Abstract
Aneuploidy, an abnormal number of copies of a genomic region, might be a significant source for neuronal complexity, intercellular diversity, and evolution. Genomic instability associated with aneuploidy, however, can also lead to developmental abnormalities and decreased cellular fitness. Here we show that neurons with a more-than-diploid content of DNA are increased in preclinical stages of Alzheimer’s disease (AD) and are selectively affected by cell death during progression of the disease. Present findings show that neuronal hyperploidy in AD is associated with a decreased viability. Hyperploidy of neurons thus represents a direct molecular signature of cells prone to death in AD and indicates that a failure of neuronal differentiation is a critical pathogenetic event in AD.
Understanding the mechanisms underlying generation of neuronal variability and complexity remains a basic challenge to neuroscience. Structural variation in the human genome is likely to be one important mechanism for neuronal diversity and brain disease.1 The genetic profile of a cell can be permanently altered by chromosomal aneuploidy (i.e., an abnormal number of copies of a genomic region). A combination of multiple different forms of aneuploid cells due to loss or gain of whole chromosomes (mosaic aneuploidy) giving rise to cellular diversity at the genomic level have been described in neurons of the normal and diseased adult human brain.2,3,4,5,6,7,8,9,10,11,12
Cells in normal individuals have basically been assumed to contain identical euploid genomes. Still, earlier hypotheses suggested that a number of mammalian somatic tissues are populated by polyploid cells. Adult neurons of mammals were assumed to be postmitotic cells characterized to some extent by a polyploid chromosome complement. Testing this hypothesis in the past through histochemical methods, however, yielded controversial results through technical limitations.13,14 However, with the recent development of molecular cytogenic techniques, aneuploid cells in the normal developing and mature brain have clearly been identified, indicating that the maintenance of aneuploid neurons in the adult CNS is a widespread, if not universal, property of organization.2,3,4,5,6,7,8,9,10,11,12,15
Recent studies of the embryonic brain have shown that approximately one-third of the dividing cells that give rise to the cerebral cortex have genetic variability, manifested as chromosome aneuploidy.3,7,12 Neurons that constitute the adult brain arise from mitotic neural progenitor cells in the ventricular zone, a proliferating region where aneuploid cells appear to be generated through various chromosome segregation defects initially.3,10 While a portion of these aneuploid cells apparently die during development,3,16,17 aneuploid neurons have been identified in the mature brain in all areas assayed2,3,4,5,6,7,8,9,10,11,12,15 indicating that aneuploidy does not necessarily impair viability.18 Aneuploid neurons in the adult have been shown to make distant connections and express markers associated with neural activity, which indicates that these neurons can be integrated into brain circuitry.4,12
Contrary to this physiological consequences of “low-level” aneuploidy potentially contributing to neuronal diversity, aneuploidy above a critical threshold might be detrimental. Genomic instability and imbalances in gene dosage associated with aneuploidy can lead to developmental abnormalities, decreased cellular and organismal fitness, and increased susceptibility to disease.19,20
Aneuploid cells have typically been associated with pathophysiological conditions such as cancer,21 and most aneuploid syndromes present brain phenotypes and show a high vulnerability for psychiatric disorders.22 Mental impairment is a characteristic feature of all recognizable autosomal aneuploidy syndromes.
Recent studies have shown an increased rate of aneuploid neurons in Alzheimer’s disease (AD), schizophrenia, autism, and ataxia-telangiectasia.2,5,23,24,25 So far, however, direct evidence for a pathogenetic role for neuronal aneuploidy in these disorders is lacking. In particular, it remains unclear whether neuronal aneuploidy affects cellular viability, thus contributing directly to neurodegeneration and cell death.
Here, we analyzed the fate of hyperploid neurons at the conversion from preclinical to mild AD and during further progression to severe stages of the disease. We can show that neurons with a more-than-diploid content of DNA are increased in preclinical stages of AD and are selectively affected by cell death during progression of the disease. Present findings show that neuronal hyperploidy in AD is associated with a decreased viability and directly linked to cell death.
Materials and Methods
Case Recruitment
Brains from 28 patients with AD (mean age ± SD: 76.3 ± 8.1 years), 12 preclinical AD (73.6 ± 6.8 years), and 14 healthy controls dying without any history of neurological or psychiatric illness (71.7 ± 10.3 years) were used. The cognitive status of subjects was assessed within a year of death using the Clinical Dementia Rating (CDR).26,27 Severity of AD pathology was scored according to three pathological standards for AD: i) the Braak stages for neurofibrillary and amyloid changes28; ii) the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) neuropathological criteria29; and iii) the National Institute on Aging/Reagan Institute criteria for likeliness of AD.30
Healthy nondemented control brains had neither amyloid plaques nor neurofibrillary tangles and were rated CDR0, “normal” on CERAD criteria, and negative with respect to likelihood for AD according to National Institute on Aging (NIA) criteria. Preclinical AD brain was defined as CDR0; with Braak stages I-II/B, rated “possible” on CERAD criteria and “low to intermediate” on NIA criteria. Mild AD was rated CDR 0.5; Braak stages III-IV/B-C; “probable” according to CERAD criteria and “intermediate” on NIA criteria. Severe AD was rated CDR 3; Braak stages V-VI/C; “definite” according to CERAD criteria and “high” on NIA criteria.
Slide-Based Cytometry
Tissue blocks from the entorhinal (Brodmann area 28) cortex were fixed in 4% phosphate-buffered paraformaldehyde (4% PFA in PBS, pH 7.4) for 9 days and cryoprotected in 30% sucrose. Sections of 30 μm thickness were cut on a freezing microtome. For slide-based cytometry (SBC), slices were processed using immunofluorescence staining as described.31 Neurons were identified with the monoclonal mouse antipan-neurofilament antibody (SMI 311; Sternberger Monoclonals Inc., Lutherville, MD; 1:750), and only neurofilament reactive cells were considered for SBC analysis. DNA was stained with propidiumiodide (PI, Sigma-Aldrich, Taufkirchen, Germany).
SBC was performed using a Laser Scanning Cytometer (LSC, CompuCyte Corporation, Cambridge, MA) and the appropriate software WinCyte, version 3.4. Each fluorescent event was recorded with respect to size, perimeter, x–y position on the object slide, and maximum (Max Pixel) and overall integral fluorescence intensity. The relative DNA content of the cells was determined by the integral PI fluorescence values, and these data were further analyzed using the cell cycle software ModfitLT, version 2.0 (Verity Software House Inc., Topsham, ME). The entire parahippocampal gyrus was scanned with 80,000 to 120,000 analyzed cells for each specimen. The determination of the numerical neuronal density was performed using the tool of WinCyte to record the x–y position of each fluorescence event. A square region with a side length of 1 mm was created, and the number of neurons within this region randomly placed ten times through the entire entorhinal cortex was determined.
Chromogenic in Situ Hybridization and Fluorescence in Situ Hybridization
Chromogenic in situ hybridization (CISH) was performed with a ZytoDotCEN 17 probe (ZytoVision, Bremerhaven, Germany), which targets α-satellite sequences of the centromere of chromosome 17. The digoxigenin-labeled probe was immunohistochemically visualized using peroxidaseconjugated Fab fragments of an anti-digoxigenin antibody from sheep (Boehringer-Mannheim, Mannheim, Germany) and nickelammoniumsulfate/DAB/0.015% H2O2 as chromogen. Fixed human lymphocytes dropped on object slides and HeLa cells, cultured under standard conditions, and grown on coverslips were used as controls. For fluorescence in situ hybridization (FISH) the same probe was visualized with a biotinylated mouse monoclonal antibody to Digoxigenin (abcam, clone BT.21H8) followed by Streptavidin-Cy2 (dianova) and DAPI (Molecular Probes, Eugene, OR) nucleic acid counterstaining.
Validation of Slide-Based Cytometry by Chromogenic in Situ Hybridization
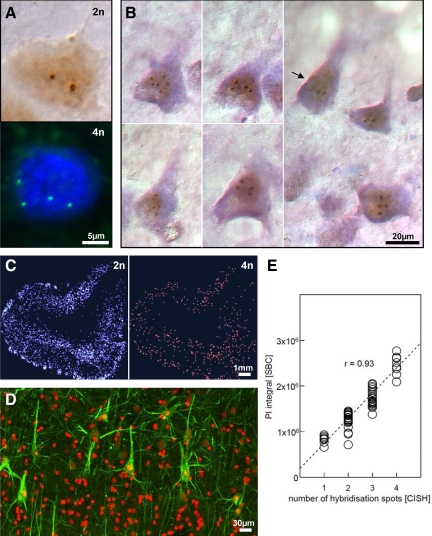
The method of DNA quantification by SBC was validated by CISH on 60 microscopically identified neurons from a randomly selected case of AD. The specimen was initially processed for immunofluorescence and analyzed by SBC. Subsequently, the slice was subjected to CISH with the ZytoDotCEN 17 probe, combined with conventional Nissl staining. For each cell, the PI integral obtained by SBC was plotted against the number of spots obtained through CISH. Linear regression analysis fit the data with r = 0.93; (P < 0.001).
Results
Hyperploid Neurons in Alzheimer’s Disease Are Elevated at Preclinical Stages and Decrease at More Advanced Stages
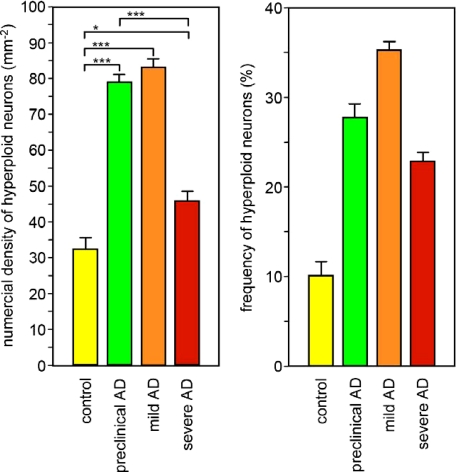
To quantify the single-neuron amount of DNA as a measure of cellular ploidy, we applied a protocol of SBC, recently developed for single-cell DNA analysis under conditions of preserved tissue architecture.5,31 Cross-validating this SBC method by CISH, a highly significant correlation was obtained between the two methods (r = 0.93; P < 0.001), confirming the validity of SBC for identification and quantification of hyperploid neurons (Figure 1, A–E). Neurons were identified by their immunofluorescence signal for neurofilaments, and the distribution of diploid and hyperploid neurons was mapped in the entorhinal cortex of healthy control, preclinical AD, and cases with mild and severe AD (Figure 2). While in control brain, hyperploid neurons were present at a frequency of about 10%, AD brain contained a significantly higher number, which is in agreement with previous observations.2,5,23 Hyperploid neurons were already elevated at preclinical stages of AD and remained high at progression toward mild AD. At more severe stages of AD, however, the number of hyperploid neurons decreased significantly, remaining only marginally different from controls.
Figure 1.
Hyperploid neurons in Alzheimer’s disease (AD). A: Examples of diploid (2n) and tetraploid (4n) neurons in AD entorhinal cortex, detected by CISH and FISH using chromosome-specific probe for chromosome 17 (ZytoDotCEN probes, ZytoVision, Germany, which target α-satellite-sequences of the centromere of the respective chromosome). B: Examples of hyperploid and diploid (arrow) pyramidal neurons in AD, identified by the abundance of Nissl substance, the typical shape of their soma and the apical dendrite approaching from the soma. Note the presence of hyperploid cells in the vicinity of a diploid pyramidal neuron in the far right panel (CISH with chromosome-specific probe for chromosome 17, counterstained for Nissl substance with cresyl echt violet). C: Mapping of neurons throughout the entorhinal cortex according to their DNA content by slide-based cytometry (SBC) in a case of mild AD (2n: diploid content of DNA; 4n: tetraploid content of DNA). D: To ensure the neuron-specificity of the SBC analysis, only cells immunoreactive for neurofilament (mab SMI 311: green) were considered (propidiumiodide signal: red). E: Intermethod reliability of SBC and quantitative CISH with a ZytoDotCEN 17 probe (ZytoVision, Bremerhaven, Germany). Linear regression analysis fits data with a correlation coefficient according to Bravais-Pearson of r = 0.93; P < 0.001.
Figure 2.
Quantification of hyperploid neurons in the entorhinal cortex by SBC in healthy controls and cases with preclinical, mild, and severe AD. Mean values ± SEM; differences are significant at the following levels: *P < 0.05, ***P < 0.001, Student t test.
This decrease in the number of hyperploid neurons from early to more advanced stages of AD can only be explained by a loss of hyperploid neurons during progression of AD as generation of hyperploidy most likely is an irreversible process.
Hyperploidy Accounts for the Majority of Cell Loss in Alzheimer’s Disease
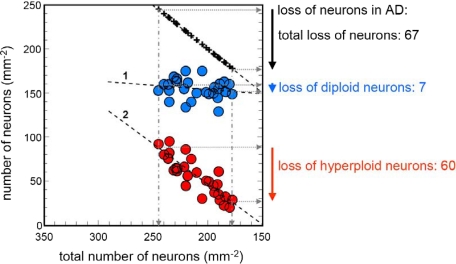
To analyze the fate of hyperploid neurons during progression of the disease, we ranked our AD cases according to the severity of total cell loss in the entorhinal cortex and plotted the changes in the number of diploid and hyperploid neurons against the total loss of neurons (Figure 3). The regression analysis for the number of diploid neurons against the total neuronal number did not reveal a significant relationship, indicating that the numerical density of diploid neurons remained stable during the process of cell loss. The loss of hyperploid neurons, however, correlated significantly with the total loss of neurons. This clearly shows that the loss of neurons in the entorhinal cortex can be explained by a loss of hyperploid neurons. While in the present sample, the total loss of neurons amounted to 67 neurons per mm2 (comparing the least and the most affected case), diploid neurons were reduced by only seven neurons and hyperploid by 60 neurons (Figure 3). Thus hyperploidy accounts for at least 89.5% of the total cell loss.
Figure 3.
Relation between changes in the number of diploid and hyperploid neurons and total neuronal loss. Total loss of neurons during progression of AD highly correlates with changes in the number of hyperploid neurons (regression line 2: r = 0.86; P < 0.001) but not with diploid neurons (regression line 1: r = 0.18; n.s.). Hyperploid neurons (red) account for a loss of 60 of 67 neurons, which corresponds to 89% of the total neuronal loss.
Elevation of Hyperploid Neurons at Early Stages of AD Precede Cell Death
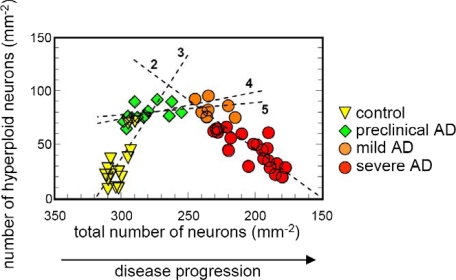
At the next step, we analyzed the transition between healthy controls and preclinical and mild AD in more detail. To visualize the relationship to cell death, the number of hyperploid neurons was plotted against total neuronal number (Figure 4). At preclinical stages of AD, the elevation of hyperploid neurons was not associated with neuronal loss. First cell loss became detectable at the transition from preclinical to mild AD. Hyperploid neurons remained stable in number at this point and subsequently decreased during disease progression from mild toward severe AD (Figure 4).
Figure 4.
Relation between changes in the number of hyperploid neurons and total neuronal loss in healthy controls and preclinical, mild, and severe AD. Hyperploid neurons are increased already in preclinical AD. They remain stable in number during transition from preclinical to mild AD and decrease with further progression of the disease. Linear regression analyses are indicated by dotted lines: 2, mild plus severe AD, r = 0.86, P < 0.001; 3, healthy controls, r = 0.71, P < 0.01; 4, preclinical AD, r = 0.50, n.s.; 5, preclinical plus mild AD, r = 0.36, n.s.
Discussion
Here we show that hyperploidy is an early event in AD and is already detectable before cell loss at the transition from control to preclinical stages of disease. At more advanced stages of the disease, which are associated with the loss of neurons, hyperploidy accounts for the majority of cell death. These findings indicate that neuronal hyperploidy in AD is associated with a decreased viability and identify hyperploidy as a major correlate of cell death. Hyperploidy is thus a direct molecular signature for cells prone to cell death in AD.
In agreement with previous studies,2,5,8,11,12 we observed aneuploid neurons in the normal human brain with a frequency of about 10%. Our data indicate a decompensation shifting this physiological into a pathological condition when the number of hyperploid neurons exceeds a certain threshold, which is in the order of two to three times the physiological “low-level.” This decompensation, occurring at preclinical stages of AD, apparently triggers a process of clearance of hyperploid neurons reducing their number back to values that are tolerated in the normal human brain. The almost stable number of diploid neurons together with the return of the frequency of hyperploid neurons during progression of AD to near normal levels suggests, furthermore, that there are no or at the most only very few new neurons added to the hyperploid population once that group has been established.
Despite much insight into the molecular pathology of AD, a direct molecular correlate for selective neuronal vulnerability and cell death has not been identified so far. It has been shown, however, that the progression of neurodegeneration in AD follows a defined pattern that inversely recapitulates ontogenetic and phylogenetic brain development.28,32 AD pathology thus preferentially affects those areas that during anthropoid evolution have recently been acquired or restructured.33,34,35 The evolutionary expansion of neocortical size in mammals, which is particularly prominent in anthropoid primates, is based on substantially more rounds of cell divisions of progenitor cells during a prolonged neurogenetic period.36,37,38,39,40 This phylogenetic prolongation of mitotic activity might provide the basis for a potentially higher rate of accumulating mitotic errors during neurogenesis that might give rise to abnormal copy numbers of a genomic region.
A recent study by Morillo et al,41 however, has directly demonstrated that DNA replication without cell division giving rise to neuronal tetraploidy in the adult vertebrate nervous system does not necessarily occur as a result of mitotic failure but rather might represent a physiological program driven by Nerve Growth Factor (NGF) and p75NTR. The fate of these neurons that re-enter the cell cycle in response to NGF (ie, apoptosis or replication without cell division and survival as tetraploid neuron instead of apoptosis) apparently depends on the cooperation and balance between NGF and Brain-Derived Neurotrophic Factor (BDNF). This is consistent with previous in vitro observations that NGF can drive DNA synthesis without replication, resulting in cells with a high DNA content,42,43 whereas BDNF can prevent neuronal apoptosis.44 In light of these observations, it might be interesting to note that an up-regulation of p75NTR has been described in AD45 together with an imbalance of neurotrophins with elevated levels of NGF and reduced levels of BDNF.46
Taken together, the present data provide direct evidence for the deleterious consequences of neuronal hyperploidy present at high frequency in AD brain. This adds hyperploidy to the list of critical molecular events that are shared between neurodegeneration and malignant cell transformation.47 Irrespectively of whether hyperploidy results from a lack of aneuploidy clearance during brain development or an aberrant attempt of cell cycle re-entry5,23,48 and DNA replication in the adult, it directs our attention to a failure of neuronal differentiation as the critical pathogenetic event and potential therapeutic target in neurodegeneration.
Footnotes
Address reprint requests to Thomas Arendt, M.D., D.Sc., University of Leipzig, Paul Flechsig Institute for Brain Research Jahnallee 59, 04109 Leipzig, Germany. E-mail: aret@medizin.uni-leipzig.de.
Supported by the Network of European Fundings for Neuroscience Research (ERA-Net NEURON-48-038).
References
- Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441:1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- Iourov IY, Vorsanova SG, Liehr T, Yurov YB. Aneuploidy in the normal. Alzheimer′s disease and ataxia-telangiectasia brain: differential expression and pathological meaning. Neurobiol Dis. 2009;34:212–220. doi: 10.1016/j.nbd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Kaushal D, Contos JJ, Treuner K, Yang AH, Kingsbury MA, Rehen SK, McConnell MJ, Okabe M, Barlow C, Chun J. Alteration of gene expression by chromosome loss in the postnatal mouse brain. J Neurosci. 2003;23:5599–5606. doi: 10.1523/JNEUROSCI.23-13-05599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Friedman B, McConnell MJ, Rehen SK, Yang AH, Kaushal D, Chun J. Aneuploid neurons are functionally active and integrated into brain circuitry. Proc Natl Acad Sci U S A. 2005;102:6143–6147. doi: 10.1073/pnas.0408171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack SD, Weil RJ, Vortmeyer AO, Zeng W, Li J, Okamoto H, Furuta M, Pak E, Lubensky IA, Oldfield EH, Zhuang Z. Individual adult human neurons display aneuploidy: detection by fluorescence in situ hybridization and single neuron PCR. Cell Cycle. 2005;4:1758–1760. doi: 10.4161/cc.4.12.2153. [DOI] [PubMed] [Google Scholar]
- Rehen SK, McConnell MJ, Kaushal D, Kingsbury MA, Yang AH, Chun J. Chromosomal variation in neurons of the developing and adult mammalian nervous system. Proc Natl Acad Sci U S A. 2001;98:13361–13366. doi: 10.1073/pnas.231487398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehen SK, Yung YC, McCreight MP, Kaushal D, Yang AH, Almeida BS, Kingsbury MA, Cabral KM, McConnell MJ, Anliker B, Fontanoz M, Chun J. Constitutional aneuploidy in the normal human brain. J Neurosci. 2005;25:2176–2180. doi: 10.1523/JNEUROSCI.4560-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra JW, Peterson SE, Yung YC, Mutoh T, Barral S, Chun J. Aneuploid mosaicism in the developing and adult cerebellar cortex. J Comp Neurol. 2008;507:1944–1951. doi: 10.1002/cne.21648. [DOI] [PubMed] [Google Scholar]
- Yang AH, Kaushal D, Rehen SK, Kriedt K, Kingsbury MA, McConnell MJ, Chun J. Chromosome segregation defects contribute to aneuploidy in normal neural progenitor cells. J Neurosci. 2003;23:10454–10462. doi: 10.1523/JNEUROSCI.23-32-10454.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, Iourov IY, Monakhov VV, Soloviev IV, Vostrikov VM, Vorsanova SG. The variation of aneuploidy frequency in the developing and adult human brain revealed by an interphase FISH study. J Histochem Cytochem. 2005;53:385–390. doi: 10.1369/jhc.4A6430.2005. [DOI] [PubMed] [Google Scholar]
- Yurov YB, Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Kutsev SI, Pellestor F, Beresheva AK, Demidova IA, Kravets VS, Monakhov VV, Soloviev IV. Aneuploidy and confined chromosomal mosaicism in the developing human brain. PLoS ONE. 2007;2:e558. doi: 10.1371/journal.pone.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregnard A, Knüsel A, Kuenzle CC. Are all the neuronal nuclei polyploid? Histochemistry. 1975;43:59–61. doi: 10.1007/BF00490154. [DOI] [PubMed] [Google Scholar]
- Swartz FJ, Bhatnagar KP. Are CNS neurons polyploid? A critical analysis based upon cytophotometric study of the DNA content of cerebellar and olfactory bulbar neurons of the bat. Brain Res. 1981;208:267–281. doi: 10.1016/0006-8993(81)90557-6. [DOI] [PubMed] [Google Scholar]
- Osada T, Kusakabe H, Akutsu H, Yagi T, Yanagimachi R. Adult murine neurons: their chromatin and chromosome changes and failure to support embryonic development as revealed by nuclear transfer. Cytogenet Genome Res. 2002;97:7–12. doi: 10.1159/000064037. [DOI] [PubMed] [Google Scholar]
- Harrison RH, Kuo HC, Scriven PN, Handyside AH, Ogilvie CM. Lack of cell cycle checkpoints in human cleavage stage embryos revealed by a clonal pattern of chromosomal mosaicism analysed by sequential multicolour FISH. Zygote. 2000;8:217–224. doi: 10.1017/s0967199400001015. [DOI] [PubMed] [Google Scholar]
- Voullaire L, Slater H, Williamson R, Wilton L. Chromosome analysis of blastomeres from human embryos by using comparative genomic hybridization. Hum Genet. 2000;106:210–217. doi: 10.1007/s004390051030. [DOI] [PubMed] [Google Scholar]
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- Torres EM, Williams BR, Amon A. Aneuploidy: Cells losing their balance. Genetics. 2008;179:737–746. doi: 10.1534/genetics.108.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, Iourov IY, Vorsanova SG, Demidova IA, Kravetz VS, Beresheva AK, Kolotii AD, Monakchov VV, Uranova NA, Vostrikov VM, Soloviev IV, Liehr T. The schizophrenia brain exhibits low-level aneuploidy involving chromosome 1. Schizophr Res. 2008;98:139–147. doi: 10.1016/j.schres.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Yurov YB, Vorsanova SG, Iourov IY, Demidova IA, Beresheva AK, Kravetz VS, Monakhov VV, Kolotii AD, Voinova-Ulas VY, Gorbachevskaya NL. Unexplained autism is frequently associated with lowlevel mosaic aneuploidy. J Med Genet. 2007;44:521–525. doi: 10.1136/jmg.2007.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- NIA criteria The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer′s disease. Neurobiol Aging. 1997;18:S1. [PubMed] [Google Scholar]
- Mosch B, Mittag A, Lenz D, Arendt T, Tárnok A. Laser scanning cytometry in human brain slices. Cytometry A. 2006;69:135–138. doi: 10.1002/cyto.a.20228. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 1996;92:197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- Arendt T. Alzheimer’s disease as a disorder of mechanisms underlaying structural brain self-organization. Neurosci. 2001;102:723–765. doi: 10.1016/s0306-4522(00)00516-9. [DOI] [PubMed] [Google Scholar]
- Arendt T, Brückner MK, Gertz HJ, Marcova L. Cortical distribution of neurofibrillary tangles in Alzheimer’s disease matches the pattern of neurons that retain their capacity of plastic remodelling in the adult brain. Neuroscience. 1998;83:991–1002. doi: 10.1016/s0306-4522(97)00509-5. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Brain evolution and Alzheimer’s disease. Rev Neurol (Paris) 1988;144:79–90. [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Kahn DM. Nature versus nurture revisited: an old idea with a new twist. Prog Neurobiol. 2003;70:33–52. doi: 10.1016/s0301-0082(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci USA. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–67. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- Morillo SM, Escoll P, de la Hera A, Frade JM. Somatic tetraploidy in specific chick retinal ganglion cells induced by nerve growth factor. Proc Natl Acad Sci USA. 2010;107:109–114. doi: 10.1073/pnas.0906121107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius MJ, Chandler CR, Shooter EM. Nerve growth factor-treated, neurite-bearing PC12 cells continue to synthesize DNA. J Neurosci. 1985;5:343–351. doi: 10.1523/JNEUROSCI.05-02-00343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer H, Thoenen H. Relationship between differentiation and terminal mitosis: chick sensory and ciliary neurons differentiate after terminal mitosis of precursor cells, whereas sympathetic neurons continue to divide after differentiation. J Neurosci. 1987;7:3739–3748. doi: 10.1523/JNEUROSCI.07-11-03739.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Bovolenta P, Martínez-Morales JR, Arribas A, Barbas JA, Rodríguez-Tébar A. Control of early cell death by BDNF in the chick retina. Development. 1997;124:3313–3320. doi: 10.1242/dev.124.17.3313. [DOI] [PubMed] [Google Scholar]
- Arendt T, Brückner MK. Perisomatic sprouts immunoreactive for nerve growth factor receptor and neurofibrillary degeneration affect different neuronal populations in the basal nucleus in patients with Alzheimer’s disease. Neurosci Lett. 1992;148(1–2):63–66. doi: 10.1016/0304-3940(92)90805-h. [DOI] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Arendt T. Differentiation and De-Differentiation – Neuronal Cell Cycle Regulation During Development and Age-Related Neurodegenerative Disorders. Lajtha A, Perez-Polo JR, Rossner S, editors. Springer; Handbook of Neurochemistry and Molecular Neurobiology. Development and Aging Changes in the Nervous System. 2008:pp. 157–213. [Google Scholar]
- Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]