Abstract
Epithelial-mesenchymal transition is an important mechanism behind initiation of cancer invasion and metastasis. This study was performed to clarify the involvement of epithelial-mesenchymal transition in the progression of cholangiocarcinoma. Cholangiocarcinoma cell lines, CCKS-1 and TFK-1, were treated with transforming growth factor-β1 (TGF-β1), and the phenotypic changes and invasive activity were examined. Immunohistochemical analysis was performed using tissue sections of cholangiocarcinoma. In vitro, TGF-β1 induced mesenchymal features in CCKS-1 and TFK-1 characterized by the reduction of E-cadherin and cytokeratin 19 expression and the induction of mesenchymal markers, such as vimentin and S100A4. TGF-β1 also induced the nuclear expression of Snail, and the invasive activity was significantly increased in both cell lines. Studies using a mouse xenograft model showed that TGF-β1 worsened the peritoneal dissemination of CCKS-1. All these changes by TGF-β1 were inhibited by the simultaneous administration of soluble TGF-β type II receptor. In vivo, six (16%) of 37 cholangiocarcinoma cases showed marked immunoreactivity of Snail in their nuclei. In these six cases, the immuno-expression of cytokeratin 19 was significantly reduced, and the expression of vimentin was significantly increased. The Snail expression significantly correlated with the lymph node metastasis and a poor survival rate of the patients. These results suggest that epithelial-mesenchymal transition induced by TGF-β1/Snail activation is closely associated with the aggressive growth of cholangiocarcinoma, resulting in a poor prognosis.
Epithelial-mesenchymal transition (EMT) is a series of events during which epithelial cells lose many of their epithelial characteristics and take on properties that are typical of mesenchymal cells. EMT has an important role in the development of many tissues during embryogenesis, and similar cell changes are recapitulated during pathological processes, such as fibrosis and cancer.1,2,3 Numerous observations support the idea that EMT has a central role in tumor progression and metastasis. Carcinoma cells acquire mesenchymal gene-expression patterns and properties, resulting in reduced cell–cell adhesion and the activation of proteolysis and motility, which promote tumor invasion and metastasis.
Members of the transforming growth factor-β (TGF-β) superfamily initiate and maintain EMT in a variety of biological systems and pathophysiological conditions by activating major signaling pathways and transcriptional regulators.4,5 TGF-β binds to TGF-β receptor type II (TβRII), and it recruits the TGF-β receptor type I (TβRI). TβRI subsequently phosphorylates Smad2 and Smad3, which form hetero-oligomers with Smad4. They translocate from the cytoplasm to the nucleus, where they regulate transcription of target genes.4,5 In addition to the well-characterized Smad signaling pathways, Smad-independent TGF-β-regulated networks also exist.4
The activation of signaling pathways results in the activation of transcriptional regulators such as Snail, Slug, and Twist, which regulate the changes in gene expression patterns that underlie EMT.6,7 Snail is a key regulator of EMT, and represses the gene expression of E-cadherin, a hallmark of the epithelial phenotype.8 Other commonly used molecular markers for EMT include reduced expression of cytokeratin (CK), increased expression of vimentin, S100A4 and N-cadherin, and nuclear localization of β-catenin.
Many studies have identified the overexpression of TGF-β1 in various types of human cancer. Since the overexpression of TGF-β1 correlates with tumor progression, metastasis, angiogenesis, and poor prognostic outcome,9,10 the inhibition of TGF-β pathway has been targeted in therapeutic strategies in cancer. For example, soluble TGF-β type II receptor (TGF-βsRII) inhibits the action of TGF-β by binding to TGF-β1 and TGF-β3 with high affinity, and the administration of a recombinant TGF-βsRII protein significantly inhibits tumor growth and metastasis.11
Bone morphogenic protein-7 (BMP-7) is a member of the TGF-β superfamily, and is also a novel TGF-β inhibitor. BMP-7 binds and activates BMP type II receptor (BMP-RII) that subsequently form complex with BMP receptor type IA (BMPR-IA). The receptors activated by BMP-7 phosphorylate Smad1, 5 and 8, which counteract Smad2/3 phosphorylation by TGF-β, and antagonize against EMT.12 Indeed, the administration of BMP-7 has been shown to reduce metastatic capability of breast cancer.13
In the gastrointestinal tract, EMT has been implicated in esophageal, gastric, colorectal, and pancreatic cancer, and hepatocellular carcinoma.14,15,16,17,18 In these reports, the occurrence of EMT was closely associated with TGF-β and/or Snail activation. Recently, it has been shown that cholangiocarcinoma acquired EMT/sarcomatoid phenotypes by epidermal growth factor (EGF)-EGF receptor signaling pathway, thereby promoting tumor progression and metastasis.19 To date, however, the occurrence of EMT mediated by TGF-β1/Snail activation has not been studied in cholangiocarcinoma. Our previous in vitro studies demonstrated that nonneoplastic cholangiocytes underwent EMT-like phenotypic changes following TGF-β1 stimulation,20,21 indicating that TGF-β1 may also be able to induce EMT in cholangiocarcinoma.
This study was performed to clarify the involvement of EMT in the progression and metastasis of cholangiocarcinoma with regard to TGF-β1/Snail activation.
Materials and Methods
Cell Culture
Two cholangiocarcinoma cell lines, CCKS-1 and TFK-1, were used. CCKS-1 was established in our laboratory from moderately differentiated adenocarcinoma,22 and TFK-1 was provided by Cell Resource Center for Biochemical Research (Tohoku University, Sendai, Japan). Cell culture was performed both in monolayer system and in three-dimensional cell culture system using a fluid gel matrix (growth factor reduced Matrigel matrix; BD Biosciences, Bendford, MA). Cells were maintained in RPMI-1640 medium (Gibco-BRL, Grand Island, NY) and Dulbecco’s-modified Eagle’s medium/F-12 (Gibco-BRL) with 10% fetal bovine serum (Gibco-BRL) and 1% penicillin-streptomycin-glutamine (Gibco-BRL) at 37°C in 5% CO2 with constant humidity.
Cells were then treated with either TGF-β1 (10 ng/ml; R&D Systems, Inc., Minneapolis, MN) alone or in combination with BMP-7 (100 ng/ml; R&D Systems, Inc.) or TGF-βsRII (400 ng/ml; R&D Systems, Inc.) for 5 days. The dosages of the molecules were determined based on results of our previous studies, as well as the manufacturer’s recommendations.23 Serum-free culture medium was used for the treatment. For comparison, human normal biliary epithelial cells (BECs), which were established and cultured as previously described,21 were used and treated in a similar manner.
Reverse Transcription (RT)-PCR and Quantitative Real-Time PCR
RT-PCR was performed using total RNA (1 μg) extracted from the cultured cells. Total RNA was extracted using an RNA extraction kit (RNeasy mini; Qiagen, Tokyo, Japan) and was used to synthesize cDNA with reverse transcriptase (ReverTra Ace; Toyobo Co., Osaka, Japan). The sequences of the primers used for the PCR analysis are shown in Table 1. The PCR products were subjected to 2% agarose gel electrophoresis and stained with ethidium bromide.
Table 1.
Sequences of the Primers Used for PCR and Real-Time PCR in This Study
| Gene | Sequences |
|---|---|
| TβRI | 5′-GGCCATTTACACTGAATGAG-3′ |
| 5′-GGCTTAGAAATGGCCCAAAA-3′ | |
| TβRII | 5′-GTGGAGACACTTACAAAGCT-3′ |
| 5′-GAAACTTGGGCTAACTGAGA-3′ | |
| BMPR-IA | 5′-GGTTTCATAGCGGCAGACAT-3′ |
| 5′-CTTTCCTTGGGTGCCATAAA-3′ | |
| BMP-RII | 5′-GCTAAAATTTGGCAGCAAGC-3′ |
| 5′-CTTGGGCCCTATGTGTCACT-3′ | |
| E-cadherin | 5′-CTGGACAGGGAGGATTTTGA-3′ |
| 5′-ACCTGAGGCTTTGGATTCCT-3′ | |
| Cytokeratin19 | 5′-GGTCAGTGTGGAGGTGGATT-3′ |
| 5′-TCAGTAACTCGGACCTGCT-3′ | |
| Vimentin | 5′-GAGAACTTTGCCGTTGAAGC-3′ |
| 5′-TCCAGCAGCTTCCTGTAGGT-3′ | |
| S100A4 | 5′-CAAGTACTCGGGCAAAGAGG-3′ |
| 5′-GCTGTCCAAGTTGCTCATCA-3′ | |
| COL1A1 | 5′-TACAGCGTCACTGTCGATGGC-3′ |
| 5′-TCAATCACTGTCTTGCCCCAG-3′ | |
| MMP-2 | 5′-CAACTACGATGATGACCGCAA-3′ |
| 5′-GTGTAAATGGGTGCCATCAGG-3′ | |
| GAPDH | 5′-GAGTCAACGGATTTGGTCGT-3′ |
| 5′-TTGATTTTGGAGGGATCTC-3′ |
BMPR-IA, bone morphogenic protein receptor type IA; BMP-RII, BMP type II receptor; MMP, matrix metalloproteinase; TβRI, transforming growth factor-β type I receptor; TβRII, TGF-β type II receptor.
Quantitative real-time PCR was performed according to a standard protocol using the SYBR Green PCR Master Mix (Toyobo Co.) and ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Warrington, UK). Cycling conditions were incubation at 50°C for 2 minutes, 95°C for 10minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Fold difference compared with glyceraldehyde-3-phosphate dehydrogenase expression was calculated. Then, the relative fold-increase in specific mRNA over the unstimulated samples was determined. Each experiment was conducted in six sets, and the mean was calculated.
Migration Assays
The migration of CCKS-1 and TFK-1 was assayed using a Matrigel invasion chamber (growth factor-reduced type; BD Biosciences). In the upper chamber, 5 × 103 cells in 0.5 ml of serum-free culture medium were treated with either TGF-β1 (10 ng/ml) alone or in combination with BMP-7 (100 ng/ml) or TGF-βsRII (400 ng/ml). After 48 hours at 37°C, the cells on the upper surface of the filter were removed using a cotton-wool swab. After fixation with 100% methanol and staining using hematoxylin, the number of cells migrating to the lower surface was counted in three fields (×100). Each experiment was conducted in six sets, and the mean was calculated.
Mouse Xenograft Model
Six-week-old BALB/cAnNCrj-nu/nu nude mice were purchased from Charles River (Charles River Japan, Inc., Yokohama, Japan). A total of 5 × 106 of CCKS-1 was suspended in 200 μl of Dulbecco’s-modified Eagle’s medium/F-12, and an intraperitoneal injection was performed. From the next day, the mice were treated daily with an intraperitoneal injection of TGF-β1 alone, TGF-β1 in combination with TGF-βsRII, or control vehicle. Each experimental group consisted of 4 or 5 mice, and the treatment was continued for 3 weeks. The dosages of recombinant TGF-β1 (R&D Systems, Inc.) and TGF-βsRII (R&D Systems, Inc.) were 200 ng and 4 μg per injection, respectively, which were determined based on the previous report as well as the manufacturer’s recommendations.24 All mice were sacrificed at the end of the study period, and the tumor size of injection site of the abdominal wall and the extent of peritoneal dissemination were examined. For histological evaluation, tumors were formalin-fixed and paraffin-embedded. The protocol was approved by the Animal Care and Use Committee of our institute.
Tissue Specimens
A total of 37 cases of cholangiocarcinoma from the surgical files in the Department of Human Pathology, Kanazawa University and its affiliated hospitals between 1987 and 2006 were used. All tumors originated from hilar and extrahepatic bile ducts, and were conventional cholangiocarcinoma. In this study, the cases of sarcomatous carcinoma and cholangiocarcinoma having a component of intraductal papillary projection of carcinoma cells (intraductal papillary neoplasm of the bile duct) were not included.25 TNM classification was applied according to the guidelines of the International Union Against Cancer.26 There was no evidence of predisposing conditions, such as hepatolithiasis and primary sclerosing cholangitis. One representative lesion from each case was used. As controls, histologically normal livers (n = 10) were used.
Immunostaining
Immunostaining was performed for formalin-fixed, paraffin-embedded sections of the surgically resected cholangiocarcinoma specimens, normal livers, and the gel matrix used in the three-dimensional cell culture of CCKS-1 and TFK-1. Immunostaining was performed using primary antibodies against E-cadherin (1:200, 4A2C7, mouse monoclonal; Zymed, South San Francisco, CA), CK19 (1:100, RCK108, mouse monoclonal; DakoCytomation), vimentin (1:600, V9, mouse monoclonal; DakoCytomation), pro-COL1A1 (1:100, goat polyclonal; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), S100A4 (1:100, rabbit polyclonal; Abcam Inc., Cambridge, MA), Snail (1:300, rabbit polyclonal; Abcam Inc.), TβRI (1:50, rabbit polyclonal; Santa Cruz Biotechnology, Inc.), and TβRII (1:50, rabbit polyclonal; Santa Cruz Biotechnology, Inc.). After deparaffinization, antigen retrieval was performed by microwaving in 10 mmol/L citrate buffer pH 6.0 for E-cadherin, CK19 and vimentin staining, by incubating with 1 mg/ml of trypsin for 10 minutes at 37°C for TβRI and TβRII staining, and by incubating with 20 mg/ml of proteinase K for 6 minutes at room temperature for S100A4 staining. To block the activity of endogenous peroxidase, sections were immersed in 0.3% hydrogen peroxidase in methanol for 20 minutes at room temperature. After pretreatment with blocking serum (DakoCytomation), sections were incubated overnight at 4°C with individual primary antibodies. Then sections were incubated with secondary antibodies conjugated to peroxidase-labeled polymer, using an EnVision+ system (DakoCytomation) or a HISTOFINE system (Nichirei, Tokyo, Japan). Color development was performed using 3,3′-diaminobenzidine tetrahydrochloride and the sections were slightly counterstained with hematoxylin. Negative controls were done by substitution of the primary antibodies with nonimmunized serum, resulted in no signal detection.
Immunofluorescence staining of Snail and phospho-Smad2/3 (pSmad2/3) was performed for the paraffin-embedded sections of CCKS-1 and TFK-1. After deparaffinization, the sections were treated with 20 mg/ml of proteinase K for 6 minutes at room temperature, and were incubated with primary antibodies against Snail (1:300 rabbit polyclonal; Abcam Inc.), and pSmad2/3 (1:50, rabbit polyclonal; Santa Cruz Biotechnology, Inc.). The protein expression was detected using the alkaline phosphatase-labeled polymer, the HISTOFINE system (Nichirei). Color development was performed using the Vector Red alkaline phosphatase substrate kit (Vector Laboratories, Burlingame, CA), and the nuclei were stained with 4′6-diamidino-2-phenylindole. The signals were detected under immunofluorescence confocal microscopy.
Histological Assessment
Semiquantitative analysis was performed for the tissue sections stained with the primary antibodies against CK19, vimentin, and Snail. The expression of CK19 and vimentin was evaluated semiquantitatively at the invasive front according to the percentage of positively stained cells: − (negative); 1+ (focal), 1% to 10% of cells in the lesion stained positive; 2+ (moderate), 11% to 50% of cells in the lesion stained positive; and 3+ (marked), more than 50% of cells in the lesion stained positive. The signal intensity of nuclear staining of Snail was evaluated using the following grade; − (negative), 1+ (mild to moderate), 2+ (marked). Representative images of the grading of Snail immunostaining are shown. The histological assessment was performed by two independent investigators (Y.S. and S.S.).
Statistics
All statistical analysis was performed using the Dr SPSS II software (version 11.01 J; SPSS Japan Inc., Tokyo, Japan) and the Statview-J5.0 software (Abacus Concepts, Inc., Berkley CA). Statistical significance was determined using the χ2 test, the Mann-Whitney U-test, and the Spearman’s rank correlation test. In the univariate analysis, the postoperative survival probability was calculated by the Kaplan-Meier method. A P value less than 0.05 was accepted as the level of statistical significance.
Results
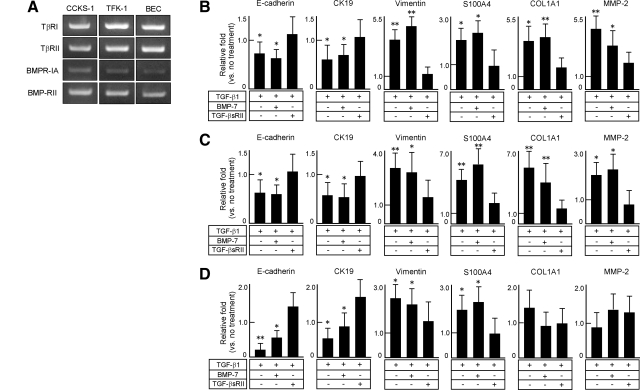
Phenotypic Alterations of CCKS-1, TFK-1 and Normal BECs by TGF-β1 at the mRNA Level
RT-PCR analysis confirmed that CCKS-1, TFK-1, and normal BECs expressed the mRNA of the receptors for TGF-β (TβRI, TβRII) and BMP-7 (BMPR-IA, BMP-RII), althoughh the expression of BMPR-1A mRNA was relatively weak (Figure 1A). Quantitative real-time PCR showed that the 5-day treatment with TGF-β1 significantly reduced the mRNA expression of epithelial markers (E-cadherin, CK19), and the mRNA expression of mesenchymal markers (vimentin, S100A4), COL1A1, and matrix metalloproteinase-2 (MMP-2) were significantly increased in CCKS-1 (Figure 1B) and TFK-1 (Figure 1C). BMP-7 had no significant effect on the changes of the expression of these markers induced by TGF-β1, while TGF-βsRII blocked the effects of TGF-β1 in CCKS-1 and TFK-1 (Figure 1, B and C).
Figure 1.
Phenotypic alterations of CCKS-1, TFK-1, and normal BECs by TGF-β1 at the mRNA level. CCKS-1, TFK-1, and human normal BECs were treated with either TGF-β1 (10 ng/ml) alone or in combination with BMP-7 (100 ng/ml) or TGF-βsRII (400 ng/ml), and phenotypic changes were examined using quantitative real-time PCR. All cell lines expressed the receptors for TGF-β (TβRI, TβRII) and BMP-7 (BMPR-IA, BMP-RII) (A). Quantitative real-time PCR showed that treatment with TGF-β1 reduced the mRNA expression of E-cadherin and CK19, and induced vimentin, S100A4, COL1A1 and MMP-2 mRNA expression in CCKS-1 (B) and TFK-1 (C). In normal BECs (D), TGF-β1 reduced the mRNA expression of E-cadherin and CK19 and induced vimentin and S100A4 mRNA expression. BMP-7 did not inhibit the phenotypic changes induced by TGF-β1 in CCKS-1, TFK-1, and normal BECs, whereas TGF-βsRII blocked them. The data represent three independent experiments (A). The relative fold-increase in specific mRNA over the unstimulated samples was determined, and the mean ± SD of six per group are shown (B–D). **P < 0.01; *P < 0.05 (vs. no treatment group).
In normal BECs, TGF-β1 reduced the mRNA expression of E-cadherin and CK19, and induced the mRNA expression of vimentin and S100A4 (Figure 1D). These phenotypic changes were blocked by the addition of TGF-βsRII, and BMP-7 had no significant effects. Contrasting to the results of CCKS-1 and TKF-1, the mRNA expression of COL1A1 and MMP-2 was not significantly affected by TGF-β1 in normal BECs (Figure 1D).
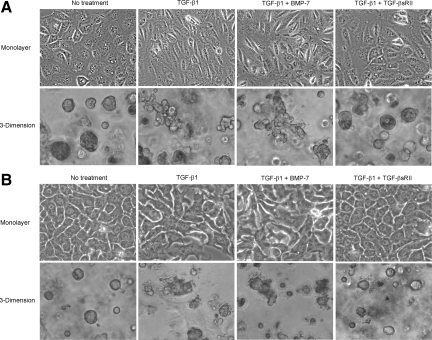
Effects of TGF-β1 on Cellular Morphology of CCKS-1 and TFK-1
In the monolayer culture system, CCKS-1 (Figure 2A) and TFK-1 (Figure 2B) grew in a form of epithelioid, sheet-like appearance under the phase-contrast microscopy, and the 5-day treatment with TGF-β1 changed the cellular morphology from epithelioid into spindle-shaped appearance in CCKS-1 (Figure 2A), and from epithelioid into pleomorphic appearance in TFK-1 (Figure 2B). In both cell lines, the addition of BMP-7 in the culture medium did not inhibit the morphological changes induced by TGF-β1, whereas the addition of TGF-βsRII preserved the epithelioid appearance (Figure 2).
Figure 2.
Effects of TGF-β1 on cellular morphology of CCKS-1 and TFK-1. CCKS-1 and TFK-1 were cultured in monolayer and three-dimensional culture systems using a Matrigel matrix and were treated with either TGF-β1 (10 ng/ml) alone or in combination with BMP-7 (100 ng/ml) or TGF-βsRII (400 ng/ml) for 5 days. CCKS-1 (A) and TFK-1 (B) grew in a form of epithelioid, sheet-like appearance in the monolayer system and in a form of spheroidal cell aggregates in the three-dimensional culture system. Treatment with TGF-β1 changed the cellular morphology of CCKS-1 from epithelioid into spindle-shaped appearance, and changed the morphology of TFK-1 from epithelioid to pleomorphic appearance in the monolayer system. The treatment also changed the cellular morphology of both cell lines from spheroid into irregular shaped cell aggregates in the three-dimensional system. Addition of BMP-7 in the culture medium did not inhibit these morphological changes of both cell lines, while TGF-βsRII preserved the epithelioid appearance. Phase-contrast microscopy. Original magnifications, ×1000.
In the three-dimensional culture system using Matrigel, CCKS-1 (Figure 2A) and TFK-1 (Figure 2B) formed spheroidal cell aggregates, and the 5-day treatment with TGF-β1 altered the cellular morphology from spheroid into irregular shaped cell aggregates, in which the morphological alterations were more remarkable in CCKS-1 (Figure 2A). Similar to the results of the monolayer culture system, the addition of BMP-7 did not inhibit the morphological changes of the cells, and the addition of TGF-βsRII blocked the effects of TGF-β1 (Figure 2).
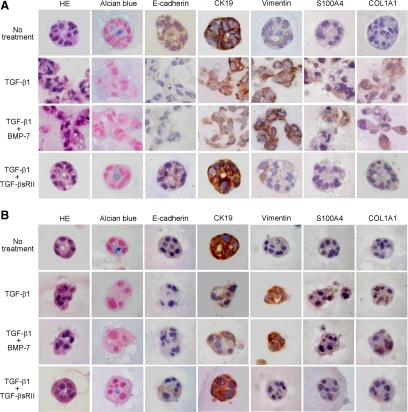
Phenotypic Alterations of CCKS-1 and TFK-1 by TGF-β1 at the Protein Level
To determine the phenotypic alterations of CCKS-1 and TFK-1 at the protein level, immunocytochemistry was performed using the paraffin-embedded sections of the Matrigel used for the three-dimensional cell culture. The results of CCKS-1 (Figure 3A) and TFK-1 (Figure 3B) were almost identical. The spheroids of CCKS-1 and TFK-1 occasionally contained acidic mucins in the central parts that were demonstrated by Alcian blue staining at pH 2.5, and the mucins were completely disappeared following the TGF-β1 treatment. Immunostaining showed that both CCKS-1 and TFK-1 originally expressed E-cadherin weakly, and expressed CK19 strongly, and the expression of vimentin, S100A4, and COL1A1 was focal and weak. TGF-β1 diminished the expression of E-cadherin, reduced the expression of CK19, and induced the expression of vimentin, S100A4, and COL1A1 in CCKS-1 and TFK-1, regardless of the presence or absence of BMP-7 (Figure 3). The phenotypic and morphological changes induced by TGF-β1 were inhibited by the addition of TGF-βsRII (Figure 3). These results were consistent with the changes of mRNA expression following the treatment (Figure 1, B and C).
Figure 3.
Phenotypic alterations of CCKS-1 and TFK-1 by TGF-β1 at the protein level. CCKS-1 and TFK-1 were cultured in the three-dimensional system using a Matrigel matrix and were treated with either TGF-β1 (10 ng/ml) alone or in combination with BMP-7 (100 ng/ml) or TGF-βsRII (400 ng/ml) for five days. The paraffin-embedded sections of the gel matrix were used for histological analysis. Spheroids of CCKS-1 (A) and TFK-1 (B) contained mucins in the central parts that were demonstrated by Alcian blue staining. Immunostaining showed that CCKS-1 and TFK-1 expressed E-cadherin and CK19, and the expression of vimentin, S100A4 and COL1A1 was focal and weak. TGF-β1 treatment diminished the mucins, reduced the expression of E-cadherin and CK19, and induced the expression of vimentin, S100A4 and COL1A1. These changes induced by TGF-β1 were completely inhibited by the addition of TGF-βsRII, while BMP-7 had no effects in both cell lines. Original magnifications, ×1000.
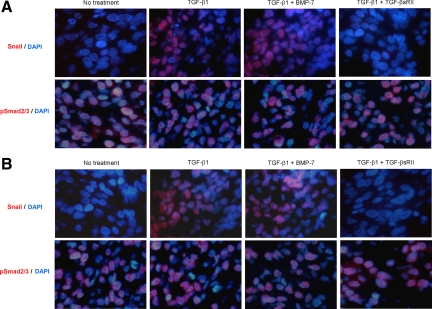
Induction of Snail Expression in CCKS-1 and TFK-1 by TGF-β1
The paraffin-embedded sections of CCKS-1 and TFK-1 were stained using the anti-Snail antibody. The immunohistochemical expression of Snail without any treatment was negligible in both CCKS-1 (Figure 4A) and TFK-1 (Figure 4B). Following the 5-day TGF-β1 treatment, approximately 50% of the cells strongly expressed Snail in their nuclei of both cell lines, which was not affected by the addition of BMP-7, and TGF-βsRII completely inhibited the nuclear expression of Snail (Figure 4), indicating that the phenotypic and morphological alterations of CCKS-1 and TFK-1 following TGF-β1 treatment were mediated by Snail.
Figure 4.
Induction of Snail expression in CCKS-1 and TFK-1 by TGF-β1. Immunofluorescence staining of Snail and pSmad2/3 was performed for the paraffin-embedded sections of CCKS-1 and TFK-1, which were treated with either TGF-β1 (10 ng/ml) alone or in combination with BMP-7 (100 ng/ml) or TGF-βsRII (400 ng/ml) for five days. The expression of Sail and pSmad2/3 was visualized using a Vector red reaction, and nuclei were stained with 4′6-diamidino-2-phenylindole (blue). The immunohistochemical expression of Snail in CCKS-1 (A) and TFK-1 (B) without any treatment was negligible, and TGF-β1 treatment induced the expression of Snail in approximately 50% of the cells in their nuclei of both cell lines. The Snail expression was not affected by the addition of BMP-7, whereas TGF-βsRII inhibited the nuclear expression of Snail. Many cells of CCKS-1 and TFK-1 originally expressed pSmad2/3, and the expression was not affected by the addition of TGF-β1, BMP-7, or TGF-βsRII. Original magnifications, ×1000.
The immunohistochemical expression of pSmad2/3 was observed in CCKS-1 (Figure 4A) and TFK-1 (Figure 4B) in more than 50% of the cells, even in the absence of TGF-β1 treatment. The expression of pSmad2/3 was not affected by any treatment with TGF-β1, BMP-7, or TGF-βsRII (Figure 4).
Activation of Invasive Potential of CCKS-1 and TFK-1 by TGF-β1
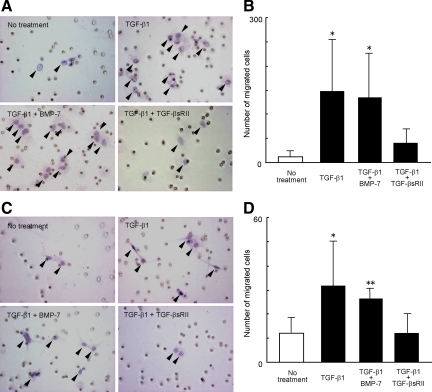
Migration assays using the Matrigel invasion chamber demonstrated that the invasive activities of CCKS-1 (Figure 5, A and B) and TFK-1 (Figure 5, C and D) were significantly increased by the treatment with TGF-β1. Again, BMP-7 did not affect the increase in the invasive potential of both cell lines induced by TGF-β1, while TGF-βsRII reduced it (Figure 5).
Figure 5.
Activation of invasive potential of CCKS-1 and TFK-1 by TGF-β1. The invasive activity of CCKS-1 and TFK-1 was assayed using the Matrigel invasion chamber. CCKS-1 and TFK-1 were treated with either TGF-β1 (10 ng/ml) alone or in combination with BMP-7 (100 ng/ml) or TGF-βsRII (400 ng/ml) for two days. Treatment with TGF-β1 significantly increased the number of migrated cells of CCKS-1 (A, B) and TFK-1 (C, D). BMP-7 did not affect the increase in the invasive potential of CCKS-1 and TFK-1 induced by TGF-β1, while TGF-βsRII reduced it. Representative images of the migrated cells through the filter of the invasion chamber are shown in A and C. Arrowheads indicate migrated carcinoma cells. Original magnification, ×400 (A, C). The data represent the mean ± SD of six per group (B, D). **P < 0.01, *P < 0.05 (vs. no treatment group).
Induction of Invasive Potential of CCKS-1 by TGF-β1 in Vivo
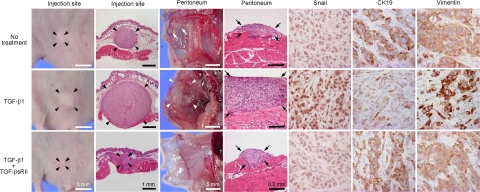
A mouse xenograft model was used to determine the effects of TGF-β1 on the invasive potential in vivo. Suspension of CCKS-1 was injected intraperitoneally to BALB/c-nu/nu nude mice, and the mice were further treated daily with recombinant TGF-β1 or TGF-β1 + TGF-βsRII by intraperitoneal administration for 3 weeks. The invasive potential and tumorigenesis were compared among the three groups of TGF-β1-treated mice, TGF-β1 + TGF-βsRII-treated mice, and vehicle control. The results are shown in Figure 6. At the site of peritoneal injection, the TGF-β1-treated mice exhibited a larger mass (average 4.7 mm in diameter) compared with that of TGF-β1 + TGF-βsRII-treated mice (average 0.6 mm) and vehicle control (average 2.5 mm) (Figure 6, black arrowheads).
Figure 6.
Induction of invasive potential of CCKS-1 by TGF-β1 in vivo. A mouse xenograft model was used to determine the effects of TGF-β1 on the invasive potential in vivo. CCKS-1 was injected intraperitoneally, and the mice were further treated with recombinant TGF-β1 or TGF-β1 + TGF-βsRII as described in the Materials and Methods. At the site of peritoneal injection, the TGF-β1-treated mice exhibited a larger mass compared with that of TGF-β1 + TGF-βsRII-treated mice and vehicle control (black arrowheads). In the peritoneum, the vehicle control group occasionally showed small disseminated tumor nodules (white allows). The surface of peritoneum in the vehicle control group was macroscopically almost sooth, and microscopically, small carcinoma foci were scattered in the peritoneum (black arrows, upper panel). By contrast, diffuse thickening of the peritoneum was macroscopically observed in the TGF-β1-treated mice (white arrowheads), and the histology confirmed diffuse and dense peritoneal dissemination (black arrows, middle panel). Addition of TGF-βsRII markedly reduced the peritoneal dissemination. Immunostaining showed that tumors of the TGF-β1-treated mice were characterized by the increased nuclear expression of Snail, the reduction of CK19 expression, and the induction of vimentin expression, and TGF-βsRII diminished these immunohistochemical alterations induced by TGF-β1. Original magnifications (immunostaining), ×400.
In the peritoneum, the vehicle control group occasionally showed small disseminated tumor nodules (Figure 6, while allows). The surface of peritoneum in the vehicle control group was macroscopically almost sooth, and microscopically, small foci of carcinoma were scattered in the peritoneum (Figure 6, black arrows, upper panel). By contrast, diffuse thickening of the peritoneum, instead of tumor nodule formation, was macroscopically observed in the TGF-β1-treated mice (Figure 6, white arrowheads), and the histology confirmed diffuse and dense peritoneal dissemination (Figure 6, black arrows, middle panel). Addition of TGF-βsRII markedly reduced the peritoneal dissemination of CCKS-1.
Immunohistochemical analysis demonstrated that tumors of the TGF-β1-treated mice were characterized by the increased nuclear expression of Snail, the reduction of CK19 expression, and the induction of vimentin expression, and TGF-βsRII diminished these immunohistochemical alterations induced by TGF-β1 (Figure 6).
Expression of Snail in Cholangiocarcinoma
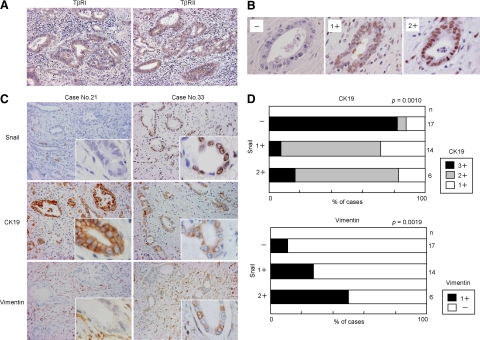
Immunostaining was performed for paraffin-embedded tissue sections of cholangiocarcinoma of hilar and extrahepatic bile ducts (n = 37) and normal livers (n = 10). The immunostaining confirmed that cholangiocarcinoma and the epithelium of normal bile ducts constantly and diffusely expressed the receptors for TGF-β (TβRI, TβRII) (Figure 7A), and the signal intensity was not significantly different among the cases.
Figure 7.
Expression of Snail and its association with CK19 and vimentin expression in cholangiocarcinoma. Immunohistochemical analysis was performed for paraffin-embedded tissue sections of cholangiocarcinoma of hilar and extrahepatic bile ducts (n = 37). Immunostaining showed that cholangiocarcinoma cells expressed the receptors for TGF-β (TβRI, TβRII) (A). The immunohistochemical expression of Snail in the nuclei of cholangiocarcinoma cells varied from case to case, and the extent was graded as negative (−), mild to moderate (1+), and marked (2+) (B). In the Snail-negative cases, marked immunohistochemical signals of CK19 were observed in cholangiocarcinoma cells, while the expression of vimentin was negative (C). By contrast, the expression of CK19 tended to be reduced in cholangiocarcinoma cells in the Snail-positive cases, and the positive immunoreactivity of vimentin was observed (C). The immunohistochemical expression of CK19 and vimentin in cholangiocarcinoma cells was semiquantitatively evaluated in four grades (−, 1+, 2+, 3+) as described in the Materials and Methods, and the association with the Snail expression was analyzed. The semiquantitative analysis showed that the Snail expression significantly correlated with the reduction of CK19 expression, and the induction of vimentin expression in cholangiocarcinoma cells (D). Original magnifications, ×200 (A, C); ×1000 (B, insets of C).
Positive immunoreactivity of Snail was detected in the nuclei of cholangiocarcinoma cells in 20 cases (54%), and 17 cases (46%) were negative (Table 2). The expression of Snail was not observed in the bile ducts of normal livers (data not shown). Among the Snail-positive 20 cholangiocarcinoma cases, 14 cases showed mild to moderate intensity (1+) of immunohistochemical signals, and six cases showed marked (2+) intensity (Table 2, Figure 7B). The nuclear signal intensity of Snail in cholangiocarcinoma cells was similar irrespective of sites in a section of each case.
Table 2.
Relationship between Immunohistochemical Expression of Snail and Clinicopathological Factors in Cholangiocarcinoma
| Clinicopathological factor | n | Snail expression
|
P value | ||
|---|---|---|---|---|---|
| − (%) | 1+ (%) | 2+ (%) | |||
| Total | 37 | 17 (46) | 14 (38) | 6 (16) | |
| Age | |||||
| ≤70 | 14 | 8 (57) | 4 (29) | 2 (14) | 0.5551 |
| >70 | 23 | 9 (39) | 10 (43) | 4 (17) | |
| Sex | |||||
| Male | 23 | 14 (61) | 8 (35) | 1 (4) | 0.0152 |
| Female | 14 | 3 (21) | 6 (43) | 5 (36) | |
| Size (cm) | |||||
| ≤2.0 | 18 | 11 (61) | 5 (28) | 2 (11) | 0.1964 |
| 2.0> | 19 | 6 (32) | 9 (47) | 4 (21) | |
| Histology | |||||
| Well | 12 | 3 (25) | 7 (58) | 2 (17) | 0.3829 |
| Moderate | 15 | 8 (53) | 5 (33) | 2 (13) | |
| Poor | 10 | 6 (60) | 2 (20) | 2 (20) | |
| Depth of invasion | |||||
| T1,2 | 20 | 8 (40) | 8 (40) | 4 (20) | 0.6794 |
| T3,4 | 17 | 9 (53) | 6 (35) | 2 (12) | |
| Perineural invasion | |||||
| Negative | 5 | 3 (60) | 2 (40) | 0 (0) | 0.5507 |
| Positive | 32 | 14 (44) | 12 (38) | 6 (19) | |
| Lymph node metastasis | |||||
| Negative | 17 | 9 (53) | 8 (47) | 0 (0) | 0.0437 |
| Positive | 20 | 8 (40) | 6 (30) | 6 (30) | |
| Stage | |||||
| Ia–IIa | 16 | 8 (50) | 8 (50) | 0 (0) | 0.0557 |
| IIb | 21 | 9 (43) | 6 (29) | 6 (29) | |
Association of Snail Expression and CK19 and Vimentin Expression in Cholangiocarcinoma
Immunostaining of CK19 and vimentin was performed for the sections of cholangiocarcinoma, and the association with the Snail expression was examined. In the Snail-negative cases, strong immunohistochemical signals of CK19 were observed in cholangiocarcinoma cells, while the expression of vimentin was mostly negative. By contrast, the expression of CK19 tended to be reduced in cholangiocarcinoma cells in the Snail-positive cases, and in these cases, positive immunostaining of vimentin was occasionally observed. The images of representative cases are shown in Figure 7C.
Semiquantitative analysis of the results showed that the Snail expression significantly correlated with the reduction of CK19 expression in cholangiocarcinoma cells, and it also closely correlated with the positive immunoreactivity of vimentin (Figure 7D).
Relationship between Snail Expression and Clinicopathological Factors in Cholangiocarcinoma
The comparisons of Snail expression and clinicopathological factors in cholangiocarcinoma cases are summarized in Table 2. Nuclear expression of Snail was significantly associated with lymph node metastasis (P = 0.0437) and sex (P = 0.0152) with a female predominance.
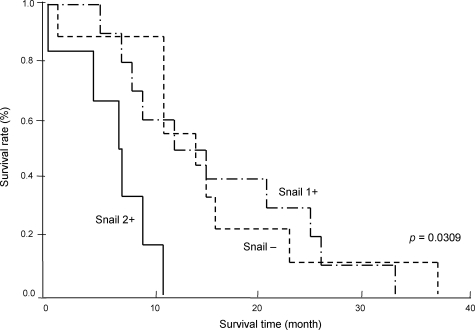
Postoperative follow-up data were available in 25 cases (15, male; 10, female). The median follow-up period was 14 months. Six patients with marked (2+) expression of Snail had a significantly poor prognosis, as compared with those with negative (−) (n = 9) and mild to moderate (1+) (n = 10) Snail expression (P = 0.0309) (Figure 8). Notably, five out of six cases with marked expression of Snail were female, although overall postoperative survival was not significantly associated with sex (P = 0.7544).
Figure 8.
Survival curve of cholangiocarcinoma cases in relation to Snail expression. Postoperative follow-up data were analyzed in 25 cholangiocarcinoma cases, and the association with the immunohistochemical expression of Snail was determined. Patients with marked (2+) immunohistochemical expression of Snail had a significantly poor prognosis, as compared with those with negative (−) and mild to moderate (1+) expression (P = 0.0309).
Discussion
This study demonstrated that EMT induced by TGF-β1/Snail activation is closely associated with the aggressive growth of cholangiocarcinoma. TGF-β1 induced mesenchymal phenotypes in cholangiocarcinoma cell lines, and increased the activity of invasive potential both in vitro and in vivo, which was accompanied by the induction of nuclear expression of Snail. The immunohistochemical expression of Snail correlated with the induction of vimentin expression in surgically resected cholangiocarcinoma cases, and also correlated significantly with the lymph node metastasis and a poor survival rate of the patients. Importantly, TGF-βsRII inhibited all of the characteristic changes of CCKS-1 and TFK-1 induced by TGF-β1, indicating that the blockage of TGF-β signaling pathways may be beneficial in patients with cholangiocarcinoma.
In contrast to the striking effects of TGF-βsRII in CCKS-1 and TFK-1, BMP-7 had no significant effects on the inhibition of EMT. BMP-7 counteracts EMT by antagonizing TGF-β, and has been shown to inhibit tumor metastasis in breast and prostatic cancer.13,27 Our recent studies using cultured microvascular endothelial cells also demonstrated that BMP-7 inhibited the phenotypic conversion of the endothelial cells (endothelial–mesenchymal transition) via TGF-β1/Smad activation.23 The results obtained in this study showed that the expression of pSmad2/3 was not affected by the treatment of TGF-β1 in vitro. These results indicate that the Smad signaling pathways do not necessarily play major roles in the induction of EMT by TGF-β1 in cholangiocarcinoma, and it is possible that EMT may be mediated by the Smad-independent pathways, including RHOA, Ras, phosphatidylinositol 3-kinase, and TGF-β-activated kinase 1.4
TGF-β-mediated growth inhibition and apoptosis can be correlated with its function as a tumor suppressor.28 However, our previous studies showed that TGF-β1 did not influence the proliferation of CCKS-1, and we identified that overexpression of cyclin D1 was an underlying causative mechanism of this phenomenon.29 Although the effects of TGF-β1 on apoptosis remain to be determined in cholangiocarcinoma, no influence on the cellular proliferative activity of CCKS-1 by TGF-β1 implies that TGF-β1 may not have apoptosis-inducing capacity. Therefore, the blockage of TGF-β signaling pathways may be able to reduce the activity of invasive potential of cholangiocarcinoma, which presumably does not activate and worsen cell proliferation.
Induction of COL1A1 expression in CCKS-1 and TFK-1 by TGF-β1 accounts for desmoplasia, which is usually seen in the stroma of conventional cholangiocarcinoma. A dynamic interaction likely exists between carcinoma cells and the host microenvironment to support cancer cell growth and invasion. Type I collagen seems to play important roles in the induction and maintenance of EMT. For example, colorectal carcinoma cells grown on type I collagen show an EMT-like phenotype and less cohesive morphology.30 During renal fibrosis, cell contact to type I collagen promotes EMT in renal tubular epithelial cells.31 From these observations, it is suggested that, in cholangiocarcinoma, TGF-β1 secreted by carcinoma cells, as well as stromal cells, induces type I collagen deposition in the stroma, which in turn may promote and/or enhance EMT.32 In these processes, MMPs including MMP-2 may also contribute greatly to the local extension of carcinoma cells.33,34
In this study, the immunohistochemical expression of Snail in cholangiocarcinoma was significantly associated with sex with a female predominance, and five out of six cases with marked (2+) expression of Snail were female. These results indicate that estrogens may relate to the occurrence of EMT in cholangiocarcinoma. Previous studies demonstrated that cholangiocarcinoma cells expressed receptors for estrogens, and estrogen modulated cancer cell growth.35,36 Snail transcription has been shown to be regulated by the estrogen receptor, and the estrogen receptor is an EMT inhibitor and is critical in maintaining the epithelial status of normal breast cells.3 In women after menopause, serum estradiol concentrations fall to values similar to or lower than those in men of similar age.37 Although this study dealt with a limited number of cases, our results suggest that the occurrence of EMT in cholangiocarcinoma may be associated with the serum estrogen concentration, and EMT tends to occur in patients with low concentration of serum estrogens, especially in the elderly women. In this regard, further study is necessary to determine whether a high risk group of cholangiocarcinoma exists in the elderly women, which are characterized by the increased expression of Snail and low serum estrogen levels.
The concept of EMT was originally defined in the cellular remodeling that occurs during development, and it implies complete transdifferentiation of the epithelial cells into mesenchymal cells. To describe the emergence of mesenchymal features during tumor progression, it has been proposed that the term, EMT-like phenotype, is more appropriate in most cases.38 In fact, the occurrence of complete EMT by TGF-β1 is a rare event in many carcinoma cell lines.39 In this context, the phenotypic alterations of cholangiocarcinoma induced by TGF-β1 observed in this study correspond to EMT-like changes rather than complete EMT; however, we used the term EMT to describe the phenotypic changes, because the definition of EMT is still in debate. Most carcinomas display phenotypic heterogeneity, probably resulting from tumor cell renewal and adaptation to specific microenvironment, and recently, it has been shown that EMT can generate cells with properties of stem cells.40 Thus, EMT and EMT-like changes seem to be involved in the complex networks of the mechanism underlining the heterogeneity of the tumors, and this may be also true in cholangiocarcinoma.
In summary, the present study provided evidence showing that human cholangiocarcinoma cells underwent EMT by TGF-β1/Snail activation, which was accompanied by the activation of invasive potential. The Snail expression significantly correlated with the lymph node metastasis and a poor survival rate of the patients, suggesting that EMT via TGF-β1/Snail activation aggravates invasive growth and metastasis of cholangiocarcinoma in vivo. The inhibition of EMT targeted for the TGF-β1/Snail pathways may improve the poor prognosis of the patients with cholangiocarcinoma.
Footnotes
Address reprint requests to Yasuni Nakanuma, M.D., Ph.D., Department of Human Pathology, Kanazawa University Graduate School of Medicine, 13-1 Takara-machi, Kanazawa 920-8640, Japan, E-mail: pbcpsc@kenroku.kanazawa-u.ac.jp.
Supported by Hokkoku Cancer Research Promotion Foundation, Hokkoku Bank, Kanazawa, Japan.
References
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail. Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K, Saitoh M. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J Biol Chem. 2009;284:245–253. doi: 10.1074/jbc.M804777200. [DOI] [PubMed] [Google Scholar]
- Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P, Cleton-Jansen AM, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clezardin P, Papapoulos SE, Cecchini MG, Lowik CW, van der Pluijm G. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- Usami Y, Satake S, Nakayama F, Matsumoto M, Ohnuma K, Komori T, Semba S, Ito A, Yokozaki H. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol. 2008;215:330–339. doi: 10.1002/path.2365. [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail. SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, Sheppard D, Oettgen P, Mercurio AM. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungert K, Buck A, von Wichert G, Adler G, Konig A, Buchholz M, Gress TM, Ellenrieder V. Sp1 is required for transforming growth factor-beta-induced mesenchymal transition and migration in pancreatic cancer cells. Cancer Res. 2007;67:1563–1570. doi: 10.1158/0008-5472.CAN-06-1670. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129:1375–1383. doi: 10.1053/j.gastro.2005.09.055. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Yu GR, Yoo HJ, Kim JH, Yoon BI, Choi YK, Kim DG. ANXA8 Down-regulation by EGF-FOXO4 signaling is involved in cell scattering and tumor metastasis of cholangiocarcinoma. Gastroenterology. 2009;137:1138–1150. doi: 10.1053/j.gastro.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Sato Y, Harada K, Ozaki S, Furubo S, Kizawa K, Sanzen T, Yasoshima M, Ikeda H, Sasaki M, Nakanuma Y. Cholangiocytes with mesenchymal features contribute to progressive hepatic fibrosis of the polycystic kidney rat. Am J Pathol. 2007;171:1859–1871. doi: 10.2353/ajpath.2007.070337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Sato Y, Ikeda H, Isse K, Ozaki S, Enomae M, Ohama K, Katayanagi K, Kurumaya H, Matsui A, Nakanuma Y. Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol. 2009;217:654–664. doi: 10.1002/path.2488. [DOI] [PubMed] [Google Scholar]
- Sugawara H, Yasoshima M, Katayanagi K, Kono N, Watanabe Y, Harada K, Nakanuma Y. Relationship between interleukin-6 and proliferation and differentiation in cholangiocarcinoma. Histopathology. 1998;33:145–153. doi: 10.1046/j.1365-2559.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- Kitao A, Sato Y, Sawada-Kitamura S, Harada K, Sasaki M, Morikawa H, Shiomi S, Honda M, Matsui O, Nakanuma Y. Endothelial to mesenchymal transition via transforming growth factor-beta1/Smad activation is associated with portal venous stenosis in idiopathic portal hypertension. Am J Pathol. 2009;175:616–626. doi: 10.2353/ajpath.2009.081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, McMurray DN. Effects of modulating TGF-β1 on immune responses to mycobatrerial infection in guinea pigs. Tubercle and Lung Dis. 1999;79:207–214. doi: 10.1054/tuld.1998.0198. [DOI] [PubMed] [Google Scholar]
- Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S, Niwa H, Mitsui T, Asada Y, Miura S, Ohta T, Nakanuma Y. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–1343. doi: 10.1002/hep.21387. [DOI] [PubMed] [Google Scholar]
- Sobin LH, Wittekind C. New York: Wiley-Liss,; Extrahepatic bile ducts. International Union Against Cancer (UICC). TNM Classification of Malignant Tumors, (6th Ed.) 2002:pp 87–89. [Google Scholar]
- Buijs JT, Rentsch CA, van der Horst G, van Overveld PG, Wetterwald A, Schwaninger R, Henriquez NV, Ten Dijke P, Borovecki F, Markwalder R, Thalmann GN, Papapoulos SE, Pelger RC, Vukicevic S, Cecchini MG, Lowik CW, van der Pluijm G. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol. 2007;171:1047–1057. doi: 10.2353/ajpath.2007.070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- Zen Y, Harada K, Sasaki M, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Intrahepatic cholangiocarcinoma escapes from growth inhibitory effect of transforming growth factor-beta1 by overexpression of cyclin D1. Lab Invest. 2005;85:572–581. doi: 10.1038/labinvest.3700236. [DOI] [PubMed] [Google Scholar]
- Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. 2009;101:320–326. doi: 10.1038/sj.bjc.6605143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira S, Itatsu K, Sasaki M, Harada K, Sato Y, Zen Y, Ishikawa A, Oda K, Nagasaka T, Nimura Y, Nakanuma Y. Local balance of transforming growth factor-beta1 secreted from cholangiocarcinoma cells and stromal-derived factor-1 secreted from stromal fibroblasts is a factor involved in invasion of cholangiocarcinoma. Pathol Int. 2006;56:381–389. doi: 10.1111/j.1440-1827.2006.01982.x. [DOI] [PubMed] [Google Scholar]
- Itatsu K, Sasaki M, Yamaguchi J, Ohira S, Ishikawa A, Ikeda H, Sato Y, Harada K, Zen Y, Sato H, Ohta T, Nagino M, Nimura Y, Nakanuma Y. Cyclooxygenase-2 is involved in the up-regulation of matrix metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis factor-alpha. Am J Pathol. 2009;174:829–841. doi: 10.2353/ajpath.2009.080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itatsu K, Zen Y, Yamaguchi J, Ohira S, Ishikawa A, Ikeda H, Sato Y, Harada K, Sasaki M, Sakamoto H, Nagino M, Nimura Y, Ohta T, Nakanuma Y. Expression of matrix metalloproteinase 7 is an unfavorable postoperative prognostic factor in cholangiocarcinoma of the perihilar, hilar, and extrahepatic bile ducts. Hum Pathol. 2008;39:710–719. doi: 10.1016/j.humpath.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S, Onetti Muda A, Dostal DE, De Santis A, Attili AF, Benedetti A, Gaudio E. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877–888. doi: 10.2353/ajpath.2006.050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, Francis H, Onori P, Franchitto A, Ginanni-Corradini S, Gaudio E, Alvaro D. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2009;41:156–163. doi: 10.1016/j.dld.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KA, Aakre ME, Gorska AE, Price JO, Eltom SE, Pietenpol JA, Moses HL. Induction by transforming growth factor-beta1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 2004;6:R215–231. doi: 10.1186/bcr778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]