;76:3944–3950. doi: 10.1021/ac049724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watt SJ, Urathamakul T, Schaeffer PM, Williams NK, Sheil MM, Dixon NE, Beck JL. Multiple Oligomeric Forms of Escherichia coli DnaB Helicase Revealed by Electrospray Ionization Mass Spectrometry. Rapid Commun Mass Spectrom. 2007;21:132–140. doi: 10.1002/rcm.2818. [DOI] [PubMed] [Google Scholar]
- 22.Siezen RJ, Bindels JG, Hoenders HJ. The Quaternary Structure of Bovine Alpha-Crystallin—Effects of Variation in Alkaline pH, Ionic-Strength, Temperature. Eur J Biochem. 1980;111:435–444. doi: 10.1111/j.1432-1033.1980.tb04958.x. [DOI] [PubMed] [Google Scholar]
- 23.Brenowitz M, Bonaventura C, Bonaventura J. Assembly and Calcium-Induced Cooperativity of Limulus-IV Hemocyanin—a Model System for Analysis of Structure–Function Relationships in the Absence of Subunit Heterogeneity. Biochemistry. 1983;22:4707–4713. doi: 10.1021/bi00289a015. [DOI] [PubMed] [Google Scholar]
- 24.Wagner R, Gonzalez DH, Podesta FE, Andreo CS. Changes in the Quaternary Structure of Phosphoenolpyruvate Carboxylase Induced by Ionic-Strength Affect Its Catalytic Activity. Eur J Biochem. 1987;164:661–666. doi: 10.1111/j.1432-1033.1987.tb11177.x. [DOI] [PubMed] [Google Scholar]
- 25.Valero E, Debonis S, Filhol O, Wade RH, Langowski J, Chambaz EM, Cochet C. Quaternary Structure of Casein Kinase-2 Characterization of Multiple Oligomeric States and Relation with Its Catalytic Activity. J Biol Chem. 1995;270:8345–8352. doi: 10.1074/jbc.270.14.8345. [DOI] [PubMed] [Google Scholar]
- 26.Smith SP, Barber KR, Dunn SD, Shaw GS. Structural Influence of Cation Binding to Recombinant Human Brain S100b: Evidence for Calcium-Induced Exposure of a Hydrophobic Surface. Biochemistry. 1996;35:8805–8814. doi: 10.1021/bi952698c. [DOI] [PubMed] [Google Scholar]
- 27.Bertenshaw GP, Norcum MT, Bond JS. Structure of Homo- and Hetero-Oligomeric Meprin Metalloproteases—Dimers, Tetramers, and High Molecular Mass Multimers. J Biol Chem. 2003;278:2522–2532. doi: 10.1074/jbc.M208808200. [DOI] [PubMed] [Google Scholar]
- 28.Batchelor JD, Doucleff M, Lee CJ, Matsubara K, De Carlo S, Heideker J, Lamers MH, Pelton JG, Wemmer DE. Structure and Regulatory Mechanism of Aquifex aeolicus NtrC4: Variability and Evolution in Bacterial Transcriptional Regulation. J Mol Biol. 2008;384:1058–1075. doi: 10.1016/j.jmb.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Batchelor JD, Sterling HJ, Hong E, Williams ER, Wemmer DE. Receiver Domains Control the Active-State Stoichiometry of Aquifex aeolicus σ54 Activator NtrC4, as Revealed by Electrospray Ionization Mass Spectrometry. J Mol Biol. 2009;393:634–643. doi: 10.1016/j.jmb.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen B, Guo Q, Guo Z, Wang X. An Improved Activity Assay Method for Arginine Kinase Based on a Ternary Heteropolyacid System. Tsinghua Sci Technol. 2003;8:422–427. [Google Scholar]
- 31.Sterling HJ, Williams ER. Origin of Supercharging in Electrospray Ionization of Noncovalent Complexes from Aqueous Solution. J Am Soc Mass Spectrom. 2009;20:1933–1943. doi: 10.1016/j.jasms.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay AR, Ruotolo BT, Ilag LL, Robinson CV. Mass Measurements of Increased Accuracy Resolve Heterogeneous Populations of Intact Ribosomes. J Am Chem Soc. 2006;128:11433–11442. doi: 10.1021/ja061468q. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M, Sandercock AM, Fraser CS, Ridlova G, Stephens E, Schenauer MR, Yokoi-Fong T, Barsky D, Leary JA, Hershey JW, Doudna JA, Robinson CV. Mass Spectrometry Reveals Modularity and a Complete Subunit Interaction Map of the Eukaryotic Translation Factor EIF3. Proc Natl Acad Sci USA. 2008;105:18139–18144. doi: 10.1073/pnas.0801313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SY, Torre A, Yan D, Kustu S, Nixon BT, Wemmer DE. Regulation of the Transcriptional Activator NtrC1: Structural Studies of the Regulatory and AAA+ ATPase Domains. Genes Dev. 2003;17:2552–2563. doi: 10.1101/gad.1125603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doucleff M, Chen B, Maris AE, Wemmer DE, Kondrashkina E, Nixon BT. Negative Regulation of AAA+ ATPase Assembly by Two Component Receiver Domains: A Transcription Activation Mechanism that is Conserved in Mesophilic and Extremely Hyperthermophilic Bacteria. J Mol Biol. 2005;353:242–255. doi: 10.1016/j.jmb.2005.08.003. [DOI] [PubMed] [Google Scholar]


