Abstract
Reactive oxygen species (ROS) are crucial for thyroid hormonogenesis, and their production is kept under tight control. Oxidative stress (OS) is toxic for thyrocytes in an inflammatory context. In vitro, Th1 pro-inflammatory cytokines have already been shown to decrease thyroid-specific protein expression. In the present study, OS level and its impact on thyroid function were analyzed in vitro in Th1 cytokine (interleukin [IL]-1α/interferon [IFN] γ)-incubated thyrocytes (rat and human), as well as in vivo in thyroids from nonobese diabetic mice, a model of spontaneous autoimmune thyroiditis. N-acetylcysteine (NAC) and prostaglandin, 15 deoxy-Δ12,14-prostaglandinJ2 (15dPGJ2), were used for their antioxidant and anti-inflammatory properties, respectively. ROS production and OS were increased in IL-1α/IFNγ-incubated thyrocytes and in destructive thyroiditis. In vitro, NAC not only reduced ROS production below control levels, but further decreased the expression of thyroid-specific proteins in addition to IL-1α/IFNγ-inhibitory effects. Thus, besides ROS, other intracellular intermediaries likely mediate Th1 cytokine effects. In vivo, NAC and 15dPGJ2 reduced OS and the immune infiltration, thereby leading to a restoration of thyroid morphology. It is therefore likely that NAC and 15dPGJ2 mainly exert their protective effects by acting on infiltrating inflammatory cells rather than directly on thyrocytes.
Thyrocytes continuously produce H2O2 and various reactive oxygen species (ROS) that are physiologically required for normal thyroid hormone synthesis. To control the toxicity resulting from ROS, thyrocytes possess several protective mechanisms. During thyroid hormone synthesis, H2O2 is produced in a limited area at the apical membrane and is immediately consumed in the peroxidation reaction catalyzed by thyroperoxidase (TPO). When ROS are produced in higher amounts, they are systematically eliminated by potent antioxidant systems such as peroxiredoxins, catalase, and glutathione peroxidases.1,2,3,4,5 Thus, a basal ROS production, which we define as oxidative load, is required to safeguard thyroid hormone synthesis, as recently demonstrated.6 Likewise, an harmless oxidative stress (OS) may also be important for cell division during goiter formation when thyrocytes are facing iodine deprivation.7
The context is quite different in the case of thyroid inflammation. Thus, in models of thyroiditis (transient or permanent), high amounts of ROS are produced and may become toxic.8,9,10,11,12,13 Using one of these models, we recently showed that increased OS associated with a strong inflammatory reaction can be controlled by 15 deoxy-Δ12,14-prostaglandin J2 (15dPGJ2),2 an anti-inflammatory prostaglandin14 that prevents OS-induced cytotoxicity.15 Iodine administration to goitrous thyrocytes produces an inflammatory reaction that is transient in most cases. However, in individuals genetically prone to develop autoimmune thyroiditis, this transient inflammation may become permanent, thereby evolving toward destructive thyroiditis. A model of destructive thyroiditis can be obtained in nonobese diabetic (NOD) mice. In this model of Hashimoto’s-like thyroiditis, the ongoing inflammatory reaction relies on pro-inflammatory Th1 cytokines16,17,18 that inhibit the expression of thyroid-specific proteins such as thyroglobulin, TPO, Na+/I− symporter (NIS), and dual oxidases (Duoxs).19,20,21,22,23,24 Mechanisms responsible for this inhibition are not yet known. In autoimmune processes targeting other cell types, such as pancreatic β cells, Th1 cytokine effects are mediated by nitric oxide (NO).25,26 In human, but not in rat thyrocytes, NO has also been identified as mediating the inhibitory actions of Th1 cytokines, but only partially.22,27,28,29,30 Intracellular factors other than NO should therefore mediate Th1 cytokine-induced inhibitory effects. ROS may represent, among others, alternative candidates, as suggested by previous studies. For instance, Th1 cytokines are known to increase ROS generation in the respiratory tract,31 in osteoarthritis,32,33 and in pancreatic islets.34,35 Up to now, nothing is known about the eventual involvement of ROS as intracellular mediators of Th1 cytokine-induced inhibitory actions in thyrocytes.
In the present study we aimed to evaluate the impact of Th1 cytokines (interleukin [IL]-1α/interferon [IFN] γ) on ROS production and how they may influence thyroid cell function in vitro. Likewise, the role played by OS in thyroiditis was analyzed in vivo in the aforementioned NOD mouse model of spontaneous autoimmune thyoiditis. In both in vitro and in vivo models, the roles of ROS were evaluated by using N-acetylcysteine (NAC), a potent antioxidant, and 15dPGJ2 for its anti-inflammatory properties. We also investigated how antioxidant systems behave in these conditions.
Materials and Methods
Cell Cultures
PCCL3 cells, a continuous line of nontransformed rat thyroid follicular cells,36 were a gift of Dr. F. Miot (Université Libre de Bruxelles, Institut de recherche interdisciplin aire en biologie humaine et moléculaire, Brussels, Belgium). They were grown to 80% to 90% confluence in Coon’s modified Ham’s F12 medium (BRL-Gibco, Paisley, Strathclyde, UK) supplemented with 5% newborn calf serum, penicillin (50 U/ml), streptomycin (50 μg/ml), fungizone (2.5 μg/ml; BRL-Gibco), 1 mU/ml thyroid stimulating hormone, 10 μg/ml insulin, and 5 μg/ml transferin (Sigma, Bornem, Belgium), in a humidified atmosphere (5% CO2). Recombinant rat IL-1α (2 ng/ml, Chemicon International, Temecula, CA) and recombinant rat IFNγ (100 U/ml, Chemicon International) were added for three additional days, in combination or not with NAC (1 mmol/L, Sigma) or 15dPGJ2 (2.5 μmol/L, Sigma) in the same medium containing 0.5% newborn calf serum and 1 mU/ml thyroid stimulating hormone. NAC, 15dPGJ2, or vehicle was added 2 hours before the cytokine cocktail. As a control, NAC or 15dPGJ2 were added on thyroid cells in the absence of cytokines.
Human thyroid tissues from patients who underwent thyroid surgery for benign multinodular goiter were obtained from the anatomopathology department after patients gave their informed consent. Thyrocytes were isolated according to Nilsson et al37 and suspended in modified Earle’s medium without phenol red containing 5% newborn calf serum, penicillin (50 U/ml), streptomycin (50 μg/ml), and fungizone (2.5 μg/ml; BRL-Gibco). They were plated in 6-well plates (50 μg DNA/well) or in multichamber glass slide (Nunc International, Naperville, IL; 7 μg DNA/chamber) and cultured in a humidified atmosphere (5% CO2) with 1 mU/ml thyroid stimulating hormone. After 1 week, cells were incubated for three additional days with cytokines in combination or not with NAC (1 mmol/L) or 15dPGJ2 (2.5 μmol/L), as described for PCCL3 cells. All experiments were repeated at least twice.
ROS Production
Thyrocytes were incubated in multichamber glass slides in appropriate medium. ROS production was measured by using a fluorescent dye, 2′, 7′ dichlorofluorescein diacetate (DCFH-DA; Molecular Probes, Paisley, UK). PBS-washed (pH 7.4) thyroid cells were incubated in Krebs-Ringer HEPES medium, pH 7.4, containing DCFH-DA (25 μmol/L) at 37°C for 1 hour. The excess of dye was removed by two washes with PBS. Cells were stained with Hoechst for 20 minutes and rinsed in PBS. Cover slides were mounted in fluorescent mounting medium (DakoCytomation, Carpinteria, CA) for microscopic observation. ROS production was visualized on a fluorescent microscope equipped with a digital camera.
Viability Assay
Cell viability was assessed by using the Alamar blue assay (Biosource International, Camarillo, CA), as previously described.22
Apoptosis Detection
Caspase activity was measured by using a CaspACE fluorescein isothiocyanate-VAD-fmk in situ marker (Promega, Madison, WI), which binds activated caspases, according to the manufacturer’s instructions. Briefly, cells were incubated with 20 μmol/L fluorescein isothiocyanate-VAD-fmk at 37°C for 20 minutes. Cells were then washed twice with PBS, fixed in 10% buffered formalin for 30 minutes, and rinsed with PBS. Coverslides were mounted in fluorescent mounting medium for microscopic observation. Cells treated with staurosporine (5 μmol/L; Sigma) were used as positive control.
Nitrite Assay
Nitrite accumulation in the medium of human thyrocytes was measured by the Griess reaction by using a commercially available kit (Promega).
Real-Time RT-PCR
Cells were suspended in TriPure isolation reagent (Roche Diagnostics GmbH, Mannheim, Germany), and total RNA was purified according to the manufacturer’s protocol. Reverse transcription was performed by incubating 2 μg RNA with 200 U Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen, Merelbeke, Belgium) in the recommended buffer containing 1 μl RNasin (Promega), 0.5 mmol/L dNTP (Promega), 2 μmol/L oligodT (Sigma), and 10 mmol/L dithiothreitol (20 μl final volume) overnight at 42°C. CDNA was diluted 1:5 in water for use in real time PCRs.
CDNAs (2 μl) were mixed with 500 nmol/L of each selected primer (Table 1) and SYBR Green reaction mix (BioRad, Herts, UK) in a final volume of 25 μl. Reactions were performed by using a iCycler apparatus (BioRad) as follows: 95°C for 1 minute, followed by 40 cycles of 95°C for 15 seconds, annealing temperature for 45 seconds (Table 1), and 81°C for 15 seconds. Amplification levels were normalized to that of β-actin.
Table 1.
Forward and Reverse Primers and Annealing Temperatures Used
| Target | Primer forward | Primer reverse | Annealing temperature, °C |
|---|---|---|---|
| Actin | 5′-CATCCTGCGTCTGGACCT-3′ | 5′-AGGAGGAGCAATGATCTTGAT-3′ | 62 |
| rDuox | 5′-GTGGCTGGAGGGAGCCAT-3′ | 5′-CCGTGAACAGACTCCTGT-3′ | 60 |
| rDuox1 | 5′-CCTGCAAGCCAAAAGAAGAC-3′ | 5′-CCACTGAAGTTTTCCCGTACA-3′ | 60 |
| rDuox2 | 5′-AGAGGGAGCCATTACCCTGT-3′ | 5′-CGCATAGCTGAGATGGATGA-3′ | 60 |
| rTPO | 5′-CAGGTTTTGGTGGGAGAA-3′ | 5′-CTGCACACTCATTAACATCTT-3′ | 58 |
| rNIS | 5′-GCGCTGCGACTCTCCCACTGAC-3′ | 5′-GGCGGTAGAAGATCGGCAAGAAGA-3′ | 60 |
| hDuox | 5′-GTGGCTGGCTGACATCAT-3′ | 5′-TGCAGGGAGTTGAAGAA-3′ | 58 |
| h Duox1 | 5′-GGACCCCCAGGACCAGGAT-3′ | 5′-CTTACACTCACCGCCCCAACAC-3′ | 60 |
| hDuox2 | 5′-AACCCAAACGTCCATCAACA-3′ | 5′-CCTTGTACCCCCTTCCACTT-3′ | 58 |
| hTPO | 5′-CACGATGCAGAGAAACCTCAA-3′ | 5′-ATAGACTGGAGGGAGCCAT-3′ | 60 |
| hNIS | 5′-ACCGCGCCCCACCTCTTTCTTATT-3′ | 5′-CCCCCTCCTGATTCTGGTTGTTG-3′ | 62 |
Western Blottings
Thyrocytes were suspended in Laemmli buffer (50 mmol/L Tris-HCl, pH 6.8, 2% SDS, and 10% glycerol), containing a protease inhibitor cocktail (Sigma), and were sonicated during 30 seconds. Protein concentration was determined by using a bicinchoninic acid protein assay kit (Pierce, Rockfort, IL). Duox (antibody provided by F. Miot, IRIBHN, Brussels), TPO (antibody provided by J. Ruf, Université de la Méditerranée, Marseille, France), catalase (Sigma), peroxiredoxin 3 and 5 (PRDX3, PRDX5; antibodies provided by B. Knoops, Université catholique de Louvain, Louvain La Neuve), and β-actin (Sigma) Western blottings were performed as previously described.22 Proteins (30 μg/lane) were heated at 95°C for 5 minutes in the loading buffer (Laemmli buffer containing 100 mmol/L dithiothreitol and 0.1% bromophenol blue), separated by 8% SDS-polyacrylamide gel electrophoresis, and transferred onto a nitrocellulose membrane (Hybond ECL, Amersham Biosciences, Rosenthaal, The Netherlands). Membranes were blocked for 1 hour at room temperature in PBS (pH 7.4), 5% nonfat dry milk, 0.1% Tween, and incubated overnight at 4°C with the primary antibody at a dilution of 1:4000 (Duox, TPO), 1:10,000 (PRDX3, PRDX5), or 1:2000 (catalase, β-actin). Membranes were incubated for 1 hour at room temperature with EnVision (1:200, DakoCytomation) peroxidase-labeled secondary antibody and visualized with enhanced chemiluminescence (SuperSignal West Pico, Pierce) on CLXposure TM films (Pierce). Western blots were scanned and quantified by densitometry using the NIH Scion Image Analysis Software (NIH, Bethesda, MD). Values were normalized by reporting the signal intensity to β-actin expression.
Immunofluorescence
Thyrocytes were cultured in multichamber glass slides in appropriate medium. Thyrocytes were fixed for 30 minutes in 4% paraformaldehyde, rinsed once with PBS, permeabilized for 15 minutes in a PBS-Triton 1% solution at room temperature, and washed with PBS supplemented with 1% bovine serum albumin. Cells were then incubated overnight with PRDX5 primary antibody (1:75) at room temperature. After being washed in PBS, fluorescein isothiocyanate-conjugated secondary antibody was added for 1 hour at room temperature at a dilution of 1:30 (anti-rabbit; DakoCytomation). Coverslides were mounted in fluorescent mounting medium for microscopic observation.
Animals and Treatments
Three-month-old female NOD mice, under a standard diet and kept under semibarrier conditions, were originally obtained from Professor Wu (Beijing, China) and inbreeded since 1989 (Proefdierencentrum, Leuven, Belgium). Animals were injected intraperitoneally with a saline solution of NAC (100 mg/kg/day) or with a saline solution of 15dPGJ2 (40 μg/kg/day) for 4 days. NMRI mice were used as control. Mice were housed and handled according to Belgian Regulation of Laboratory Animal Welfare.
Preparation of Tissue Samples for Microscopy and Morphometric Analysis
Five animals of each group were anesthetized with pentothal, and thyroid lobes were dissected. One thyroid lobe was fixed in paraformaldehyde (4% in PBS) for 24 hours and embedded in paraffin. The second lobe was frozen for cryostat sections. Thick sections (5 μm) were used for morphology analysis and for immunohistochemistry. Morphometric measurements were performed by using the point-counting method described by Weibel et al.38
For each thyroid, 1000 points were counted, and the relative volumes of epithelium, colloid, vessels, and interstitium were measured.
Immunohistochemistry
4-hydroxynonenal (4-HNE), PRDX5, and catalase immunostainings were performed on paraffin sections. Sections were dewaxed and rehydrated. Except for PRDX5 detection, paraffin sections were pretreated in a microwave oven in citrate buffer (pH 6.6) for one cycle of 3 minutes at 750 W, followed by four cycles of 3.5 minutes, each at 350 W. Endogenous peroxidases were quenched with 1% H2O2 for 30 minutes.
Then, paraffin sections were washed with PBS supplemented with 1% or 5% bovine serum albumin and thereafter incubated in PBS supplemented with 1% or 5% bovine serum albumin containing 2% or 5% normal goat serum at room temperature. Sections were incubated with the first antibody (4-HNE, PRDX5, and catalase) at room temperature (Table 2). The binding of antibodies was detected by using a second antibody conjugated to a peroxidase-labeled polymer (EnVision detection, DakoCytomation, Carpinteria, CA) or a biotinylated second antibody for 30 minutes followed by an avidin-biotin peroxidase complex for 30 minutes (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). The peroxidase activity was measured with 3-amino-9-ethylcarbazole substrate (DakoCytomation). Sections were counterstained with Mayer’s hematoxylin, rinsed, and mounted in Faramount Aqueous mounting medium (DakoCytomation). To verify the binding specificity, some sections were incubated with the second antibody alone.
Table 2.
Experimental Conditions for Immunohistochemistry
| Antibody | First antibody | Second antibody | Revelation substrate |
|---|---|---|---|
| 4-HNE (Calbiochem, Darmstadt, Germany) | Rabbit polyclonal | EnVision rabbit (DakoCytomation) | AEC (DakoCytomation) |
| Dilution: 1:800 | |||
| Incubation time: overnight | |||
| PRDX5 (Polyclonal rabbit, B. Knoops, Université catholique de Louvain) | Rabbit polyclonal | EnVision rabbit (DakoCytomation) | AEC (DakoCytomation) |
| Dilution: 1:200 | |||
| Incubation time: 1 hour | |||
| PRDX3 (Polyclonal rabbit, B. Knoops, Université catholique de Louvain) | Rabbit polyclonal | EnVision rabbit (DakoCytomation) | AEC (DakoCytomation) |
| Dilution: 1:500 | |||
| Incubation time: 1 hour | |||
| Catalase (Sigma) | Monoclonal mouse | EnVision mouse (DakoCytomation) | AEC (DakoCytomation) |
| Dilution: 1:200 | |||
| Incubation time: 3 hours |
Data Analysis and Statistics
Data were expressed as mean ± SEM, n = 6 for all experiments. Each experiment was repeated at least twice. Statistical analyses were performed by using analysis of variance followed by Tukey-Kramer Multiple Comparison Test (GraphPad InStat, San Diego, CA), or by unpaired t-test. P < 0.05 was considered as statistically significant.
Results
IL-1α/IFNγ Increase Intracellular ROS Production without Affecting Cell Viability In Vitro: Differential Effects of NAC and 15dPGJ2
Although Th1 cytokines are known to induce ROS production in various cell types,31,32,34,35 this has not been yet described in thyrocytes. IL-1α/IFNγ-induced ROS, and nitrite production was therefore analyzed both in rat and human thyroid cells.
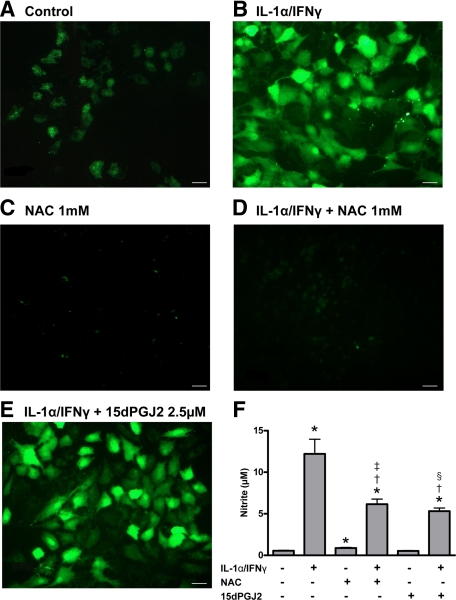
In rat PCCL3 control cells, ROS detected by DCFH-DA fluorescence were observed as granules within the cytoplasm (Figure 1A). The staining was greatly enhanced in Th1 cytokine-treated cells (Figure 1B), whereas ROS were detected both in the cytoplasm and in nuclei. In cells treated with NAC alone, and in accordance with NAC anti-oxidant properties, ROS fluorescence was strongly reduced compared with control cells (Figure 1C). In cells co-incubated with IL-1α/IFNγ together with NAC, ROS fluorescence was below control levels (Figure 1D). By contrast, 15dPGJ2 influenced ROS production, neither in control cells (data not shown) nor in Th1 cytokine-treated cells (Figure 1E). Similar results were obtained in human primary cells (data not shown).
Figure 1.
Detection of intracellular ROS in PCCL3 cells (A–E). ROS production was measured in control cells (A), in IL-1α/IFNγ-treated cells (B), in NAC-treated cells (C), in IL-1α/IFNγ-treated cells co-incubated with NAC (D), and with 15dPGJ2 (E). Scale bars = 200 μm. Nitrite accumulation in the culture medium of human thyrocytes (F) is shown. Results are expressed as mean ± SEM of six individual wells of one representative experiment (n = 6). *P < 0.05 versus control cells; †P < 0.05 versus IL-1α/IFNγ-treated cells; ‡P < 0.05 versus NAC-treated cells; §P < 0.05 versus 15dPGJ2-treated cells.
According to previous results, nitrite levels, the stable end-product of NO generation, were low in media from control human thyroid cells, but greatly enhanced in Th1 cytokine-treated cells (Figure 1F).22,30 When cells were co-incubated with IL-1α/IFNγ and NAC or 15dPGJ2, nitrite levels were significantly reduced as compared with Th1-treated cells, but remained higher than in control cells (Figure 1F).
Cell viability was not affected, and no change in apoptosis was detected whatever the treatment used, indicating that the observed effects were not resulting from cell death (data not shown).
IL-1α/IFNγ Induce a Down-Regulation of Thyroid Cell Function In Vitro: Differential Effects of NAC and 15dPGJ2
As already reported,19,22 IL-1α/IFNγ induced a down-regulation of the thyrocyte function, as indicated by decreased Duox (Duox1 and Duox2), TPO, and NIS expression, at mRNA or protein levels in rat PCCL3 and human cells (Figure 2; Supplemental Figure S1, see http://ajp.amjpathol.org).
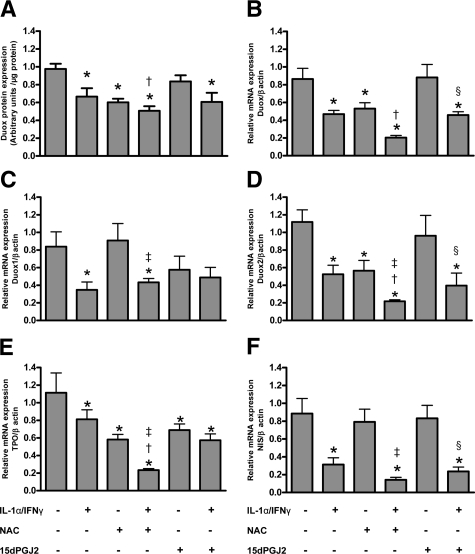
Figure 2.
Quantification of Duox protein expression in PCCL3 cells (A). Densitometric values of Western Blots are expressed as mean ± SEM of one representative experiment (n = 6). Relative expression of Duox (B), Duox 1 (C), Duox 2 (D), TPO (E), and NIS (F) mRNAs in PCCL3 cells, adjusted to the β-actin signal, are expressed as mean ± SEM of one representative experiment (n = 6). *P < 0.05 versus control cells; †P < 0.05 versus IL-1α/IFNγ-treated cells; ‡P < 0.05 versus NAC-treated cells; §P < 0.05 versus 15dPGJ2 treated-cells.
Our data also confirm that Duox protein and mRNA expression was strongly decreased in PCCL3 cells incubated with NAC alone.6 When incubated together with IL-1α/IFNγ, NAC further aggravated Th1-induced inhibitory effects (Figure 2, A and B). In human thyroid cells, NAC alone or in combination with Th1 cytokines significantly reduced Duox protein and mRNA expression without additional effect on Th1-induced down-regulation of Duox protein expression (Supplemental Figure S1, A and B, see http://ajp.amjpathol.org).
The distinct analysis of Duox1 and Duox2 expression in PCCL3 cells showed a different pattern. NAC influenced neither basal nor Th1-induced down-regulation of Duox1 mRNA expression (Figure 2C), but induced a decrease in Duox2 mRNA expression and further impaired Th1 cytokine-induced down-regulating effects (Figure 2D).
As for Duox and already reported,6 NAC alone induced a down-regulation of TPO mRNA and protein expression both in PCCL3 and human thyroid cells (Figure 2E; Supplemental Figure S1, C and D, see http://ajp.amjpathol.org). In cells incubated together with NAC and IL-1α/IFNγ, an additive effect of their respective inhibitory action was observed.
Although NAC alone had no effect on NIS mRNA, it further aggravated IL-1α/IFNγ-induced NIS down-regulation, at least in human cells (Figure 2F; Supplemental Figure S1E, see http://ajp.amjpathol.org).
Both in PCCL3 and human cells, 15dPGJ2, when administered alone or together with Th1 cytokines, had no specific effect on Duox nor on NIS expression (Figure 2A–D and F; Supplemental Figure S1A, B, and E, see http://ajp.amjpathol.org). The only isolated modification we observed was on TPO mRNA expression in PCCL3 cells (Figure 2E).
The Expression and Intracellular Localization of PRDX3 and PRDX5 Antioxidant Enzymes Are Strongly Influenced by Th1 Cytokines, NAC, and 15dPGJ2
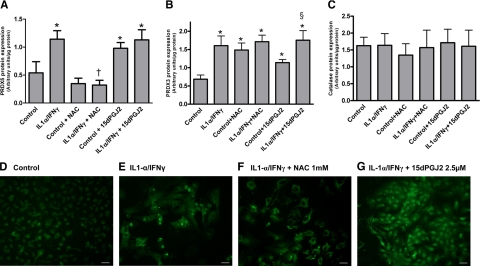
PRDX5 and PRDX3 protein expression in PCCL3 cells was significantly increased by IL-1α/IFNγ (Figure 3, A and B). The subcellular localization of PRDX5 also changed. In control cells, PRDX5 was expressed only as granules in the cytoplasm (Figure 3D). In Th1 cytokine-treated cells, PRDX5 expression not only increased in the cytoplasm, but also lighted up in the nuclei (Figure 3E). NAC treatment differentially regulated both PRDXs. NAC alone or together with Th1 cytokines reduced PRDX5 expression (Figure 3, A and F), but increased the expression of PRDX3 (Figure 3B). 15dPGJ2 significantly increased PRDX5 and PRDX3 expression in PCCL3 without affecting their expression induced by Th1 cytokines, or their localization (Figure 3A, B, and G). Other antioxidant enzymes reacted differently, as catalase protein expression remained stable in all experimental conditions (Figure 3C).
Figure 3.
Expression of PRDX5, PRDX3, and catalase antioxidant enzymes. Quantification of PRDX5 (A), PRDX3 (B), and catalase (C) protein expression in PCCL3 cells. Densitometric values of Western blots are expressed as mean ± SEM of one representative experiment (n = 6). *P < 0.05 versus control cells; †P < 0.05 versus IL-1α/IFNγ-treated cells; §P < 0.05 versus 15dPGJ2-treated cells. Immunofluorescence of PRDX5 in PCCL3 cells in control (D), in IL-1α/IFNγ-treated cells (E), in IL-1α/IFNγ-treated cells co-incubated with NAC (F), and in IL-1α/IFNγ-treated cells co-incubated with 15dPGJ2 (G). Scale bars = 200 μm.
The Strong Increase of Both OS and Inflammatory Reaction in the NOD Mouse Model of Spontaneous Thyroiditis Is Down-Regulated by NAC and 15dPGJ2
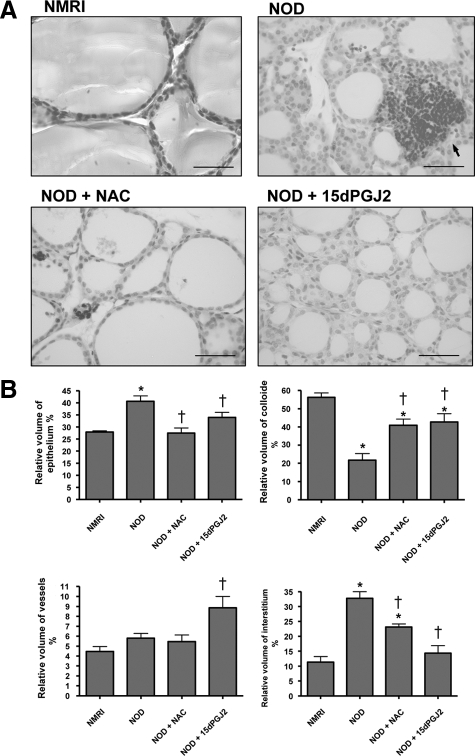
To study the role of OS in thyroiditis in vivo, we used the NOD mouse model of spontaneous thyroiditis. At 3 months, 100% of mice exhibited an inflammatory infiltrate in the thyroid together with variable stages of tissue destruction. Instead of large and regular follicles filled with colloid and lined by cuboidal epithelial cells as observed in normal thyroids, the thyroids of NOD mice consisted of small follicles with narrowed follicular lumina lined by thicker cell walls. Signs of cell destruction were observed in some areas: dead cells were observed in follicular lumina, some follicles were completely destroyed, and the interstitium was massively occupied by inflammatory cells (Figure 4A). These observations were confirmed by the morphometric analysis that showed a decrease in the relative volume of the colloid together with an increase in the relative volume of both epithelium and interstitium (Figure 4B).
Figure 4.
Thyroid morphology (A) and morphometric analysis (B) of thyroids from NMRI and NOD mice. Thyroiditis was observed in thyroids from NOD mice as shown by an interstitium massively infiltrated by inflammatory cells (arrows). In addition, follicles were small and narrowed compared with NMRI mice, and dead cells were observed. By contrast, when NOD mice were treated with NAC or with 15dPGJ2, the inflammatory reaction was markedly decreased: follicles were more regular, and lumina were filled with colloid and lined by flattened epithelial cells. Scale bars = 50 μm. These observations were confirmed by morphometric analysis. *P < 0.05 versus NMRI mice; †P < 0.05 versus NOD mice.
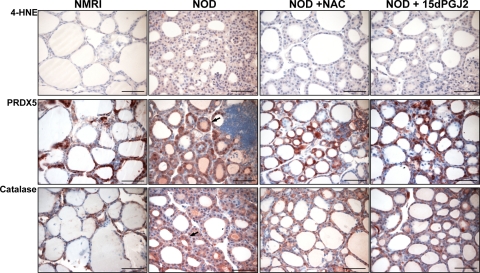
4-HNE, a toxic product resulting from lipid peroxidation, was used as a OS marker.39 Although 4-HNE immunostaining was fairly detected in thyroids from NMRI mice, it strongly increased in NOD mice, with localization in thyrocytes, as well as in some interstitial cells (Figure 5).
Figure 5.
Immunohistochemical detection of 4-HNE, PRDX5, and catalase in thyroids from NMRI and NOD mice. The high expression of 4-HNE observed in NOD thyroid glands was strongly decreased after treatment with NAC or 15dPGJ2. PRDX5 and catalase were expressed in control thyroids from NMRI mice. In thyroids from NOD mice, their expression was heterogeneously distributed with a weak expression in follicles with flattened epithelium and a stronger expression in those with thicker epithelial cells (arrows). Scale bars = 50 μm.
In NAC-treated NOD thyroid glands, OS was drastically reduced, as confirmed by the strong decrease in 4-HNE expression, although remaining higher than in NMRI mice (Figure 5). Follicles were larger and more regular in size. Follicular lumina were filled with colloid and lined by flattened epithelial cells. Although the inflammatory reaction was still observed in the interstitium, it was markedly less pronounced compared with thyroids of untreated NOD mice (Figure 4A). Hence, the colloid relative volume was significantly increased, but remained lower than in NMRI mice. The relative volume of the epithelium decreased to values similar to NMRI mice. The relative volume of the interstitium also decreased, but remained higher than in NMRI mice (Figure 4B).
As with NAC, the follicles of NOD mice treated with 15dPGJ2 were larger and more regular in size. Their lumina were filled with more colloid and lined by flattened epithelial cells (Figure 4A). The interstitium compartment was also significantly reduced. These morphological changes were associated with a decrease in 4-HNE expression, indicating a reduced OS (Figure 5).
Because of the strong OS induction in NOD thyroid glands, we analyzed the in vivo expression of two potent enzymatic antioxidant systems, namely PRDX5 and catalase (Figure 5). They were weakly expressed in the cytoplasm of control thyrocytes from NMRI mice. In thyroids from NOD mice, their expression was heterogeneously distributed, with a weak expression in follicles with flattened epithelium and a stronger expression in those with thicker epithelial cells. The total expression of PRDX5 and catalase was weaker in NOD mice treated either with NAC or with 15dPGJ2.
Discussion
In this study, we show for the first time that, in vivo, thyroiditis is associated with increased toxic OS and that, in vitro, Th1 pro-inflammatory cytokines induce intracellular ROS production in rat and human thyrocytes. This intracellular accumulation of ROS is quite different from that observed under physiological conditions. Hence, in a normal thyroid, H2O2 is produced by Duox in a limited area called thyroxisome that is located at the apical pole of the cell in microvilli.40 H2O2 is either consumed during the hormone synthesis process or detoxified by potent antioxidant systems, thereby being harmless for cells.1,2,41,42 The outcome for thyrocytes is quite different when ROS are heavily produced in cells incubated with Th1 cytokines. ROS may then become toxic, being detected both in the cytoplasm and in nuclei. In previous studies, we have shown that Th1 cytokines induce a down-regulation of Duox,22 and a reduction in the production of extracellular H2O2 in the FRTL5 cell line (unpublished data). It is therefore likely that Th1 cytokine-induced ROS in thyrocytes are not generated from Duox enzymes, but instead from a source that remains to be discovered.
Another question that remains to be sorted out concerns the exact nature of ROS produced when thyrocytes are incubated with Th1 cytokines. The DCFH-DA probe used in our study is not sensitive enough to distinguish H2O2 from other ROS, likely more toxic, including peroxynitrite and hydroxyl radicals. It is, however, clear that the nature of ROS will be determinant in terms of cell survival, some of them being more deleterious than others.41 Among reactive oxygen species, reactive nitrogen species, especially NO, are known to be induced by IL-1α/IFNγ in human thyrocytes27,30,43 and are partially responsible for the inhibitory effects of Th1 cytokines on thyrocytes.22 In addition, although NAC partially reduces the production of nitrite, ROS immunoflorescence detected by DCFH-DA fluorescence is completely abolished. Thus, ROS other than NO are likely produced in Th1 cytokine-incubated thyrocytes.
In vivo, the production of ROS was evaluated indirectly by measuring the induction of OS. In NOD mice, both toxic OS and inflammatory reaction affecting the whole thyroid gland were observed, as described in other models such as osteoarthritis, autoimmune encephalomyelitis, and lung diseases.33,44,45 Here, OS results from ROS produced by thyrocytes themselves facing Th1 cytokines (intrafollicular OS), but also from inflammatory cells colonizing the interstitium (extrafollicular OS). A way to explain the toxicity of OS in thyroiditis in NOD mice is the induction of intracellular adhesion molecule-1 by ROS.46
In the presence of NAC, the intracellular ROS production and OS were significantly reduced both in vitro and in vivo. As precursor of glutathione, NAC may decrease cell OS directly or indirectly by restoring glutathione content.47,48 In vitro, NAC alone exerts an inhibitory effect on thyroid cell function.6 Combined with Th1 cytokines, the inhibitory effects were additive, suggesting that although ROS are produced in Th1 cytokine-treated cells, other intracellular mediators are involved in Th1 cytokine inhibitory effects. In vivo, the paradigm is not exactly the same as NAC reduces the inflammatory reaction, thereby protecting indirectly thyrocytes against the cell destruction induced by the extrathyroidal autoimmune reaction. This could be due to the ability of NAC to decrease the inflammation by inhibiting various cytokines (tumor necrosis factor-α, IFNγ, IL-8, and IL-6)45 and/or to restore the cellular redox-status and to modulate the activity of redox sensitive cell signaling pathways such as nuclear factor κB that regulates pro-inflammatory genes.44 The absence of NAC effects directly on thyrocytes in vivo compared with the in vitro experimental conditions could also be due to differences in terms of local concentration of NAC, probably reaching lower concentrations in thyroids when administered systemically in vivo than when added in vitro directly to cultured thyrocytes.
The effects of 15dPGJ2 were different from those of NAC, at least in vitro, as no reduction of ROS production was observed. By contrast, in vivo, as for NAC, OS was decreased, thereby allowing the recovery of a near normal thyroid morphology. It is not the first time that such protective effects are observed as they have been already reported in a nonautoimmune model of iodine-induced thyroid involution.2 This is likely due to the ability of 15dPGJ2 to inhibit the expression of a variety of pro-inflammatory factors including cyclooxygenase-2, NOS2, and several cytokines (IL-6, IL-12, and tumor necrosis factor-α).49,50,51 15dPGJ2 may also modulate or inhibit the nuclear factor κB system51,52,53 and activate the mitogen activated protein kinase pathway through PPARγ-dependent and independent mechanisms.54 Because 15dPGJ2 has no direct effect on thyroid cells in vitro, but is able to reduce inflammation in vivo, we suggest that 15dPGJ2 may favorably influence OS in the thyroid gland by acting directly on infiltrating inflammatory cells.
In this present study we report for the first time a differential regulation of Duox1 and Duox2 mRNA levels in PCCL3 rat thyroid cells. The respective roles of these two proteins encoded by two different genes remain unclear. Rigutto et al55 have reported in PCCL3 cells that Duox1 alone is able to generate H2O2 and that the amount of Duox1 present is sufficient to generate enough H2O2. By contrast, other arguments suggest that in humans, thyroid Duox2 is the main H2O2 generator.55 A study in the respiratory tract epithelium demonstrated that IL-4, a Th2 cytokine, increases Duox1 mRNA expression and that IFNγ, a Th1 cytokine, markedly induces Duox2 mRNA expression.31 In our study, the results were quite different. Here, Th1 cytokines inhibited both Duox genes. On the other hand, the antioxidant NAC negatively influenced only Duox2 mRNA. Thus, Duox2 expression seems to require a minimal oxidative load to be adequately expressed, whereas Duox1 expression does not depend on the thyroid cell ROS content. Obviously, further investigations are required to clarify the exact underlying mechanisms.
In conclusion, our results confirm that the maintenance of a minimal oxidative load, as in control cells, is essential to safeguard thyroid cell function. In addition, ROS are not the sole intracellular mediators of Th1 cytokine-induced inhibitory effects of thyroid cell function in vitro. In vivo, both the antioxidant NAC and the anti-inflammatory prostaglandin 15dPGJ2 protect the thyroid against toxic OS mainly by acting on infiltrating inflammatory cells, thereby contributing to the reduction of the extrafollicular toxic OS. The intracellular OS remains under the control of efficient intracellular antioxidant systems, thereby allowing the thyroid cell function and morphology to recover.
Footnotes
Address reprint requests to Anne-Catherine Gérard, Ph.D., Unité de Morphologie Expérimentale (MOEX), Université catholique de Louvain, UCL-5251, 52 Av. E.Mounier, B-1200, Brussels, Belgium. E-mail: anne-catherine.gerard@uclouvain.be.
Supported by institutional funding.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Gerard AC, Many MC, Daumerie C, Knoops B, Colin IM. Peroxiredoxin 5 expression in the human thyroid gland. Thyroid. 2005;15:205–209. doi: 10.1089/thy.2005.15.205. [DOI] [PubMed] [Google Scholar]
- Poncin S, Gerard AC, Boucquey M, Senou M, Calderon PB, Knoops B, Lengele B, Many MC, Colin IM. Oxidative stress in the thyroid gland: from harmlessness to hazard depending on the iodine content. Endocrinology. 2008;149:424–433. doi: 10.1210/en.2007-0951. [DOI] [PubMed] [Google Scholar]
- Mano T, Shinohara R, Iwase K, Kotake M, Hamada M, Uchimuro K, Hayakawa N, Hayashi R, Nakai A, Ishizuki Y, Nagasaka A. Changes in free radical scavengers and lipid peroxide in thyroid glands of various thyroid disorders. Horm Metab Res. 1997;29:351–354. doi: 10.1055/s-2007-979052. [DOI] [PubMed] [Google Scholar]
- Mutaku JF, Many MC, Colin I, Denef JF, van den Hove MF. Antigoitrogenic effect of combined supplementation with dl-alpha-tocopherol, ascorbic acid, and beta-carotene and of dl-alpha-tocopherol alone in the rat. J Endocrinol. 1998;156:551–561. doi: 10.1677/joe.0.1560551. [DOI] [PubMed] [Google Scholar]
- Nadolnik LI, Valentyukevich OI. Peculiarities of the antioxidant status of the thyroid gland. Bull Exp Biol Med. 2007;144:529–531. doi: 10.1007/s10517-007-0369-3. [DOI] [PubMed] [Google Scholar]
- Poncin S, Colin IM, Gerard AC. Minimal oxidative load: a prerequisite for thyroid cell function. J Endocrinol. 2009;201:161–167. doi: 10.1677/JOE-08-0470. [DOI] [PubMed] [Google Scholar]
- Poncin S, Van ES, Humblet K, Colin IM, Gerard AC. Oxidative stress: a required condition for thyroid cell proliferation. Am J Pathol. 2010;176:1355–1363. doi: 10.2353/ajpath.2010.090682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Many MC, Mestdagh C, van den Hove MF, Denef JF. In vitro study of acute toxic effects of high iodide doses in human thyroid follicles. Endocrinology. 1992;131:621–630. doi: 10.1210/endo.131.2.1639011. [DOI] [PubMed] [Google Scholar]
- Belshaw BE, Becker DV. Necrosis of follicular cells and discharge of thyroidal iodine induced by administering iodide to iodine-deficient dogs. J Clin Endocrinol Metab. 1973;36:466–474. doi: 10.1210/jcem-36-3-466. [DOI] [PubMed] [Google Scholar]
- Wollman SH, Breitman TR. Changes in DNA and weight of thyroid glands during hyperplasia and involution. Endocrinology. 1970;86:322–327. doi: 10.1210/endo-86-2-322. [DOI] [PubMed] [Google Scholar]
- Mahmoud I, Colin I, Many MC, Denef JF. Direct toxic effect of iodide in excess on iodine-deficient thyroid glands: epithelial necrosis and inflammation associated with lipofuscin accumulation. Exp Mol Pathol. 1986;44:259–271. doi: 10.1016/0014-4800(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Many MC, Maniratunga S, Varis I, Dardenne M, Drexhage HA, Denef JF. Two-step development of Hashimoto-like thyroiditis in genetically autoimmune prone non-obese diabetic mice: effects of iodine-induced cell necrosis. J Endocrinol. 1995;147:311–320. doi: 10.1677/joe.0.1470311. [DOI] [PubMed] [Google Scholar]
- Mutaku JF, Poma JF, Many MC, Denef JF, van den Hove MF. Cell necrosis and apoptosis are differentially regulated during goitre development and iodine-induced involution. J Endocrinol. 2002;172:375–386. doi: 10.1677/joe.0.1720375. [DOI] [PubMed] [Google Scholar]
- Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- Garg TK, Chang JY. 15-deoxy-delta 12, 14-Prostaglandin J2 prevents reactive oxygen species generation and mitochondrial membrane depolarization induced by oxidative stress. BMC Pharmacol. 2004;4:6. doi: 10.1186/1471-2210-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport B, McLachlan SM. Thyroid autoimmunity. J Clin Invest. 2001;108:1253–1259. doi: 10.1172/JCI14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassi G, De MR. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol. 2002;2:195–204. doi: 10.1038/nri750. [DOI] [PubMed] [Google Scholar]
- Colin IM, Isaac J, Dupret P, Ledant T, D'Hautcourt JL. Functional lymphocyte subset assessment of the Th1/Th2 profile in patients with autoimmune thyroiditis by flowcytometric analysis of peripheral lymphocytes. J Biol Regul Homeost Agents. 2004;18:72–76. [PubMed] [Google Scholar]
- Ajjan RA, Watson PF, Findlay C, Metcalfe RA, Crisp M, Ludgate M, Weetman AP. The sodium iodide symporter gene and its regulation by cytokines found in autoimmunity. J Endocrinol. 1998;158:351–358. doi: 10.1677/joe.0.1580351. [DOI] [PubMed] [Google Scholar]
- Rasmussen AK, Bendtzen K, Feldt-Rasmussen U. Thyrocyte-interleukin-1 interactions. Exp Clin Endocrinol Diabetes. 2000;108:67–71. doi: 10.1055/s-2000-5797. [DOI] [PubMed] [Google Scholar]
- Caraccio N, Giannini R, Cuccato S, Faviana P, Berti P, Galleri D, Dardano A, Basolo F, Ferrannini E, Monzani F. Type I interferons modulate the expression of thyroid peroxidase, sodium/iodide symporter, and thyroglobulin genes in primary human thyrocyte cultures. J Clin Endocrinol Metab. 2005;90:1156–1162. doi: 10.1210/jc.2004-1173. [DOI] [PubMed] [Google Scholar]
- Gerard AC, Boucquey M, van den Hove MF, Colin IM. Expression of TPO and ThOXs in human thyrocytes is downregulated by IL-1alpha/IFN-gamma, an effect partially mediated by nitric oxide. Am J Physiol Endocrinol Metab. 2006;291:E242–E253. doi: 10.1152/ajpendo.00439.2005. [DOI] [PubMed] [Google Scholar]
- Sato K, Satoh T, Shizume K, Ozawa M, Han DC, Imamura H, Tsushima T, Demura H, Kanaji Y, Ito Y. Inhibition of 125I organification and thyroid hormone release by interleukin-1, tumor necrosis factor-alpha, and interferon-gamma in human thyrocytes in suspension culture. J Clin Endocrinol Metab. 1990;70:1735–1743. doi: 10.1210/jcem-70-6-1735. [DOI] [PubMed] [Google Scholar]
- Poncin S, Lengele B, Colin IM, Gerard AC. Differential interactions between Th1/Th2, Th1/Th3, and Th2/Th3 cytokines in the regulation of TPO and DUOX expression, and of thyroglobulin secretion in thyrocytes in vitro. Endocrinology. 2008;149:1534–1542. doi: 10.1210/en.2007-1316. [DOI] [PubMed] [Google Scholar]
- Corbett JA, Wang JL, Hughes JH, Wolf BA, Sweetland MA, Lancaster JR, Jr, McDaniel ML. Nitric oxide and cyclic GMP formation induced by interleukin 1 beta in islets of Langerhans: evidence for an effector role of nitric oxide in islet dysfunction. Biochem J. 1992;287(Pt 1):229–235. doi: 10.1042/bj2870229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Strynadka K, Schulz R, Rabinovitch A. Mechanisms of cytokine-induced destruction of rat insulinoma cells: the role of nitric oxide. Endocrinology. 1994;134:1006–1010. doi: 10.1210/endo.134.3.8119136. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hattori Y, Nakanishi N, Manaka K, Banba N, Motohashi S, Shimoda S. Regulation of inducible nitric oxide production by cytokines in human thyrocytes in culture. Endocrinology. 1995;136:4261–4270. doi: 10.1210/endo.136.10.7545102. [DOI] [PubMed] [Google Scholar]
- Motohashi S, Kasai K, Banba N, Hattori Y, Shimoda S. Nitric oxide inhibits cell growth in cultured human thyrocytes. Life Sci. 1996;59:L227–L234. doi: 10.1016/0024-3205(96)00437-7. [DOI] [PubMed] [Google Scholar]
- Reimers JI, Rasmussen AK, Karlsen AE, Bjerre U, Liang H, Morin O, Andersen HU, Mandrup-Poulsen T, Burger AG, Feldt-Rasmussen U, Nerup J. Interleukin-1 beta inhibits rat thyroid cell function in vivo and in vitro by an NO-independent mechanism and induces hypothyroidism and accelerated thyroiditis in diabetes-prone BB rats. J Endocrinol. 1996;151:147–157. doi: 10.1677/joe.0.1510147. [DOI] [PubMed] [Google Scholar]
- van den Hove MF, Stoenoiu MS, Croizet K, Couvreur M, Courtoy PJ, Devuyst O, Colin IM. Nitric oxide is involved in interleukin-1alpha-induced cytotoxicity in polarised human thyrocytes. J Endocrinol. 2002;173:177–185. doi: 10.1677/joe.0.1730177. [DOI] [PubMed] [Google Scholar]
- Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases: Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Darlington LG, Stone TW. Antioxidants and fatty acids in the amelioration of rheumatoid arthritis and related disorders. Br J Nutr. 2001;85:251–269. doi: 10.1079/bjn2000239. [DOI] [PubMed] [Google Scholar]
- Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008;16:624–630. doi: 10.1016/j.joca.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Strynadka K, Rabinovitch A. Destruction of rat pancreatic islet beta-cells by cytokines involves the production of cytotoxic aldehydes. Endocrinology. 1996;137:5290–5296. doi: 10.1210/endo.137.12.8940348. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Lakey JR, Rajotte RV. Human pancreatic islet beta-cell destruction by cytokines involves oxygen free radicals and aldehyde production. J Clin Endocrinol Metab. 1996;81:3197–3202. doi: 10.1210/jcem.81.9.8784069. [DOI] [PubMed] [Google Scholar]
- Fusco A, Berlingieri MT, Di Fiore PP, Portella G, Grieco M, Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7:3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Husmark J, Nilsson B, Tisell LE, Ericson LE. Primary culture of human thyrocytes in Transwell bicameral chamber: thyrotropin promotes polarization and epithelial barrier function. Eur J Endocrinol. 1996;135:469–480. doi: 10.1530/eje.0.1350469. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Kistler GS, Scherle WF. Practical stereological methods for morphometric cytology. J Cell Biol. 1966;30:23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L, Szweda PA, Vinogradova O, Szweda LI. Structural characterization and immunochemical detection of a fluorophore derived from 4-hydroxy-2-nonenal and lysine. Proc Natl Acad Sci USA. 1998;95:7975–7980. doi: 10.1073/pnas.95.14.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Driessens N, Costa M, De Deken X, Detours V, Corvilain B, Maenhaut C, Miot F, Van SJ, Many MC, Dumont JE. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007;92:3764–3773. doi: 10.1210/jc.2007-0660. [DOI] [PubMed] [Google Scholar]
- Denef JF, Many MC, van den Hove MF. Iodine-induced thyroid inhibition and cell necrosis: two consequences of the same free-radical mediated mechanism? Mol Cell Endocrinol. 1996;121:101–103. doi: 10.1016/0303-7207(96)03848-8. [DOI] [PubMed] [Google Scholar]
- Taurog A, Dorris ML, Doerge DR. Mechanism of simultaneous iodination and coupling catalyzed by thyroid peroxidase. Arch Biochem Biophys. 1996;330:24–32. doi: 10.1006/abbi.1996.0222. [DOI] [PubMed] [Google Scholar]
- Rasmussen AK, Di MR, Diamant M, Feldt-Rasmussen U, Bendtzen K. Nitric oxide production is not involved in the effects of interleukin-1 beta on cAMP, thyroglobulin, and interleukin-6 in TSH-stimulated human thyroid cells. Autoimmunity. 1994;19:239–245. doi: 10.3109/08916939409071349. [DOI] [PubMed] [Google Scholar]
- Sadowska AM, Manuel YK, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther. 2007;20:9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Stanislaus R, Gilg AG, Singh AK, Singh I. N-acetyl-L-cysteine ameliorates the inflammatory disease process in experimental autoimmune encephalomyelitis in Lewis rats. J Autoimmune Dis. 2005;2:4. doi: 10.1186/1740-2557-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Traore K, Trush MA, Rose NR, Burek CL. Intracellular adhesion molecule-1 up-regulation on thyrocytes by iodine of non-obese diabetic H2(h4) mice is reactive oxygen species-dependent. Clin Exp Immunol. 2008;152:13–20. doi: 10.1111/j.1365-2249.2008.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen A, Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med. 1998;92:609–623. doi: 10.1016/s0954-6111(98)90506-6. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S. Peroxisome proliferator-activated receptors gamma ligands and ischemia and reperfusion injury. Vascul Pharmacol. 2004;41:187–195. doi: 10.1016/j.vph.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-Delta12,14-prostaglandin J2. Proc Natl Acad Sci USA. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EH, Surh YJ. 15-deoxy-Delta12,14-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factors. Biochem Pharmacol. 2006;72:1516–1528. doi: 10.1016/j.bcp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Vaidya S, Somers EP, Wright SD, Detmers PA, Bansal VS. 15-Deoxy-Delta12,1412,14-prostaglandin J2 inhibits the beta2 integrin-dependent oxidative burst: involvement of a mechanism distinct from peroxisome proliferator-activated receptor gamma ligation. J Immunol. 1999;163:6187–6192. [PubMed] [Google Scholar]
- Hortelano S, Castrillo A, Alvarez AM, Bosca L. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J Immunol. 2000;165:6525–6531. doi: 10.4049/jimmunol.165.11.6525. [DOI] [PubMed] [Google Scholar]
- Rigutto S, Hoste C, Dumont JE, Corvilain B, Miot F, De Deken X. Duox1 is the main source of hydrogen peroxide in the rat thyroid cell line PCCl3. Exp Cell Res. 2007;313:3892–3901. doi: 10.1016/j.yexcr.2007.06.011. [DOI] [PubMed] [Google Scholar]