Abstract
In the spring of 2009, a novel influenza A (H1N1) virus emerged in North America and spread worldwide to cause the first influenza pandemic since 1968. During the first 4 months, over 500 deaths in the United States had been associated with confirmed 2009 pandemic influenza A (H1N1) [2009 H1N1] virus infection. Pathological evaluation of respiratory specimens from initial influenza-associated deaths suggested marked differences in viral tropism and tissue damage compared with seasonal influenza and prompted further investigation. Available autopsy tissue samples were obtained from 100 US deaths with laboratory-confirmed 2009 H1N1 virus infection. Demographic and clinical data of these case-patients were collected, and the tissues were evaluated by multiple laboratory methods, including histopathological evaluation, special stains, molecular and immunohistochemical assays, viral culture, and electron microscopy. The most prominent histopathological feature observed was diffuse alveolar damage in the lung in all case-patients examined. Alveolar lining cells, including type I and type II pneumocytes, were the primary infected cells. Bacterial co-infections were identified in >25% of the case-patients. Viral pneumonia and immunolocalization of viral antigen in association with diffuse alveolar damage are prominent features of infection with 2009 pandemic influenza A (H1N1) virus. Underlying medical conditions and bacterial co-infections contributed to the fatal outcome of this infection. More studies are needed to understand the multifactorial pathogenesis of this infection.
In April 2009, novel influenza A (H1N1) virus infection was first reported in two US children,1 followed by identification of cases with acute respiratory illness caused by the identical virus infection in Mexico.2 Global transmission of 2009 influenza A (H1N1) [2009 H1N1] virus led to the first influenza pandemic since 1968. Although most case-patients of 2009 H1N1 had mild-to-moderate illness, severe and fatal disease has been reported,3,4,5 with an estimated mortality of 0.048% among symptomatic US cases.6
In previous influenza pandemics, studies of autopsy specimens revealed histopathological findings of bronchitis, thrombosis, interstitial inflammation, hyaline membrane formation, and various degrees of intra-alveolar edema, hemorrhage, and inflammation.7,8,9,10 In contrast, studies of fatal seasonal influenza cases illustrated that viral localization is primarily in major airways, with rare involvement of alveolar cells and lung parenchyma.11,12 Several studies of previous pandemics concluded that the majority of deaths were likely due to viral infection concurrent with bacterial pneumonia.10,13 A few histopathological and immunohistologic studies of fatal 2009 H1N1 cases have been recently described.14,15,16,17 In this report, we describe some of the unique clinicopathologic and epidemiological aspects of a large number of US deaths associated with 2009 H1N1 virus infection. We performed histopathological, immunohistochemical (IHC), and other laboratory methods to study viral distribution and cellular localization and to provide further insights into the pathogenesis of this disease.
Materials and Methods
Patient Characteristics and Specimens
This study includes autopsy specimens from confirmed 2009 H1N1 case-patients submitted to the Infectious Diseases Pathology Branch, Centers for Disease Control and Prevention (CDC), for evaluation from May 1 to October 1, 2009. A confirmed case of 2009 H1N1 was defined as a patient with influenza-like illness and 2009 H1N1 virus infection confirmed by real-time RT-PCR (rRT-PCR) of either pre- or postmortem respiratory specimens at outside laboratories or CDC. Demographic data, laboratory test results for influenza virus and bacteria, and other relevant clinical information were collected from medical records and preliminary autopsy reports when available. Tissues were received as unfixed fresh or frozen, fixed in formalin, or as formalin-fixed, paraffin-embedded (FFPE) blocks. Autopsy specimens from 202 deaths were received by the Infectious Diseases Pathology Branch, and 104 of these were confirmed as described above. Four case-patients who did not have sufficient representative respiratory tissues available were excluded from the evaluation. Nonrespiratory tissues were evaluated from some case-patients, including heart (n = 30), liver (n = 23), brain (n = 19), kidney (n = 14), spleen (n = 12), gastrointestinal tract (n = 10), muscle (n = 4), and pancreas (n = 4).
Nucleic Acid Extraction
Nucleic acid extracts were prepared by using either fresh or frozen tissue samples or FFPE tissues. Briefly, for fresh or frozen tissues, a 1 to 3 mm3 portion of tissue was minced, and nucleic acids were extracted per protocol by using the RNeasy Mini Kit (Qiagen, Valencia, CA) for RNA and EZ1 DNA Tissue Mini Kit for DNA. For FFPE sections, the EZ1 RNA, DNA Tissue Mini Kits, MagAttract RNA, or DNA Tissue Mini M48 Kits (Qiagen) were used.
RT-PCR Assays for Influenza A Virus
The CDC rRT-PCR Protocol for Detection and Characterization of Swine Influenza, (http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/index.html, last accessed May 11, 2010), which universally detects influenza A viruses, swine influenza A viruses, and swine H1 influenza, was used to determine whether respiratory tissue samples from all case-patients and select nonrespiratory tissues were positive for 2009 H1N1. RNase P or β2-microglobulin gene targets were used as an internal control to ensure effective RNA extraction. In cases where the samples were identified as influenza A/unsubtypeable using this assay, primers and probes from a recently published assay that discriminates seasonal H1 from pandemic H1 in a single tube were used with modified cycling conditions.18
PCR Assays for Additional Respiratory Viruses
PCR assays for respiratory syncytial virus, parainfluenza viruses, and adenovirus were also performed by using the extracted nucleic acids.
PCR Assays for Bacterial Agents
The DNA extracts from respiratory tissues from all case-patients were evaluated with a broad-range eubacterial PCR assay targeting the 16S rRNA gene.19 Conventional single stage or nested PCR assays for Streptococcus pneumoniae targeting the pneumolysin gene20; Streptococcus pyogenes targeting the Streptococcal pyrogenic exotoxin B (speB) gene21; and Haemophilus influenzae targeting the outer membrane protein P6 gene22 were performed by using DNA from select case-patients. Amplified PCR products were sequenced on a CEQ 8000 automated sequencer (Beckman Coulter, Fullerton, CA) for confirmation. DNA extracts from select cases were tested for methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) with two real-time multiplex PCR assays: (1) S. aureus toxin genes PCR assay targets Panton-Valentine Leukocidin gene and toxic shock syndrome toxin 1 gene; (2) PCR assay, which detects mecA (exogenous gene encoding methicillin resistance in staphylococci), nuc (heat stable DNA nuclease, specific for S. aureus), and femA (factor essential for methicillin resistance, specific for Staphylococcus epidermidis) genes.23 The presence of amplifiable DNA in all extracts was verified by amplification of human house-keeping genes β-globin and glyceraldehyde-3-phosphate dehydrogenase.
Histopathological Evaluation
Routine H&E stains of available major organs were evaluated for histopathological changes. Lillie-Twort Gram stain and Warthin-Starry silver impregnation stain were performed on selected sections of all case-patients to examine for the presence of bacterial infection.
IHC Assays
IHC assays were performed by using a polymer-based colorimetric indirect immunoalkaline phosphatase method. Respiratory and available nonrespiratory tissues from all case-patients were evaluated with a monoclonal antibody against the nucleoprotein of influenza A virus.24 Select respiratory sections from all case-patients were evaluated with a polyclonal antibody against Group A Streptococcus (S. pyogenes)21; a polyclonal antibody against S. pneumoniae25; a polyclonal antibody against H. influenzae; and a monoclonal antibody against S. aureus. The antibody/polymer conjugate was visualized by applying UltraVision LP system with Napthol Phosphate Substrate (Thermo Scientific/Lab Vision) to tissue sections. Negative controls consisted of sequential tissue sections incubated with normal serum pertinent to the primary antibody. Double-stain IHC was performed by using peroxidase polymer-labeled antibodies (Dako, Carpinteria, CA) against cytokeratin, surfactant, or CD68, followed by the mouse anti-influenza A nucleoprotein antibody labeled with immunoalkaline phosphatase polymer.26
Electron Microscopic Examination
Formalin fixed tissues or FFPE sections from five cases with duration of illness <7 days and abundant immunostaining were processed for transmission electron microscopy as previously described.27,28
Viral Culture
Confluent monolayers of MDCK cells grown in Dulbecco’s modified Eagle’s medium were inoculated with fresh respiratory tissue and monitored daily for cytopathic effect. Immunofluorescence testing by using the monoclonal antibody against nucleoprotein was performed when cytopathic effect was identified or 8 days postinoculation if no cytopathic effect was observed.
Results
Patient Characteristics
One hundred confirmed case-patients with fatal 2009 H1N1 virus infection were evaluated at the Infectious Diseases Pathology Branch during May 12 to October 1, 2009. Fifty-three (53%) of these case-patients had 2009 H1N1 confirmed by postmortem specimen testing. The median age of fatal case-patients was 36 years (range, 2 months to 84 years), 80% were aged 20 to 60 years, and 51 (51%) were male patients (Table 1). The majority (85%) of case-patients with known previous medical history had at least one underlying comorbidity. Obesity (46%), cardiovascular disease (25%), and asthma (22%) were the three most frequent conditions reported. Fever (82%), cough (67%), and shortness of breath (58%) were the most common signs and symptoms reported, with a median duration from illness onset to death of 8 days (range, 1 to 44 days). Fifty-eight (67%) of 87 case-patients with available clinical history were hospitalized before death. Forty-two (74%) of 57 case-patients with available hospital records required mechanical ventilation. Radiographical diagnosis of pneumonia was documented in 59% (38 of 64) of case-patients.
Table 1.
Characteristics of Confirmed 2009 H1N1 Case-Patients with Autopsy Samples Evaluated for Pathologic Features
| Characteristic | No. (%) |
|---|---|
| Male patients | 51 (51) |
| Age group | |
| 0–9 | 4 (4) |
| 10–19 | 13 (13) |
| 20–29 | 17 (17) |
| 30–39 | 24 (24) |
| 40–49 | 22 (22) |
| 50–59 | 17 (17) |
| ≥60 | 3 (3) |
| Medical history (n = 93) | |
| At least one pre-existing condition | 79 (85) |
| Obesity* | 43 (46) |
| Extreme obesity* | 16 (17) |
| Cardiovascular disease | 23 (25) |
| Asthma | 20 (22) |
| Diabetes | 12 (13) |
| Pregnancy | 6 (6) |
| HIV infection | 4 (4) |
| Duration of illness, median days (range) | 8 (1–44 days) |
| Clinical symptoms (n = 72) | |
| Fever | 59 (82) |
| Cough | 48 (67) |
| Shortness of breath | 42 (58) |
| Headache | 11 (15) |
| Fatigue/weakness | 10 (14) |
| Sore throat | 11 (15) |
| Vomiting | 16 (22) |
| Diarrhea | 8 (11) |
| Hospitalized (n = 87) | 58 (67) |
| Invasive mechanical ventilation (n = 57) | 42 (74) |
| Antiviral treatment (n = 67) | 44 (66) |
Obesity is defined as body mass index ≥30 and extreme obesity as body mass index ≥40.
Histopathological Findings
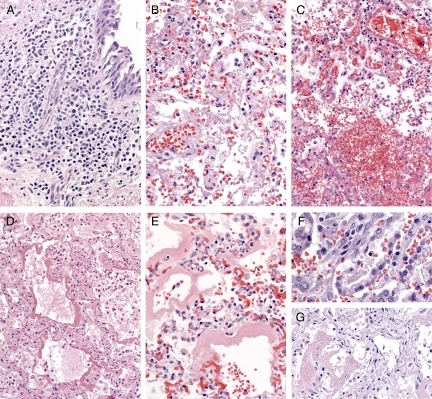
Table 2 presents the histopathological features observed in airways and lungs. Eighty-five case-patients had airway tissues available for evaluation. The most frequent histopathological findings in airways were inflammation and edema (66%). The inflammation was usually mild and consisted predominantly of mononuclear cells (Figure 1A). Necrosis of epithelium (26%) and hemorrhage (18%) were less frequently observed. Lung tissues in all case-patients showed a spectrum of histopathological changes of diffuse alveolar damage (DAD), including edema, hyaline membranes, inflammation, and fibrosis (Figure 1, B–G). The nature and extent of DAD generally corresponded to the duration of clinical illness of the patients. Forty-one case-patients had paratracheal or hilar lymph nodes available for evaluation, and hemophagocytosis was noted in 25 (61%) of these case-patients. Pulmonary thromboemboli were noted in gross autopsy findings or microscopically in 17 case-patients. No histopathological evidence of myocarditis or encephalitis was observed in any of the case-patients with heart (n = 30) or brain samples (n = 19) available for evaluation. Histopathological findings in other organs were nonspecific and likely associated with the patients’ underlying medical conditions. These findings included prominent eosinophils in patients with history of asthma, enlarged nuclei of cardiac myocytes in patients with history of hypertension, and fatty metamorphosis in the liver in obese patients.
Table 2.
Histopathological Features in Respiratory Tract Specimens of 2009 H1N1 Fatal Case-Patients (N = 100)
| Histopathologic feature | No. (%) of case-patients with feature |
|---|---|
| Trachea and bronchi (n = 85) | |
| Inflammation and edema | 56 (66) |
| Necrosis | 22 (26) |
| Hemorrhage | 15 (18) |
| Lung* (n = 100) | |
| Edema | 63 (63) |
| Hyaline membranes | 59 (59) |
| Fibrin | 58 (58) |
| Hemorrhage | 58 (58) |
| Inflammation | 48 (48) |
| Type II pneumocyte hyperplasia | 46 (46) |
| Neutrophilic bronchopneumonia | 29 (29) |
| Organizing fibrosis | 28 (28) |
| Squamous metaplasia | 12 (12) |
Edema, fibrin, and hemorrhage were seen in alveolar spaces; inflammation and organizing fibrosis primarily involved interstitium but were also focally seen within alveolar spaces.
Figure 1.
Histopathological findings in airway and lung; H&E staining. Original magnification: ×50 (A, B, E, and G); ×25 (C and D); ×100 (F). A: Inflammation in the airway is usually mild and predominantly composed of mononuclear cells. B: The lungs show various phases of diffuse alveolar damage, including fibrinous inflammation, intra-alveolar hemorrhage (C), intra-alveolar edema and hyaline membrane formation (D and E), type II pneumocytes hyperplasia (F), and organizing fibrosis (G).
Viral Localization and Cellular Targets
The IHC assay results for influenza A were positive in respiratory tissues from 44 of the 100 case-patients (44%); the amount of influenza virus antigen varied, with abundant immunostaining in nine case-patients and rare in 24 case-patients. Viral nucleoprotein antigens were localized in the nuclei and cytoplasm of infected cells, including epithelial cells in airways (Figure 2A), submucosal glands (Figure 2B), and pneumocytes, either detached or lining alveoli (Figure 2, C–E). Antigens were also seen in association with hyaline membranes (Figure 2F) and in endothelial cells in rare cases (Figure 2G). Double staining revealed that the major cellular targets of viral infection were pneumocytes (co-labeled with cytokeratin; Figure 2H), predominantly type II (co-labeled with surfactant; Figure 2I), and occasionally macrophages (co-labeled with CD68; Figure 2J). No immunostaining of influenza A viral antigen was detected in any of the nonrespiratory tissue samples available. Influenza rRT-PCR testing on a limited number of these nonrespiratory samples was also negative. Electron microscopic examination of lung tissue identified rare infected cells with extracellular virus particles in the alveolar space (Figure 2K). Virions were round to oblong-shaped and averaged 88 nm in diameter; some particles were surrounded by spikes approximately 12 nm in length (Figure 2L).
Figure 2.
Immunolocalization of 2009 H1N1 influenza viral antigens and ultrastructural features: immunoalkaline phosphate staining, naphthol fast red substrate with light hematoxylin counterstain (A–G); double immunostaining with immunoperoxidase and immunoalkaline phosphatase, naphthol fast red substrate with light hematoxylin counterstain (H–J); and thin-sectioned electron microscopy (K and L). Original magnification: ×50 (A, D, and F); ×25 (B); ×12.5 (C); ×100 (E and G); ×158 (H–J). Scale bars = 500 nm (K); 100 nm (L). A: Viral antigens are detected in the nuclei of infected airway epithelial cells. B: Viral antigens are seen in submucosal glands. C–E: Viral antigens are predominantly present in the nuclei of alveolar lining cells, including type I and type II pneumocytes; many of them have detached and are in the alveolar space. F: Immunostaining of viral antigens is seen in hyaline membranes lining alveoli. G: Viral antigens are located in endothelial cells. H and I: Double staining reveals influenza viral antigens (brown) are predominantly in pneumocytes (red, co-labeled with cytokeratin [H]), specifically in type II pneumocytes (red, co-labeled with surfactant [I]). Viral antigen (brown) can also be seen in occasional macrophages (red, co-labeled with CD68 [J]). K: Electron micrograph showing infected cell (asterisk) with extracellular virus particles, associated with dense material. L: Virions consist of internal nucleocapsids surrounded by an envelope with surface projections.
Bacterial and Viral Co-Infections
Overall, 26 (26%) case-patients had confirmatory test results of bacterial co-infection. Twenty-nine case-patients showed histopathological evidence of bronchopneumonia with prominent alveolar polymorphonuclear cells, indicating a possibility of bacterial co-infection (Figure 3A, D, and G). Of these, 22 (76%) case-patients had a specific bacterial pathogen identified. Bacterial agents were identified in an additional four case-patients that did not show histopathological evidence of bronchopneumonia in the tissue sections examined. Gram-positive cocci were the most frequent bacteria identified by using special stains (Figure 3C and F). IHC and PCR assays were positive as follows: nine case-patients positive for S. pneumoniae (Figure 3B); three case-patients for S. pyogenes (Figure 3E); one case-patient for both S. pyogenes and S. pneumoniae; one case-patient for both S. pyogenes and S. mitis; one case-patient for S. mitis; one case-patient for S. agalactiae; four case-patients for MRSA (Figure 3H); one case-patient for both MRSA and S. pyogenes; one case-patient for both MRSA and H. influenzae; and four case-patients for MSSA. None of the case-patients were found to have evidence of a co-infection with respiratory syncytial virus, parainfluenza viruses 1 to 3, or adenovirus.
Figure 3.
Bacterial co-infections detected with histopathology, special stains, and IHC. A, D, and G: H&E staining; B, E, and H: immunoalkaline phosphate staining, naphthol fast red substrate with light hematoxylin counterstain; C and F: Lilly-Twort tissue Gram staining. Original magnification: ×25 (A, D, and G); ×100 (B and E); ×158 (C and F); ×50 (H). A: Abundant neutrophilic infiltrate in the alveoli indicative of an acute pneumonic process. B: Extracellular and intracellular immunostaining of S. pneumoniae antigens. C: Gram-positive cocci in the serial section as B. D: Abundant neutrophilic infiltrate in the alveoli of another patient with pneumonia. E: Extracellular and intracellular immunostaining of Group A Streptococcus antigens. F: Gram-positive cocci in the serial section as E. G: Prominent necrosis and neutrophilic infiltrate indicative of a necrotizing pneumonia. H: Abundant immunostaining of S. aureus.
Concordance of Diagnostic Test Results
Of the 100 case-patients with confirmed 2009 H1N1, testing of respiratory tissue by rRT-PCR assays at CDC were positive for influenza A virus in 90, including 87 for H1N1 and 3 nonsubtypeable virus (Table 3). Based on available records for 80 case-patients with known duration of illness, rRT-PCR results were positive for 2009 H1N1 in respiratory tissue specimens of 42 (53%) case-patients with illness duration <10 days and in 28 case-patients (35%) with illness >10 days when death occurred. Negative rRT-PCR results for 2009 H1N1 were not observed in any case-patients with known illness duration <10 days, but results were negative in seven case-patients (9%) with duration >10 days. In these same 80 case-patients with known duration of illness, positive IHC results for influenza A viral antigen were observed in respiratory tissues of 31 case-patients (39%) with illness duration <10 days and in five case-patients (6%) with illness >10 days. In contrast, negative IHC results for influenza A were observed in respiratory tissues of 14 case-patients (18%) with time from onset to death <10 days and in 31 case-patients (39%) who died after an illness >10 days. The 2009 H1N1 virus was isolated from fresh lung tissue specimens in 5 of 30 case-patients tested.
Table 3.
Diagnostic Results for Respiratory Tissues Evaluated from 100 Fatal Cases of 2009 H1N1
| Influenza A
|
Bacterial co-infections
|
Total no. of case-patients | ||
|---|---|---|---|---|
| RT-PCR* | IHC | PCR | IHC | |
| + | − | − | − | 33 |
| + | + | − | − | 29 |
| + | + | + | + | 14 |
| + | − | + | + | 10 |
| + | + | − | + | 1 |
| A/unsub | − | + | + | 2 |
| A/unsub | − | − | − | 1 |
| − | − | − | − | 8 |
| − | − | + | + | 1 |
| − | − | − | + | 1 |
| Total | 100 | |||
Of the 100 case-patients included in this study, 90 were positive for influenza A in respiratory tissue evaluated, either for 2009 H1N1 (+) or nonsubtypeable (A/unsub). The 10 case-patients that were PCR negative (−) were confirmed by outside laboratory results from clinical samples.
Discussion
This report presents pathological studies on autopsy samples from 100 patients with fatal 2009 H1N1 virus infection that occurred between May and October 2009 in the United States. Histopathological evaluations revealed DAD as the most significant and consistent finding, and immunolocalization showed viral antigens predominantly in the lung parenchyma. This is somewhat similar to the pattern observed in fatal case-patients of highly pathogenic avian influenza (H5N1).29,30,31,32 However, a significant proportion of 2009 H1N1 case-patients in this report also showed viral localization along with inflammation or other histopathological changes in trachea, bronchi, or bronchioles (Table 2), a pattern more commonly seen in severe or fatal cases of seasonal influenza.11 Lung parenchyma had a remarkably high amount of viral antigen observed in close association with DAD when compared with areas with less histopathological damage. The amount of viral antigen was variable and was more abundant in case-patients with a shorter duration of illness; this may reflect clearance of viral antigens by host immune responses later in the clinical course.
Our results indicate that 2009 H1N1 virus can target the lower respiratory tract, resulting in DAD, as manifested clinically by severe adult respiratory distress syndrome with refractory hypoxemia.5,16,33,34 These findings reinforce current recommendations to initiate early empirical neuraminidase inhibitor treatment of hospitalized patients with suspected 2009 H1N1 infection.35 The viral immunolocalizations in lower airway and lung parenchyma are in agreement with studies of receptor binding demonstrating the ability for 2009 H1N1 virus to target both upper and lower respiratory tract tissue.36,37,38,39 The differential presence of receptors and nature of the virus may account, at least in part, for the dissimilarity of histopathological changes and viral antigen distribution among seasonal influenza A, avian influenza A (H5N1), and 2009 H1N1 viral infections.36,40,41,42
The cellular localization of viral antigens was primarily in pneumocytes, especially type II, and was demonstrated by double immunostaining for influenza virus and cell markers, such as surfactant and cytokeratin. Many of these antigen-laden pneumocytes were seen detached in alveolar spaces. Morphologically these can be extremely difficult to distinguish from alveolar macrophages, but double staining confirmed their identity as pneumocytes. Type II pneumocytes play an important role in tissue restitution after acute lung injury and are known to secrete pulmonary surfactants. Surfactants reduce surface tension to preserve the integrity of alveolar space and also play important roles in modulating inflammation and enhancing pathogen clearance.43 In general, immunolocalization of viral antigens does not indicate active viral replication; however, nuclear immunostaining is highly suggestive of the replication process. Furthermore, the finding of viral antigen within pneumocytes and in association with hyaline membranes suggests a direct viral cytopathic effect as a major pathogenic mechanism in this disease.
Besides the primary viral pneumonia, other factors, such as underlying medical conditions and bacterial co-infections may also contribute to DAD observed with severe 2009 H1N1 virus infection. The vast majority of fatal case-patients assessed in this study with available medical history had underlying medical conditions. Obesity (body mass index ≥ 30) was the most significant associated underlying condition (Table 1), and 17% of case-patients were extremely obese (body mass index ≥ 40). Obesity is associated with several disorders including obstructive sleep apnea and is a risk factor for the development of asthma, deep vein thrombosis, pulmonary embolism, pulmonary hypertension, and pneumonia. There is also a clear association between obesity and prevalence of metabolic disorders; however, very little is known about the effect of obesity on immune function, especially during viral infection. Smith et al44 reported that diet-induced obese mice were more susceptible to morbidity and mortality with influenza virus infection than lean mice. In this model, obesity is postulated to interfere with cellular responses to influenza virus infection, leading to selective impairment in dendritic cell function and alterations in T-cells, but the impact on the human immune response is unknown. Other medical conditions, such as asthma, cardiovascular diseases, diabetes, and pregnancy, which were prevalent among case-patients, have all been associated with a higher morbidity and mortality with influenza.45,46,47 Further studies are needed to elucidate the pathophysiologic effect of obesity and other medical conditions on patients with 2009 H1N1 virus infection.
Co-infections may also affect the nature and severity of clinical manifestations and disease outcome. There was no evidence of other common respiratory viral infections in this case series; however, these results may be biased by the seasonality of these viruses. On the other hand, bacterial pneumonia was present in 29% of case-patients in this series. Bacterial co-infections in severe and fatal influenza case-patients have been well documented in previous influenza pandemics and in seasonal epidemics.8,10,11 As previously reported,11 it is often difficult to correlate bacterial culture results with clinicopathologic features due to confounding issues, such as sampling, postmortem contamination, culture techniques, and antibiotic treatment. IHC assays performed by using FFPE tissues allow identification of specific respiratory bacterial pathogens in lung tissues with bronchopneumonia and confirmed by paneubacterial 16S rRNA PCR and agent-specific PCR assays. Bacterial co-infections with S. pneumoniae and S. aureus were the most frequent identified in this study. The prevalence of invasive S. aureus, either MRSA or MSSA, was high among younger patients. For 20 of 26 case-patients where bacteria were identified from a tissue sample and duration of illness could be determined, 16 case-patients (80%) had duration less than 10 days and four case-patients (20%) more than 10 days, suggesting a correlation between bacterial co-infections and shorter duration of illness before death. Our results strongly support, in addition to antiviral medications, managing influenza patients with suspected bacterial pneumonia with empirical antibacterial therapy.48 Additionally, expanded efforts are indicated to improve pneumococcal vaccine coverage among targeted risk groups.49
There was no evidence of myocardial inflammation in any case-patient, although myocarditis is associated with seasonal influenza11,50 and is likely to occur in 2009 H1N1 patients. Histopathological findings in nonrespiratory tissues were nonspecific and most likely due to underlying conditions. Although viral antigens were seen in rare endothelial cells in lung and most likely represent phagocytosed antigens, no evidence of virus was seen outside of the respiratory system. Hemophagocytosis was present in over half of the case-patients with lymph nodes available for evaluation. Hemophagocytosis is a nonspecific histopathological finding that can be observed in many infectious diseases, including influenza, and may be triggered by cytokines.32,51,52 In severe H5N1 virus infection, high viral replication in the lower respiratory tract is associated with cytokine dysregulation.53 Several studies of natural and experimental influenza infections demonstrated correlation between symptoms and elevated cytokines, including interleukin-4, interleukin-6, and tumor necrosis factor-α, in plasma or nasopharyngeal specimens in the absence of viremia.30,54 Some systemic symptoms and complications of 2009 H1N1, such as encephalopathy or encephalitis, may be a result of cytokine dysregulation and not direct viral invasion of extrapulmonary tissues.
Many respiratory viral infections can cause DAD, especially at the end stage of critical illness. Some of these viruses, such as adenoviruses, human herpesviruses, and respiratory syncytial virus, may show distinct cytopathic effects or inclusions in the lung. Because these distinct features are not observed with influenza virus infection, a combination of clinical, epidemiological, and laboratory data are necessary to diagnose influenza virus infection in fatal cases. In this series, confirmatory laboratory diagnosis was established primarily by rRT-PCR, and confirmation of 2009 H1N1 was obtained only by postmortem evaluation in over half of the case-patients. This underscores the important role of medical examiners, coroners, and pathologists in infectious disease surveillance.
In summary, the most prominent histopathological feature observed in 2009 H1N1 deaths is diffuse alveolar damage associated with viral antigen localization in type II pneumocytes and alveolar lining cells. Bacterial co-infections also contributed to the severity of 2009 H1N1 infection. Additionally, the severe or fatal outcome of 2009 H1N1 infection may be attributable to unknown immunological factors and underlying medical conditions, such as obesity and asthma. More studies, including building on experimental animal studies,37,38,55,56 are clearly needed to assess other potential host and genetic risk factors to further characterize the pathogenesis and to determine effective treatment strategies for severe illness caused by 2009 H1N1 virus infection.
Acknowledgments
We greatly acknowledge all state and local public health departments, state and local public health laboratories, and all pathologists and medical examiners who submitted specimens to the Infectious Diseases Pathology Branch. We also thank Dr. Nancy Cox and other colleagues in the Influenza Division, CDC, for helpful discussions and critical review of the article.
Footnotes
Address reprint requests to Wun-Ju Shieh, M.D., Ph.D., Centers for Disease Control and Prevention, 1600 Clifton Road, MS G-32, Atlanta, GA 30333. E-mail: wbs9@cdc.gov.
See related Commentary on page 13
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- Echevarria-Zuno S, Mejia-Arangure JM, Mar-Obeso AJ, Grajales-Muniz C, Robles-Perez E, Gonzalez-Leon M, Ortega-Alvarez MC, Gonzalez-Bonilla C, Rascon-Pacheco RA, Borja-Aburto VH. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet. 2009;374:2072–2079. doi: 10.1016/S0140-6736(09)61638-X. [DOI] [PubMed] [Google Scholar]
- Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States. April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, Samuel MC, Rosenberg J, Talarico J, Hatch D. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- Miller RR, 3rd, Markewitz BA, Rolfs RT, Brown SM, Dascomb KK, Grissom CK, Friedrichs MD, Mayer J, Hirshberg EL, Conklin J, Paine R, 3rd, Dean NC. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A (H1N1) infection. Chest. 2010;137:752–758. doi: 10.1378/chest.09-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presanis AM, Lipsitch MA, De Angelis D, Hagy A, Reed C, Riley S, Cooper B, Swine Flu Investigation Team, New York City Department of Health and Mental Hygiene The severity of pandemic H1N1 influenza in the United States, April–July 2009. PLoS Curr Influenza. 2009:RRN1042. doi: 10.1371/currents.RRN1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers JF, Mulder J. Broad aspects of the pathology and pathogenesis of human influenza. Am Rev Respir Dis. 1961;83:84–97. doi: 10.1164/arrd.1961.83.2P2.84. [DOI] [PubMed] [Google Scholar]
- Louria DB, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. Studies on influenza in the pandemic of 1957–1958. II Pulmonary complications of influenza. J Clin Invest. 1959;38:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseasohn R, Adelson L, Kaji M. Clinicopathologic study of thirty-three fatal cases of Asian influenza. N Engl J Med. 1959;260:509–518. doi: 10.1056/NEJM195903122601101. [DOI] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J, Paddock CD, Shieh WJ, Packard MM, Patel M, Montague JL, Uyeki TM, Bhat N, Balish A, Lindstrom S, Klimov A, Zaki SR. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine. 2008;26 Suppl 4:D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay MI, Jr, Herrmann EC, Jr, Morrow GW, Jr, Brown AL., Jr Hong Kong influenza: clinical, microbiologic, and pathologic features in 127 cases. JAMA. 1970;214:1825–1832. doi: 10.1001/jama.214.10.1825. [DOI] [PubMed] [Google Scholar]
- Gill J, Sheng ZM, Ely SF, Guinee DGJ, Beasley MB, Suh J, Deshpande C, Mollura DJ, Morens DM, Bray M, Travis WD, Taubenberger JK. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:E1–E9. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, Galas FR, Alves VA, Malheiros DM, Auler JO, Jr, Ferreira AF, Borsato MR, Bezerra SM, Gutierrez PS, Caldini ET, Pasqualucci CA, Dolhnikoff M, Saldiva PH. Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med. 2010;181:72–79. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- Soto-Abraham MV, Soriano-Rosas J, Diaz-Quinonez A, Silva-Pereyra J, Vazquez-Hernandez P, Torres-Lopez O, Roldan A, Cruz-Gordillo A, Alonso-Viveros P, Navarro-Reynoso F. Pathological changes associated with the 2009 H1N1 virus. N Engl J Med. 2009;361:2001–2003. doi: 10.1056/NEJMc0907171. [DOI] [PubMed] [Google Scholar]
- Wang R, Sheng ZM, Taubenberger JK. Detection of novel (swine origin) H1N1 influenza A virus by quantitative real-time reverse transcription-PCR. J Clin Microbiol. 2009;47:2675–2677. doi: 10.1128/JCM.01087-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrit K, Goldfischer M, Wang J, Green J, Levine J, Lombardo J, Hong T. Identification of bacteria in formalin-fixed, paraffin-embedded heart valve tissue via 16S rRNA gene nucleotide sequencing. J Clin Microbiol. 2006;44:2609–2611. doi: 10.1128/JCM.00572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch DR, Anderson TP, Beynon KA, Chua A, Fleming AM, Laing RT, Town GI, Mills GD, Chambers ST, Jennings LC. Evaluation of a PCR assay for detection of Streptococcus pneumoniae in respiratory and nonrespiratory samples from adults with community-acquired pneumonia. J Clin Microbiol. 2003;41:63–66. doi: 10.1128/JCM.41.1.63-66.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J, Sumner J, Paddock CD, Shieh WJ, Greer PW, Reagan S, Fischer M, Van Beneden CA, Zaki SR. Diagnosis of invasive group a streptococcal infections by using immunohistochemical and molecular assays. Am J Clin Pathol. 2006;126:148–155. doi: 10.1309/KHGV-R72C-BRM4-FQ58. [DOI] [PubMed] [Google Scholar]
- Stralin K, Backman A, Holmberg H, Fredlund H, Olcen P. Design of a multiplex PCR for Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, and Chlamydophila pneumoniae to be used on sputum samples. APMIS. 2005;113:99–111. doi: 10.1111/j.1600-0463.2005.apm1130203.x. [DOI] [PubMed] [Google Scholar]
- Thomas LC, Gidding HF, Ginn AN, Olma T, Iredell J. Development of a real-time Staphylococcus aureus and MRSA (SAM-) PCR for routine blood culture. J Microbiol Methods. 2007;68:296–302. doi: 10.1016/j.mimet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Guarner J, Shieh WJ, Dawson J, Subbarao K, Shaw M, Ferebee T, Morken T, Nolte KB, Freifeld A, Cox N, Zaki SR. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114:227–233. doi: 10.1309/HV74-N24T-2K2C-3E8Q. [DOI] [PubMed] [Google Scholar]
- Guarner J, Packard MM, Nolte KB, Paddock CD, Shieh WJ, Tondella ML, McGee L, Zaki SR. Usefulness of immunohistochemical diagnosis of Streptococcus pneumoniae in formalin-fixed, paraffin-embedded specimens compared with culture and gram stain techniques. Am J Clin Pathol. 2007;127:612–618. doi: 10.1309/J3LD0RBP788W1TM8. [DOI] [PubMed] [Google Scholar]
- Shieh WJ, Hsiao CH, Paddock CD, Guarner J, Goldsmith CS, Tatti K, Packard M, Mueller L, Wu MZ, Rollin P, Su IJ, Zaki SR. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada JC, Selim MA, Miller SR. TEM of tissue from paraffin-embedded H&E-stained 6-micron sections for diagnosis (an unusual papovavirus case). Microsc Microanal. 2005;11:964CD–965CD. [Google Scholar]
- Goldsmith CS, Whistler T, Rollin PE, Ksiazek TG, Rota PA, Bellini WJ, Daszak P, Wong KT, Shieh WJ, Zaki SR. Elucidation of Nipah virus morphogenesis and replication using ultrastructural and molecular approaches. Virus Res. 2003;92:89–98. doi: 10.1016/s0168-1702(02)00323-4. [DOI] [PubMed] [Google Scholar]
- Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau LT, Lu J, Zhang B, McNutt MA, Lu M, Anderson VM, Gong E, Yu AC, Lipkin WI. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370:1137–1145. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol. 2008;172:1155–1170. doi: 10.2353/ajpath.2008.070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WF, To KF. Pathology of human H5N1 infection: new findings. Lancet. 2007;370:1106–1108. doi: 10.1016/S0140-6736(07)61490-1. [DOI] [PubMed] [Google Scholar]
- To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, Cheng AF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernandez M, Stewart TE, Fowler RA. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- Uyeki T. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1). N Engl J Med. 2009;361:e110. doi: 10.1056/NEJMopv0910738. [DOI] [PubMed] [Google Scholar]
- Childs RA, Palma AS, Wharton S, Matrosovich T, Liu Y, Chai W, Campanero-Rhodes MA, Zhang Y, Eickmann M, Kiso M, Hay A, Matrosovich M, Feizi T. Receptor-binding specificity of pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nature Biotechnol. 2009;27:797–799. doi: 10.1038/nbt0909-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Qi L, Kash JC, Dugan VG, Wang R, Jin G, Cunningham RE, Taubenberger JK. Role of sialic acid binding specificity of the 1918 influenza virus hemagglutinin protein in virulence and pathogenesis for mice. J Virol. 2009;83:3754–3761. doi: 10.1128/JVI.02596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol. 2007;171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology. 2009;126:268–279. doi: 10.1111/j.1365-2567.2008.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–1175. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000;137:856–864. doi: 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- Valdez R, Narayan KM, Geiss LS, Engelgau MM. Impact of diabetes mellitus on mortality associated with pneumonia and influenza among non-Hispanic black and white US adults. Am J Public Health. 1999;89:1715–1721. doi: 10.2105/ajph.89.11.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1), United States, May–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1071–1074. [PubMed] [Google Scholar]
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- Nolte KB, Alakija P, Oty G, Shaw MW, Subbarao K, Guarner J, Shieh WJ, Dawson JE, Morken T, Cox NJ, Zaki SR. Influenza A virus infection complicated by fatal myocarditis. Am J Forensic Med Pathol. 2000;21:375–379. doi: 10.1097/00000433-200012000-00016. [DOI] [PubMed] [Google Scholar]
- La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- Uyeki TM. Human infection with highly pathogenic avian influenza A (H5N1) virus: review of clinical issues. Clin Infect Dis. 2009;49:279–290. doi: 10.1086/600035. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KP, Lee SM, Cheung CY, Ng IH, Poon LL, Guan Y, Ip NY, Lau AS, Peiris JS. Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J Immunol. 2009;182:1088–1098. doi: 10.4049/jimmunol.182.2.1088. [DOI] [PubMed] [Google Scholar]
- Kirkeby S, Martel CJ, Aasted B. Infection with human H1N1 influenza virus affects the expression of sialic acids of metaplastic mucous cells in the ferret airways. Virus Res. 2009;144:225–232. doi: 10.1016/j.virusres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]