Abstract
Deficiency in Cathepsin D (CtsD), the major cellular lysosomal aspartic proteinase, causes the congenital form of neuronal ceroid lipofuscinoses (NCLs). CtsD-deficient mice show severe visceral lesions like lymphopenia in addition to their central nervous system (CNS) phenotype of ceroid accumulation, microglia activation, and seizures. Here we demonstrate that re-expression of CtsD within the CNS but not re-expression of CtsD in visceral organs prevented both central and visceral pathologies of CtsD−/− mice. Our results suggest that CtsD was substantially secreted from CNS neurons and drained from CNS to periphery via lymphatic routes. Through this drainage, CNS-expressed CtsD acts as an important modulator of immune system maintenance and peripheral tissue homeostasis. These effects depended on enzymatic activity and not on proposed functions of CtsD as an extracellular ligand. Our results furthermore demonstrate that the prominent accumulation of ceroid/lipofuscin and activation of microglia in brains of CtsD−/− are not lethal factors but can be tolerated by the rodent CNS.
Cathepsin D (CtsD), the cell’s major aspartic protease, is a ubiquitously expressed lysosomal protein, but very little is know about its physiological functions. CtsD appears to be dispensable for bulk proteolysis in lysosomes,1 but to date only very few specific substrates have been defined or ruled out in cellular contexts.2,3,4,5,6,7 Earlier studies suggesting a prominent role for CtsD in antigen processing were disproved recently.8,9 CtsD is heavily secreted from certain tumor cells and has been proposed to have a multitude of pathophysiological functions independent of its enzymatic activity by acting as a ligand to as yet undiscovered receptors.10,11 Deficiency in CtsD causes the congenital form of neuronal ceroid lipofuscinosis (NCL) in humans, dogs, sheep, and mice.12,13,14,15 CtsD knockout mice develop normally through their first two weeks of life but start to lose weight and become blind during the third week. Animals die at day p26 ± 1 presenting central nervous system (CNS) pathology closely resembling human cNCL in terms of neuron loss, blindness, deposition of autofluorescent ceroid/lipofuscin, astrogliosis and microglia activation, and seizures.16 Pronounced microglia activation and nitric oxide (NO) synthesis were suggested as contributing directly to neuronal degeneration,17,18 but application of NO synthase inhibitors could not prevent the severe CNS phenotype and prolonged life-time of the animals by only 1 to 2 days.19
In contrast to mice deficient in other NCL-related proteins, CtsD−/− mice develop a severe peripheral pathology characterized by lymphopenia, degeneration of the intestinal mucosa, and atrophy of liver and spleen.1 If and how central and visceral pathology are interrelated or depending on each other is unknown, although the concurrent appearance of neurodegeneration and loss of CD4+/CD8+ double positive thymocytes suggests a putative common trigger. We redelivered CtsD to different body compartments of CtsD−/− mice by means of viral vector–mediated gene transfer to elucidate in which tissues CtsD activity might be needed to overcome the severe visceral phenotype. Unexpectedly, we found that CtsD expressed within the CNS but not CtsD expressed in visceral organs was capable of substantially postponing appearance of lymphopenia and other visceral lesions. Here we describe for the first time drainage of a CNS-expressed protein to the periphery, which thereby provides essential functions in immune system maintenance and tissue homeostasis.
Materials and Methods
Experimental Animals
All experimental animal procedures were conducted according to approved experimental animal licenses issued by the responsible animal welfare authority (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit) and controlled by the local animal welfare committee of the University Medicine Göttingen.
CtsD−/− mice were bred from heterozygous founders1 maintained in a C57B6×129SV background and genotyped at day p2. Vector injections into neonate mouse CNS or visceral organs was performed at day p3. Two μl corresponding to 6 × 109 vector genomes were injected into either one or into both hemispheres at position 1 mm rostral to bregma and 1 mm lateral to midline. Depth of injection was ≈2 mm, resulting in application of the viral suspension to the frontal cortex/anterior dorso-lateral striatum. Vector applications into periphery consisted of one injection into liver (50 μl, corresponding to 3 × 1011 vector genomes) and one intraluminal injection into stomach (50 μl, 3 × 1011 vector genomes).
Viral Vector Preparations
Recombinant AAV vectors of mosaic serotype 1/2 were produced essentially as described20 and expressed either enhanced green fluorescent protein (EGFP) or mouse CtsD under control of the CMV/human β-actin hybrid (HBA) promoter.
Tissue Processing and Analysis
For immunohistochemical analysis mice were perfused with PBS followed by 4% paraformaldehyde, tissue was postfixed in 4% paraformaldehyde for 4 hours, cryoprotected in sucrose, and sectioned to 18-μm slices on a cryostat (Leica, Nussloch, Germany). Antibodies used for immunohistochemistry were as follows: anti-NeuN (Chemicon, Temecula, CA), custom made rabbit polyclonal anti-CtsD,1 anti-CD11b (MCA711, Düsseldorf, Germany), anti-activated Caspase-3 (Cell Signaling Technologies, Germany). Cy2- or Cy3-conjugated secondary antibodies (Dianova, Germany) were used for visualization. Wide-field images were recorded on a Zeiss Axiovision microscope equipped with cooled CCD camera and Axiovision 6 software. Confocal-like images (1-μm section depth) were recorded on a Zeiss Apotome microscope. Tissue for protein analysis was sampled after perfusion of mice with PBS and quick frozen on dry ice. Tissue was crushed in a Potter-Elvehjem homogenizator in 50 mmol/L Tris, pH 8.0, 1 mmol/L dithiothreitol, and complete protease inhibitors (Roche Diagnostics, Penzberg, Germany) and after addition of SDS to 0.5% final concentration was homogenized by ultrasound on ice. Equal amounts of protein were electrophoresed on SDS-PAGE gels and blotted onto nitrocellulose membranes. A polyclonal goat anti-CtsD antibody (#6486, Santa Cruz Biotechnology, Santa Cruz, CA) was used for detection and visualized with alkaline phosphatase coupled secondary antibody by chemo luminescence.
FACS Analysis of Thymocytes
The thymus was removed, thymocytes were prepared as single cell preparation, and 5 × 106 thymocytes were used for staining. Lymphocytes from peripheral blood were purified using lymphocyte separation medium (PAA Laboratories GmbH, Pasching, Austria). The following clones were used for fluorescence-activated cell sorting (FACS) analysis: anti-CD4 (clone GK1.5), anti-CD8 (53-6.7). Cells were incubated with the appropriate antibodies for 15 minutes on ice in 100 μl FACS buffer (PBS supplemented with 0.1% bovine serum albumin and 0.1% sodium azide), washed once with 3 ml FACS puffer and analyzed on a FACSCalibur using CellQuest software (BD Biosciences, Franklin Lakes, NJ).
Results
Central but not Visceral Application of AAV-CtsD Prolongs Lifespan of CtsD−/− Mice
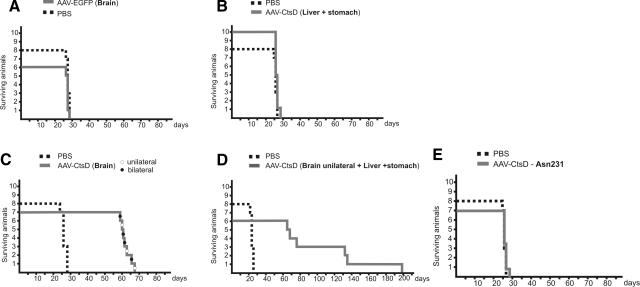
In a first set of experiments we analyzed survival times of CtsD−/− mice injected with AAV vectors into the CNS, into visceral organs, or into CNS plus visceral organs. Injections of AAV-EGFP control vector into the brain or injection of AAV-CtsD into visceral organs did not lead to any prolongation of the lifespan of CtsD−/− mice, which died at p26 ± 1 day as did untreated littermates (Figure 1, A and B). In contrast, animals injected into the CNS with AAV-CtsD demonstrated a roughly doubled lifespan and died at p63 ± 3 days (Figure 1C). Three of six animals injected into both CNS and periphery also died around p63, while two animals survived for further 60 days (death at p132 and p134), and one animal died at p198, which represents another 60 days in survival time (Figure 1D).
Figure 1.
CNS application of AAV-CtsD prolonged lifetime of CtsD−/− mice. Survival times in days are shown for CtsD−/− mice injected with control virus (n = 6) or PBS (n = 8) (A), injected into visceral organs with AAV-CtsD (n = 10) (B), injected into brain either unilaterally (n = 4, black circles) or bilaterally (n = 3, white circles) with AAV-CtsD (total n = 7) (C), injected into brain plus periphery with AAV-CtsD (n = 6) (D), and injected with the enzymatically inactive CtsDAsn231 mutant into the brain (n = 7) (E). Survival times were significantly different between controls (PBS/AAV-EGFP) and AAV-CtsD brain-injected animals, and between animals survived for about 60, 130, or 190 days after AAV-CtsD brain + visceral injection, as assessed by Student’s t test at P < 0.01.
The life-prolonging effect of AAV-CtsD application into the CNS appeared to depend on delivery of the vector to the forebrain, as application of a second vector dose to the midbrain/hindbrain did not result in further enhanced survival times (not shown). In addition, survival times did not differ between unilaterally and bilaterally brain injected animals (Figure 1C), arguing against a pronounced effect of vector dosage on survival of animals. Thus, forebrain CNS injections were performed throughout the study, as this mode of application also prevented any loss of viral particles into the ventricular system.
Rescue of CtsD−/− Mice Depends on Catalytic Activity of CtsD
AAV vector-mediated expression of the nonenzymatically active CtsDAsn231 mutant21 did not have any beneficial effects on the CtsD−/− phenotype. Immunohistochemistry detected CtsD reactivity in the brains after AAV-CtsDAsn231 injection as being indistinguishable from AAV-CtsD injections (not shown). However, AAV-CtsDAsn231 injected−/− mice died at day p26 ± 1 day (Figure 1E), presenting with typical CtsD−/− pathology, suggesting that survival of AAV-CtsD–injected animals depended on the enzymatic activity of CtsD and not on extracellular ligand–receptor interactions.
CNS Application of AAV-CtsD Prevents Ceroid Accumulation and Microglia Activation in the Brain
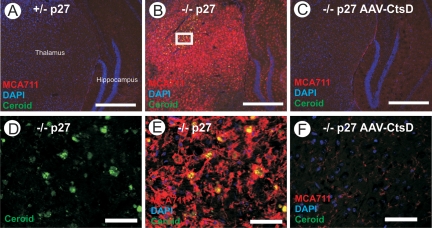
The pronounced accumulation of ceroid and activation of microglia seen in the thalamus of untreated CtsD−/− animals were completely prevented in animals injected into both hemispheres with AAV-CtsD (Figure 2, A–F), and only very mild ceroid accumulations and microglial activation was detected in the contralateral side in unilaterally injected mice (not shown). CtsD−/− mice injected with AAV-CtsD either unilaterally or bilaterally into the CNS were completely healthy at day p27 and phenotypically undistinguishable from +/+ and +/− littermates except for a slightly reduced body weight (Figure 3G).
Figure 2.
AAV-mediated CtsD expression prevents ceroid accumulation and microglia activation in CtsD−/− mice. Immunohistochemical staining for activated microglia (MCA711, red, as overlay with nuclear DAPI stain, blue) at day p27 in the thalamus of CtsD+/− mice (A), CtsD−/− mice (B), and CtsD−/− mice injected into CNS with AAV-CtsD (C). High-power micrographs show deposition of autofluorescent ceroid (green) in CtsD−/− mice (D) and fulminate accumulation of microglia in vicinity to these deposits (E), whereas both ceroid and activated microglia are absent in AAV-CtsD injected CtsD−/− mice (F). D and E represents the rectangle in panel B. Scale bar = 500 μm (A–C), 50 μm (D–F). Representative pictures taken from n = 4 or 5 per condition.
Figure 3.
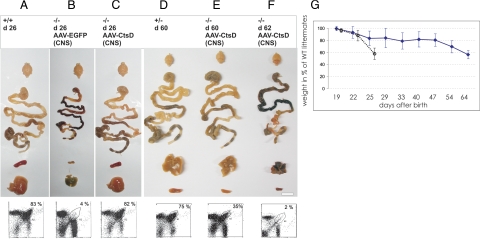
Integrity of visceral organs after re-expression of CtsD in CNS and recurrence of disease after adolescence. Overview of brain, gastro-intestinal system, spleen, and liver morphology and FACS analysis of CD4+/CD8+ double positive thymocytes, at day p 26 of CtsD +/+ mice (A), CtsD−/− mice AAV-EGFP injected (B), and CtsD−/− mice AAV-CtsD injected into CNS (C). Tissue morphology around day p60 is shown for CtsD+/− mice (D), for AAV-CtsD brain injected−/− mice of healthy phenotype (E), and for AAV-CtsD brain injected mice of moribund phenotype (F). Scale bar = 12 mm. Weight loss over time of AAV-CtsD brain injected animals (solid line) and untreated CtsD−/− animals (dotted line) as compared with sex and age matched littermates is shown (G).
CNS Application of AAV-CtsD Maintains Visceral Organ Integrity
Visceral re-expression of CtsD did not prevent any of the visceral pathologies in CtsD−/− mice. In pronounced contrast, CNS re-expression maintained visceral organs in a healthy state as examined at p26 both on macroscopic level (Figure 3, A–C) and histochemically (see Supplemental Figure S1 at http://ajp.amjpathol.org). While untreated and viscerally injected CtsD−/− mice demonstrated severe distortion of intestinal mucosa, and atrophic liver and spleen, no such phenotype was detected in CtsD−/− mice that had received AAV-CtsD injection into the brain. The only degenerative phenotype detected in the CNS of these animals was seen in the retina (see Supplemental Figure S1, M–P at http://ajp.amjpathol.org), which was not transduced by the AAV vector according to EGFP tracing, and also showed no CtsD immunostaining (not shown).
CNS Application of AAV-CtsD Allows for Proper Immune System Maintenance
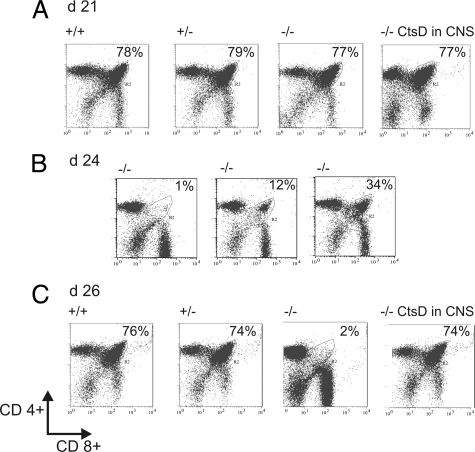
To evaluate the functional restoration of the immune system after CtsD re-expression within the CNS we assessed T cell development in the thymus by quantification of T cell subpopulations. Whereas thymic cellularity in untreated CtsD−/− mice dropped to one tenth of that of controls and the CD4+/CD8+ double positive compartment virtually disappeared from day 21 to day 26, thymocytes developed identically in terms of absolute numbers and relative subset distribution as compared with +/+ or +/− littermates after CNS application of AAV-CtsD (Figure 4, A–C).
Figure 4.
Re-expression of CtsD within CNS prevents lymphopenia in CtsD−/− mice. The percentage of CD4+/CD8+ double positive thymocytes was determined from thymi isolated at days p21 (A), p24 (B), and p26 (C) from +/+, +/−, untreated −/−, and AAV-CtsD brain injected −/− mice by FACS analysis. n = 3 to 5 animals for each diagram in A, n = 1 for each diagram in B, demonstrating the spectrum of CD4+/CD8+ thymocytes depletion at this state, and n = 4 to 6 animals for each diagram in C.
CtsD Is Secreted by CNS Neurons but not by Visceral Cell Types
Coinjection of equal titers of AAV-EGFP and AAV-CtsD into the brain demonstrated large numbers of CtsD immunoreactive neurons far remote from EGFP-positive cells, arguing for secretion of CtsD from vector-transduced neurons and reuptake by remote neurons in the contralateral hemisphere and in deeper brain areas like thalamus and hypothalamus (see Supplemental Figure S2, H–L, at http://ajp.amjpathol.org). Formal proof that cortical neurons robustly secrete CtsD was provided by transduction of cultured cortical neurons (see Supplemental Figure S2M at http://ajp.amjpathol.org), where we detected large amounts of CtsD in the culture supernatant. Importantly, intracellular overexpressed CtsD localized exclusively to lysosomes and not to regulated secretory vesicles (see Supplemental Figure S3 at http://ajp.amjpathol.org). In contrast, after visceral coinjection of AAV-EGFP and AAV-CtsD CtsD immunoreactivity was mainly confined to vector-transduced (i.e., EGFP-positive) cells as shown exemplarily for liver, choroid plexus, thymus, and intestine smooth muscle (see Supplemental Figure S4 at http://ajp.amjpathol.org). Nonetheless, visceral vector application resulted in organism-wide transduction (liver, skeletal and cardiac muscle, kidneys, cortex of the adrenal gland and of the thymus, pancreas, spleen, stomach and intestine, including the enteric nervous system and the choroid plexus of the CNS; see Supplemental Figure S4 at http://ajp.amjpathol.org).
CtsD Protein Is Drained from CNS to Periphery
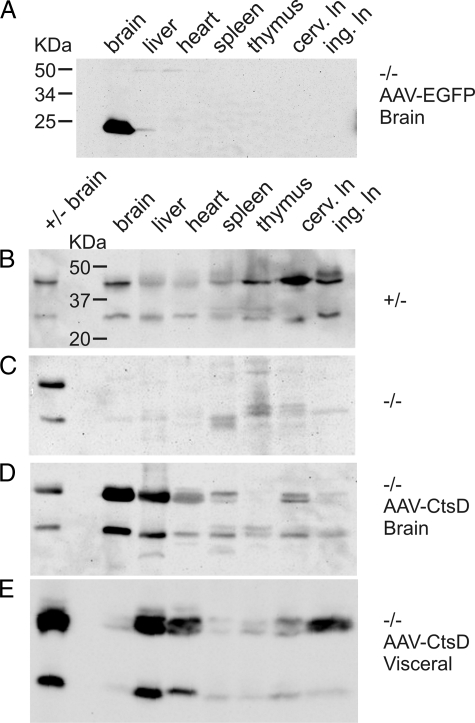
Injection of AAV-EGFP into postnatal brains of CtsD−/− mice resulted in only minor leakage of the viral vector to periphery. Quantitative analysis by Western blotting revealed that only about 1 to 2% of EGFP protein was detected outside the CNS (Figure 5A), arguing for retention of the majority of viral vector within the CNS and for an intact blood–brain barrier in CtsD−/− mice. In pronounced contrast, intracranial application of AAV-CtsD resulted in detection of almost 40 to 50% of total CtsD outside the CNS (Figure 5, C and D). Western blotting did not detect CtsD in blood samples, but there was strong evidence that CtsD was drained from CNS via lymphatic routes. Much higher levels of CtsD were detected in cervical lymph nodes, into which cerebro-spinal fluid is directly drained, as compared with inguinal lymph nodes. This finding closely resembles the wild-type situation, where highest levels of CtsD were found in cervical lymph nodes (Figure 5B). After CNS re-expression, CtsD was also detected in heart muscle, liver, and spleen. Lymphatics are finally drained into vasculature, which might explain accumulation of CtsD in these organs with high blood supply. Note that no specific immunoreactivity for CtsD was detected in the thymus after CtsD re-expression in CNS. Western blotting demonstrated considerable overlap of CtsD detection between visceral and CNS re-expression (Figure 5, D and E), with the distinction that visceral vector application resulted in only minor levels of CtsD in CNS but detectable CtsD levels in thymus (Figure 5E).
Figure 5.
Quantitative analysis of viral vector spread and CtsD delivery to CNS and visceral tissues. Representative Western blot for detection of EGFP in visceral tissues after CNS injection of AAV-EGFP into CtsD−/− mice is shown (A). Western blot analysis of CtsD levels at p22 is shown for control mice (B), for untreated −/− mice (C), for −/− mice injected into CNS with AAV-CtsD (D), and for −/− mice injected into periphery with AAV-CtsD (E). Each blot shown is representative of tissue analysis of n = 3 animals per condition. Blot A was probed with an anti-EGFP antibody, blots B–E were probed with an anti-CtsD antibody. In B–E a size standard for CtsD from control brain was used to distinguish CtsD immunoreactivity from background signals. Please note that in E a batch of standard containing higher protein concentration than those used in B–D was used. Otherwise, each lane of the Western blots represents 40 μg of loaded protein.
CNS-Independent Recurrence of Disease Symptoms in AAV-CtsD-Treated CtsD−/− Mice
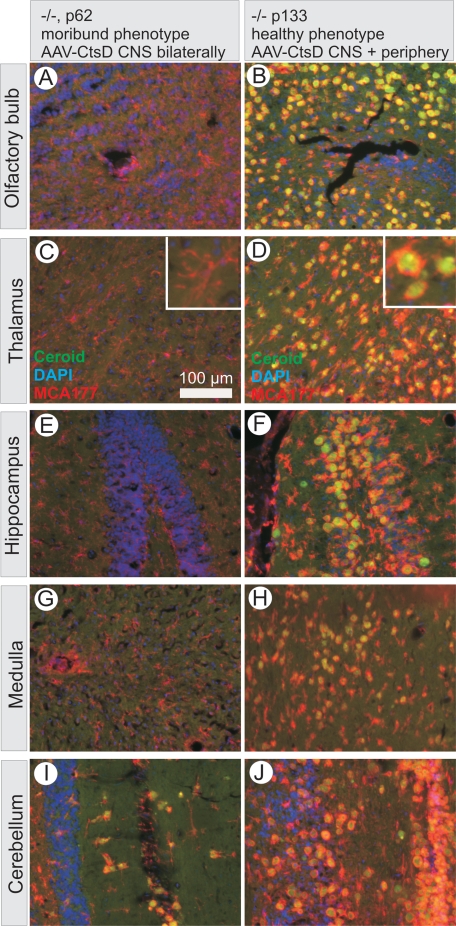
Animals receiving either unilateral or bilateral CNS application of AAV-CtsD developed normally through adolescence but started to moderately lose weight around day p50 despite normal feeding and drinking behavior. Animals died around day p63, with no significant difference between those injected into only one hemisphere or into both hemispheres (Figure 1C). Around day 62 to 63 their phenotype strikingly resembled that of untreated −/− mice at day p26, demonstrating atrophy of liver and spleen, necrosis of intestinal mucosa, and severe depletion of CD4+/CD8+ thymocytes (Figure 3, D–F). It should be noted that we did not detect any decline in CtsD immunoreactivity in their brains and that persistent and sufficiently functional CtsD expression within the CNS was clearly documented by almost complete absence of ceroid accumulations and microglia activation in brains of bilaterally AAV-CtsD injected−/− mice at p62 (Figure 6, A, C, E, G, and I).
Figure 6.
CNS ceroid accumulation is not responsible for death of CtsD−/− mice. Representative brain sections from CtsD−/− mouse injected with AAV-CtsD bilaterally into CNS and presenting with a completely moribund phenotype at day p62 are shown in A, C, E, G, and I, whereas contralateral brain sections from a CtsD−/− mouse injected with AAV-CtsD unilaterally into CNS and additionally into periphery presenting with a healthy phenotype at p133 is shown in B, D, F, H, and J. Note that J was taken from a more rostral part of the cerebellum as compared with I. Pictures are overlays of wide field micrographs showing ceroid autofluorescence in green, activated microglia stained by MCA711 in red, and nuclear counter stain by DAPI in blue.
Two lines of evidence suggest that visceral pathology develops independently from the CNS. First, we did not detect any impairments of autonomous control in CtsD−/− mice, as demonstrated by a functional HPA axis, which did not mediate aberrant release of corticosterone (see Supplemental Figure S5 at http://ajp.amjpathol.org). We also detected no obvious neurodegeneration in brains of bilaterally injected mice at times of recurrence of visceral pathology. Within the hindbrain, which contains many of the neuronal cell bodies projecting toward peripheral autonomous nuclei only the reticular-tegmental nuclei of the pons was found to be affected by ceroid accumulation, microglia activation and a lack of NeuN immunoreactivity (see Supplemental Figure S6 at http://ajp.amjpathol.org). These nuclei control saccadic eye movements but are not involved in autonomous circuits of visceral innervations. We also did not detect neuronal FluoroJade staining or immunoreactivity for activated Caspase-3 in these brains (Supplemental Figure S7 at http://ajp.amjpathol.org). These data suggest that AAV-CtsD injection into the forebrain is sufficient to prevent neurodegeneration in the whole brain, and that recurrence of visceral pathology and death of these animals thus was CNS-independent. We also did not detect a decline in CtsD activity in liver over time after AAV-CtsD injection into the brain (see Supplemental Figure S8 at http://ajp.amjpathol.org), arguing against a general loss of CtsD activity in visceral organs as well.
The Rodent CNS Tolerates Enormous Accumulations of Ceroid and Microglia Activation
An important lesson from analysis of CtsD−/− mice injected into CNS with AAV-CtsD was the finding that mice could tolerate massive accumulations of ceroid together with correspondingly massive activation of microglia throughout whole brain hemispheres. Such whole brain accumulation of ceroid was found in animals that have been injected with AAV-CtsD unilaterally into brain and additionally into visceral organs and survived for more than 60 days. A representative example is shown in Figure 6, which compares levels of ceroid accumulation and microglia activation in corresponding brain sections from an animal with complete moribund phenotype at day p62 after bilateral AAV-CtsD injection into CNS (Figure 6, A, C, E, G, and I) with an animal sacrificed at day p133 at still normal phenotype after unilateral CNS injection plus peripheral injection (Figure 6, B, D, F, H, and J). While the former animal was moribund without significant ceroid accumulation or microglia activation, the latter was moving freely and showed normal behavior with respect to food uptake and exploration but demonstrated massive ceroid accumulation accompanied by microglia activation. As such, neither the accumulation of large amounts of ceroid nor the long-lasting activation of microglia is primarily responsible for death of CtsD−/− animals. The lack of ceroid accumulation in AAV-CtsD injected brain hemispheres around p60 was evident in all animals dying around this time (n = 12) while the massive ceroid accumulation in the contralateral hemisphere was seen in all 4 animals surviving for this extended period of time. It should be noted that accumulation of ceroid specifically took place in neurons, accompanied by a gradual loss of NeuN immunoreactivity (see Supplemental Figure S6, K–N, at http://ajp.amjpathol.org) and that these neurons then seemed to be engulfed by activated microglia.
Discussion
The most intriguing result of this study was the finding that CtsD re-expressed in CNS but not CtsD re-expressed in visceral organs of CtsD−/− mice was able to prevent the severe lymphopenia and other visceral pathologies of CtsD−/− mice. This finding was striking because visceral application of the recombinant AAV vector resulted in organism-wide transduction including brain astrocytes and ventricle epithelia, gut lamina propria, and thymus. Maturation and maintenance of CD4+/CD8+ double positive thymocytes is a thymus-autonomous process taking place in the thymic cortex, where thymocytes are selected by antigen presentation through cortical thymic epithelial cells.22 Tracing of AAV transduction showed that the thymic cortex was efficiently transduced by visceral vector application, but this did not prevent degeneration of the CD4+/CD8+ double positive compartment. Similarly, gut and liver were effectively transduced, but this peripheral CtsD expression did not result in any relief from pathology. In contrast, re-expression of CtsD within the CNS not only prevented CNS pathology but also all visceral pathology. Thus, on first sight this would argue for a lysosomal processing of an as yet unknown substrate in CNS neurons, which might be crucial for critical immune functions like maintenance of CD4+/CD8+ double positive thymocytes. Such a processed peptide might then diffuse from the brain to periphery to exert its actions. However, as it is difficult to envisage why such a processing should only occur in brain lysosomes but not in lysosomes of visceral tissues, our data argue for a different mode of how brain-derived CtsD maintains immune functions and visceral tissue homeostasis. First, CtsD is secreted from CNS neurons, allowing the protein to be distributed throughout large brain areas despite localized vector transduction. This secretion and reuptake of the protein explains the almost complete prevention of CNS related pathology in all brain areas reached by the protein. It has to be emphasized, however, that a single AAV-CtsD injection into the mouse brain was not sufficient to deliver curative amounts of CtsD to the contralateral hemisphere, indicating limitations in CtsD distribution probably by physical barriers. Second, CtsD is drained from the brain, at least partially by lymphatic routes, as it was detected in cervical rather than in inguinal lymph nodes after CNS expression. Substantial CtsD was also detected in liver, heart, and spleen so that a direct contribution of vasculature to distribution of CtsD cannot be ruled out, but lymphatics are draining into vasculature as well, leaving the relative contribution of both routes unclear. Given the forebrain injection of the viral vector, the olfactory nerve, which leaves the brain through the cribriform plate and through its perineural spaces provides direct continuity between subarachnoid space and olfactory sub mucosa lymphatics,23 appears to be a good candidate for the route by which CtsD is drained from CNS to periphery. In any case, after CNS expression, CtsD appears to be targeted to sites within the organism that are not reached by visceral vector application despite the ubiquitous transduction. This theory is strengthened by the finding that CtsD is apparently much less effectively secreted and remotely reuptaken by peripheral cell types compared with CNS neurons. CtsD can potentially be secreted from all cell types but is reendocytosed through the MPR300,24 resulting in very low levels of CtsD and other lysosomal hydrolases outside the cell. This situation may be different in neurons where MPR-independent pathways exist and the bulk flow of CtsD to extracellular space may be higher. Third, in wild-type mice, highest levels of CtsD are found in cervical lymph nodes, into which cerebro-spinal fluid is directly drained. Studies in bone marrow chimeras demonstrated that CtsD is dispensable within hematopoietic cells for their development and maturation in otherwise CtsD+/+ mice25 and thus there would be no biological reason for these high levels in lymph nodes. Thus, it seems possible that drainage of CtsD from brain via lymphatics may be a naturally occurring process.
Altogether, these results suggest that temporal prevention of visceral pathology due to CtsD re-expression within the CNS depends on drainage of the protein itself to the periphery, where CtsD activity in as yet unknown cells allows for proper maintenance of thymocytes and tissue homeostasis. While a potential contribution of a CNS-processed CtsD substrate to visceral tissue integrity cannot be formally ruled out, this is unlikely insofar as at times of recurrence of visceral pathology CtsD activity in the CNS persisted as demonstrated by prevention of ceroid accumulation.
Secretion of CtsD is described here for the first time from CNS neurons and also for the first time in the living animal, but secretion and remote reuptake of lysosomal hydrolases seems to be not an unusual process in the CNS of rodents as demonstrated (e.g., for β-glucoronidase and tripeptidyl peptidase 1).26,27 In contrast, the substantial drainage of CtsD from CNS to periphery represents the first report to our knowledge of such a feature for a protein that is intracellularly sorted to lysosomes. CNS and periphery are not completely shielded from each other as it has long been shown that peptides can be drained from CNS to serum via the arachnoid villi of dural blood sinuses and to lymph nodes of the head and neck along cranial nerves.28,29,30 Such drainage from CNS to viscera, however, did not occur after CNS gene transfer of acid sphingomyelinase in the mouse model of Nieman-Pick disease, where systemic re-expression of the protein was essential to correct for visceral lesions.31 Thus, at least as investigated so far for other lysosomal hydrolases, CtsD is the only representative of this class of proteins, which is prominently drained from CNS to periphery.
Secreted CtsD has been demonstrated to act as a mitogenic ligand to as yet unidentified receptors in promoting tissue remodeling like metastasis and tumor growth.32,33,34 This mitogenic activity of CtsD is independent of its enzymatic activity, as is the described role of CtsD in sensitization of cells to apoptosis.35 Our results clearly rule out such roles of CtsD for the NCL-like phenotype, because re-expression of secreted but enzymatically inactive CtsD did not prevent any symptoms of central or visceral pathology in CtsD−/− mice. However, the mode of CtsD action might be indirect insofar as not CtsD activity directly but a processed substrate would be responsible for, for example, immune maintenance.
Reasons for the sudden recurrence of the visceral disease phenotype are enigmatic, as is its apparent 60 days periodicity in animals receiving both central and visceral vector injections. Ceasing transgene expression cannot be the cause, because brains were still protected from ceroid accumulation at times of recurrence of lymphopenia and the AAV vector with respect to serotype and promoter demonstrated stable transgene expression for more than a year in mice.20 Furthermore, CtsD activity as measured in liver did not decline after CtsD expression in brain. As the cell type that in periphery may depend on CtsD activity is unknown, we cannot rule out that in a particular cell type CtsD activity is ceasing over time, and forthcoming studies will have to demonstrate whether at certain times after adolescence drainage of CtsD from the brain may be impaired, or whether the protein is differentially distributed to certain tissues. Likewise, loss or decline of CtsD activity through cell divisions may have occurred. More detailed tracing studies of CtsD tissue distribution using an epitope-tagged version of the protein36 might help to clarify this issue. Our results nevertheless show that all visceral pathology in CtsD−/− is triggered by a common initiator, because at recurrence of disease all symptoms appear concomitantly.
Although long-term survivors are rare under the current treatment regimen with AAV-CtsD, these animals offer a unique opportunity to study mechanisms taken by brain tissue to deal with accumulations of ceroid and with pronounced microglia activation, and for the first time demonstrate that the CNS can tolerate very prominent accumulation of ceroid. These findings also demonstrate that neither ceroid accumulation nor continuous microglia activation are lethal factors for CtsD−/− mice.
Taken together, our results demonstrate several novel features of CtsD: i) the protein can be secreted and reuptaken very efficiently by CNS neurons but not by a variety of peripheral cell types, ii) the protein is drained from CNS to periphery at least partially through lymphatic routes, iii) this drainage temporally provides immune system maintenance and tissue homeostasis, iv) all observed effects of CtsD depend on the protein’s enzymatic activity and not on proposed functions as a ligand, v) the rodent CNS can tolerate severe accumulations of ceroid and microglia activation, arguing for vi) that CNS pathology in CtsD mice is not a lethal factor and does not initiate visceral pathology. This work also demonstrates that CtsD activity is only essential in one or more special peripheral cell types where it remote controls immune system maintenance and tissue homeostasis, and opens the venue for identification of these cells by differential analysis of CtsD distribution after central versus visceral re-expression.
Acknowledgments
We are grateful to Monika Zebski, Ulrike Schöll, and Martina Weig for expert technical assistance.
Footnotes
Address reprint requests to Sebastian Kügler, Ph.D., Department of Neurology, University Medicine Göttingen, Waldweg 33, 37073 Göttingen, Germany. E-mail: sebastian.kuegler@med.uni-goettingen.de.
Supported by the Deutsche Forschungsgemeinschaft (DFG) research center Molecular Physiology of the Brain and European Research Training Network contract No. MRTN-CT-2003-504636.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossmann H, Koster A, Hess B, Evers M, von Figura K. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 1995;14:3599–3608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechle D, Flad T, Cansier A, Steffen H, Schittek B, Tolson J, Herrmann T, Dihazi H, Beck A, Mueller GA, Mueller M, Stevanovic S, Garbe C, Mueller CA, Kalbacher H. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. J Biol Chem. 2006;281:5406–5415. doi: 10.1074/jbc.M504670200. [DOI] [PubMed] [Google Scholar]
- Saftig P, Peters C, von Figura K, Craessaerts K, Van Leuven F, De Strooper B. Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J Biol Chem. 1996;271:27241–27244. doi: 10.1074/jbc.271.44.27241. [DOI] [PubMed] [Google Scholar]
- Lkhider M, Castino R, Bouguyon E, Isidoro C, Ollivier-Bousquet M. Cathepsin D: released by lactating rat mammary epithelial cells is involved in prolactin cleavage under physiological conditions. J Cell Sci. 2004;117:5155–5164. doi: 10.1242/jcs.01396. [DOI] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Wickel M, Schneider-Brachert W, Trauzold A, Hethke A, Schutze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- Benes P, Vetvicka V, Fusek M. Cathepsin D–many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss CX, Villadangos JA, Watts C. Destructive potential of the aspartyl protease cathepsin D in MHC class II-restricted antigen processing. Eur J Immunol. 2005;35:3442–3451. doi: 10.1002/eji.200535320. [DOI] [PubMed] [Google Scholar]
- Deussing J, Roth W, Saftig P, Peters C, Ploegh HL, Villadangos JA. Cathepsins B and D: are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc Natl Acad Sci USA. 1998;95:4516–4521. doi: 10.1073/pnas.95.8.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusek M, Vetvicka V. Mitogenic function of human procathepsin D: the role of the propeptide. Biochem J. 1994;303:775–780. doi: 10.1042/bj3030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes P, Vashishta A, Saraswat-Ohri S, Fusek M, Pospisilova S, Tichy B, Vetvicka V. Effect of procathepsin D activation peptide on gene expression of breast cancer cells. Cancer Lett. 2006;239:46–54. doi: 10.1016/j.canlet.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, Lehesjoki AE, Tyynela J. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129:1438–1445. doi: 10.1093/brain/awl107. [DOI] [PubMed] [Google Scholar]
- Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Bruck W, Saftig P, Gartner J. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am J Hum Genet. 2006;78:988–998. doi: 10.1086/504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyynela J, Sohar I, Sleat DE, Gin RM, Donnelly RJ, Baumann M, Haltia M, Lobel P. A mutation in the ovine cathepsin D gene causes a congenital lysosomal storage disease with profound neurodegeneration. EMBO J. 2000;19:2786–2792. doi: 10.1093/emboj/19.12.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritchie K, Siintola E, Armao D, Lehesjoki AE, Marino T, Powell C, Tennison M, Booker JM, Koch S, Partanen S, Suzuki K, Tyynela J, Thorne LB. Novel mutation and the first prenatal screening of cathepsin D deficiency (CLN10). Acta Neuropathol. 2009;117:201–208. doi: 10.1007/s00401-008-0426-7. [DOI] [PubMed] [Google Scholar]
- Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, Ohsawa Y, Schulz-Schaeffer W, Watanabe T, Waguri S, Kametaka S, Shibata M, Yamamoto K, Kominami E, Peters C, von Figura K, Uchiyama Y. Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J Neurosci. 2000;20:6898–6906. doi: 10.1523/JNEUROSCI.20-18-06898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Shibata M, Ohsawa Y, Nakanishi H, Koga T, Kametaka S, Waguri S, Momoi T, Kominami E, Peters C, Figura K, Saftig P, Uchiyama Y. Involvement of two different cell death pathways in retinal atrophy of cathepsin D-deficient mice. Mol Cell Neurosci. 2003;22:146–161. doi: 10.1016/s1044-7431(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Yamasaki R, Zhang J, Koshiishi I, Sastradipura Suniarti DF, Wu Z, Peters C, Schwake M, Uchiyama Y, Kira J, Saftig P, Utsumi H, Nakanishi H. Involvement of lysosomal storage-induced p38 MAP kinase activation in the overproduction of nitric oxide by microglia in cathepsin D-deficient mice. Mol Cell Neurosci. 2007;35:573–584. doi: 10.1016/j.mcn.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Zhang J, Koike M, Nishioku T, Okamoto Y, Kominami E, von Figura K, Peters C, Yamamoto K, Saftig P, Uchiyama Y. Involvement of nitric oxide released from microglia-macrophages in pathological changes of cathepsin D-deficient mice. J Neurosci. 2001;21:7526–7533. doi: 10.1523/JNEUROSCI.21-19-07526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kügler S, Hahnewald R, Garrido M, Reiss J. Long-term rescue of a lethal inherited disease by adeno-associated virus-mediated gene transfer in a mouse model of molybdenum-cofactor deficiency. Am J Hum Genet. 2007;80:291–297. doi: 10.1086/511281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glondu M, Coopman P, Laurent-Matha V, Garcia M, Rochefort H, Liaudet-Coopman E. A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene. 2001;20:6920–6929. doi: 10.1038/sj.onc.1204843. [DOI] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Zakharov A, Papaiconomou C, Johnston M. Lymphatic vessels gain access to cerebrospinal fluid through unique association with olfactory nerves. Lymphat Res Biol. 2004;2:139–146. doi: 10.1089/lrb.2004.2.139. [DOI] [PubMed] [Google Scholar]
- Ni X, Canuel M, Morales CR. The sorting and trafficking of lysosomal proteins. Histol Histopathol. 2006;21:899–913. doi: 10.14670/HH-21.899. [DOI] [PubMed] [Google Scholar]
- Tulone C, Uchiyama Y, Novelli M, Grosvenor N, Saftig P, Chain BM. Haematopoietic development and immunological function in the absence of cathepsin D. BMC Immunol. 2007;8:22. doi: 10.1186/1471-2172-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi D, Peterson DA, Edelstein AM, del Fierro K, Hackett NR, Crystal RG. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp Neurol. 2008;213:18–27. doi: 10.1016/j.expneurol.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Martins I, Wemmie JA, Chiorini JA, Davidson BL. Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J Neurosci. 2005;25:9321–9327. doi: 10.1523/JNEUROSCI.2936-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion MF, Banks WA, Bost KL, Kastin AJ. Transmission routes of HIV-1 gp120 from brain to lymphoid tissues. Brain Res. 1999;822:26–33. doi: 10.1016/s0006-8993(99)01069-0. [DOI] [PubMed] [Google Scholar]
- Dickstein JB, Moldofsky H, Lue FA, Hay JB. Intracerebroventricular injection of TNF-alpha promotes sleep and is recovered in cervical lymph. Am J Physiol. 1999;276:R1018–R1022. doi: 10.1152/ajpregu.1999.276.4.R1018. [DOI] [PubMed] [Google Scholar]
- Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2:269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Passini MA, Bu J, Fidler JA, Ziegler RJ, Foley JW, Dodge JC, Yang WW, Clarke J, Taksir TV, Griffiths DA, Zhao MA, O'Riordan CR, Schuchman EH, Shihabuddin LS, Cheng SH. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc Natl Acad Sci USA. 2007;104:9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusek M, Vetvicka V. Dual role of cathepsin D: ligand and protease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:43–50. doi: 10.5507/bp.2005.003. [DOI] [PubMed] [Google Scholar]
- Laurent-Matha V, Lucas A, Huttler S, Sandhoff K, Garcia M, Rochefort H. Procathepsin D interacts with prosaposin in cancer cells but its internalization is not mediated by LDL receptor-related protein. Exp Cell Res. 2002;277:210–219. doi: 10.1006/excr.2002.5556. [DOI] [PubMed] [Google Scholar]
- Berchem G, Glondu M, Gleizes M, Brouillet JP, Vignon F, Garcia M, Liaudet-Coopman E. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene. 2002;21:5951–5955. doi: 10.1038/sj.onc.1205745. [DOI] [PubMed] [Google Scholar]
- Beaujouin M, Baghdiguian S, Glondu-Lassis M, Berchem G, Liaudet-Coopman E. Overexpression of both catalytically active and -inactive cathepsin D by cancer cells enhances apoptosis-dependent chemo-sensitivity. Oncogene. 2006;25:1967–1973. doi: 10.1038/sj.onc.1209221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsova Z, Malik JM, Michel U, Scholl U, Bähr M, Kügler S. Evaluation of epitope tags for protein detection after in vivo CNS gene transfer. Eur J Neurosci. 2006;23:1961–1969. doi: 10.1111/j.1460-9568.2006.04725.x. [DOI] [PubMed] [Google Scholar]