Abstract
Decreased blood flow to the brain in humans is associated with altered Alzheimer’s disease (AD)-related pathology, although the underlying mechanisms by which hypoperfusion influences AD neuropathology remains unknown. To try to address this question, we developed an oligemic model of cerebral hypoperfusion in the 3xTg-AD mouse model of AD. We bilaterally and transiently occluded the common carotid artery and then examined the molecular and cellular pathways by which hypoperfusion influenced tau and amyloid-β proteins. We report the novel finding that a single, mild, transient hypoperfusion insult acutely increases Aβ levels by enhancing β-secretase protein expression. In contrast, transient hypoperfusion markedly decreases total tau levels, coincident with activation of macroautophagy and ubiquitin-proteosome pathways. Furthermore, we find that oligemia results in a significant increase specifically in tau phosphorylated at serine212 and threonine214, a tau epitope associated with paired helical filaments in AD patients. Despite the mild and transient nature of this hypoperfusion injury, the pattern of decreased total tau, altered phosphorylated tau, and increased amyloid-β persisted for several weeks postoligemia. Our study indicates that a single, mild, cerebral hypoperfusion event produces profound and long lasting effects on both tau and amyloid-β. This finding may have implications for the pathogenesis of AD, as it indicates for the first time that total tau and amyloid-β are differentially impacted by mild hypoperfusion.
Alzheimer’s Disease (AD), a progressive, age-related neurodegenerative disorder, currently affects more than 5.3 million people in the United States.1 Pathologically, AD is characterized by the accumulation of two hallmark brain lesions: amyloid-β (Aβ) deposits, which can accumulate intracellularly but mainly occur as plaques composed of fibrillar aggregates of the 40- to 42-amino acid Aβ peptide, and intraneuronal neurofibrillary tangles, consisting of hyperphosphorylated and insoluble species of the microtubule-binding protein tau. The causes of sporadic AD are poorly understood, as are the factors that affect disease progression. A combination of lifestyle, environmental, dietary, and genetic and epigenetic factors, in concert with natural changes occurring in the aged brain, all likely influence the development and progression of sporadic AD. These factors can be broadly considered risk factors if they influence the initiation of disease, and co-morbidities when they influence the progression of AD.
The effect of mild hypoperfusion on Aβ has been largely unstudied, however, it is known that major hypoperfusion injuries up-regulate Aβ.2,3,4,5,6 Although the underlying mechanism remains unclear, positron emission tomography scans show that patients exhibit cerebral hypometabolism many years before being diagnosed with AD.7,8 Work in rodent models of AD indicates that severe ischemic insults, such as middle cerebral artery occlusion, increase both Aβ9,10,11 and phosphotau levels.12,13,14 Although the mechanism by which middle cerebral artery occlusion induces tau pathology remains to be defined, the elevation of Aβ following middle cerebral artery occlusion is linked to up-regulation of β-secretase (BACE1), and/or increased levels of the amyloid precursor protein (APP).4,5,15
There is a documented relationship between hypoperfusion injuries and the development of dementia and AD in humans. For example, patients who suffer an ischemic stroke are 2 to 5 times more likely to develop AD and dementia than other patients,16,17,18,19,20,21,22 and other insults that induce cerebral hypoperfusion, such as traumatic brain injury, also show similar trends.23,24,25 Although ischemic strokes are common in aged individuals, mild hypoperfusion events are far more prevalent but less studied. Mild hypoperfusion can result from a number of clinical conditions including mild ischemic stroke (ie, oligemia), ischemic stroke penumbra, migraines, traumatic brain injury, cardiac arrest, atherosclerosis, and excessively low or high cerebral blood pressure. Oligemia, sometimes called mild ischemia, refers to an episode of low blood flow that causes molecular changes, but does not produce an infarct or cell death. These hypoperfusion insults occur in a large cohort of the elderly population,17,26 yet their effects on AD neuropathology have not been systematically investigated. Understanding the consequences of mild hypoperfusion on AD pathology may help to identify populations that have a high risk of developing AD and could allow further insight into the pathogenesis and early treatment of AD.
Here, for the first time, we determined the impact of mild hypoperfusion injury on both tau and Aβ in a transgenic mouse model. We induced a transient global oligemia event by bilaterally occluding the common carotid artery in pre-symptomatic 3xTg-AD mice. Our results clearly show that oligemia elevates brain levels of Aβ42, clears intraneuronal total tau, and activates macroautophagy and ubiquitin-proteosomal pathways within the affected brain region for a prolonged period of time. These findings are critical because they show that oligemia/mild hypoperfusion produces a long lasting effect on AD-related proteins, lowering total and select phosphotau levels via autophagy and ubiquitin-proteosome dependent pathways and increasing Aβ levels via enhanced BACE1 expression. Taken together, we show that even a single, short, mild hypoperfusion event results in profound and long-lasting changes in AD-related proteins and may influence disease progression.
Materials and Methods
Global Oligemia
Male 3xTg-AD mice, 3 months of age, were deeply anesthetized with isoflurane mixed with 20% oxygen, placed on a temperature-feedback controlled heating pad and maintained at a core body temperature of 37° ± 2°C. The bilateral common carotid artery occlusion was performed based on previously described global ischemic injuries.27 Briefly, an incision was made on the skin at the midline of the ventral neck. The common carotid arteries were exposed, bilaterally isolated, and temporarily clamped (Fine Science Tools, Foster City, CA) for 4-minutes to occlude blood flow as confirmed by visual inspection. After the occlusion, the clips were removed and the skin sutured. Antibiotic cream was placed on the wound and the animals were allowed to recover in a temperature-controlled cage.
Animal Treatments
All rodent experiments were performed in accordance with animal protocols approved by the Institutional Animal Care and Use Committee at the University of California, Irvine. The triple-transgenic mice (3xTg-AD) have been described previously.28 After the treatment, the animals were sacrificed and the brains removed. The brains were immediately dissected in half sagittally. One-half was frozen for biochemical analysis and the other fixed in 4% paraformaldehyde; 48 hours later, brains were sliced into 50 μmol/L sections using a vibratome.
Immunoblotting
Protein extracts were prepared from complete half brain samples cut sagittally or microdissected hippocampal samples homogenized in T-per (Pierce Biotechnology, Rockford, IL) extraction buffer and Complete Mini Protease Inhibitor Tablets (Roche, Indianapolis, IN) followed by high-speed centrifugation at 100,000 × g for 1 hour. The supernatant was taken as the soluble protein extract. The insoluble fraction was taken by collecting the supernatant after resuspending the pellet remaining from the soluble fraction in 70% formic acid and recentrifuging the sample at 100,000 × g for 1 hour. Protein concentrations were determined by the Bradford method. Equal concentrations of protein were separated by SDS-polyacrylamide gel electrophoresis on a 4 to 12%, 12%, or 10% Bis/Tris gel using 2-(N-morpholino)ethanesulfonic acid (MES) or 3-(N-morpholino)propanesulfonic acid (MOPS) running buffer (Invitrogen, Carlsbad, CA) , transferred to 0.2 mmol/L nitrocellulose membranes, blocked for 1 hour in 5% (v/v) nonfat milk in Tris-buffered saline (pH 7.5) supplemented with 0.2% Tween20. Antibodies and dilutions used in this study include hypoxia-inducible factor HIF1α 1:1000 (Novus Biological, Littleton, CO) 6E10 (1:1000 Signet, Dedham, MA), CTF20 (1:5000 Calbiochem, San Diego, CA), HT7 (1:3000; Innogenetics, Gent, Belgium), Phosphotau at S212 T214 (AT100), Thr231 (AT180), and Thr181 (AT270) (1:1000, Pierce Biotechnology, Rockford, IL), phosphotau at S199 S202 (AT8) (1:1000, Pierce Biotechnology, Rockford, IL & 1:1000 EMD Gibbstown, NJ), PHF1 (1:1000 Thermo Scientific, Waltham, MA), anti-BACE (1:1000 Calbiochem, San Diego, CA), anti-ADAM10 (1:1000 Chemicon, Temecula, CA), anti-GGA3 (1:1000 Abcam ab10553), anti-Furin (1:500 Affinity Bioreagents Rockford, IL), anti-GSK3β (1:1000 Cell Signaling Danvers, MA), anti-PP2A (1:1000 Millipore, Billerica, MA), anti-HSP70 (1:1000 Calbiochem, San Diego, CA), anti-CHIP (1:1000 Affinity BioReagents Rockford, IL), anti-LC3 (1:1000 Affinity BioReagents Rockford, IL), FK2 (1:1000 DAKO Carpinteria, CA), anti-Beclin1 (1:1000 Santa Cruz, CA), anti mTOR (1:1000 Sigma-Aldrich, St. Louis MO), anti-PmTOR (1:1000 Cell Signaling, Danvers MA), anti-p62 (1:1000 BIOMOL International, Plymouth Meeting, PA), anti-ATG12 (1:1000 Novus Biological Littleton, CO), anti-ATG5 (1:000 Abcam Cambridge, MA), and anti-actin (1:10,000; Sigma-Aldrich). Quantitative densiometric analyses were performed on digitized images of immunoblots with ImageJ. All error bars represent SEM.
Aβ Enzyme-Linked Immunosorbent Assay
Aβ1-40 and Aβ1-42 were measured using a sensitive sandwich enzyme-linked immunosorbent assay (ELISA) system. Soluble and insoluble Aβ was isolated from entire half brain homogenates using T-per Extraction Buffer (Pierce Biotechnology, Rockford, IL) and 70% formic acid respectively. Soluble fractions were loaded directly onto ELISA plates and formic acid fractions were diluted 1:20 in neutralization buffer (1 mol/L Tris base; 0.5 mol/L NaH4PO4) before loading. MaxiSorp immunoplates (Nunc, Rochester, NY) were coated with mAB20.1 (William Van Nostrand, Stony Brook, NY) antibody at a concentration of 25 mg/ml in Coating Buffer (0.1 mol/L NaCO3 buffer, pH 9.6), and blocked with 3% bovine serum albumin. Standards of both Aβ40 and 42 were made in Antigen Capture Buffer (ACB; 20 mmol/L NaH2PO4; 2 mmol/L EDTA, 0.4 mol/L NaCl; 0.5 g 3[(3-Cholamidopropyl)dimethylammonio]-propanesulfonic acid (CHAPS); 1% bovine serum albumin, pH 7.0), and loaded onto ELISA plates in duplicate. Samples were then loaded in duplicate and incubated overnight at 4°C. Plates were washed and then probed with either horseradish peroxidase-conjugated anti-Aβ 35–40 (MM32-13.1.1, for Aβ1-40) or anti-Aβ 35–42 (MM40-21.3.4, for Aβ1-42) overnight at 4°C. 3,3′,5,5′-tetramethylbenzidine was used as the chromogen, and the reaction stopped by 30% O-phosphoric acid, and read at 450 nm on a Molecular Dynamics plate reader.
Immunostaining
Light-level immunohistochemistry was performed using an avidin-biotin immunoperoxidase technique (ABC kit; Vector Laboratories Inc., Burlingame, CA) and was visualized with diaminobenzidine as previously described.28 The following antibodies were used: anti-Aβ, 6E10 (Signet Laboratories, Dedham, MA) and anti-Tau HT7 (Innogenetics, Gent, Belgium). Primary antibodies were applied at dilutions of 1:1000.
Confocal Microscopy
Fluorescent immunolabeling followed a standard two-way technique (primary antibody followed by fluorescent secondary antibody). Free-floating sections were rinsed in Tris-buffered saline (pH 7.4) and then blocked (0.25% Triton X-100, 5% normal goat serum in Tris-buffered saline) for 1 hour. Sections were incubated in primary antibody overnight (4°C), rinsed in PBS, and incubated (1 hour) in either fluorescently labeled anti-rabbit (Alexa 555, 1:200; Molecular Probes Inc., Eugene, OR) or anti-mouse secondary antibodies (Alexa 488, 1:200; Molecular Probes Inc.). Antibodies were diluted as follows: HT7 (1:1000 Innogenetics, Gent, Belgium), anti-cathepsin D (1:1000 DAKO Carpinteria, CA), FK2 (Enzo, Plymouth Meeting, PA) LC3 (1:1000 BioRegents, Rockford, IL). Nuclear stain TOTO-red (1:1000 Invitrogen) was incubated for 20 minutes. Omission of primary antibody or use of pre-immune IgG eliminated all labeling (data not shown). Confocal images were captured on a Biorad Radiance 2100 (Bio-Rad, Hercules, California) confocal system. To prevent signal bleed-through, all fluorophores were excited and scanned separately using lambda strobing.
Statistical Analyses
Biochemical data were analyzed by Student’s t-tests. Results were considered significant only when P ≤ 0.05.
Results
Induction of Oligemia in 3xTg-AD Mice
To model a mild hypoperfusion injury, an incomplete global cerebral oligemic insult was induced by transiently and bilaterally occluding the common carotid arteries using removable clamps. The occlusion lasted 4 minutes and was performed in pre-symptomatic 3-month-old male 3xTg-AD mice.
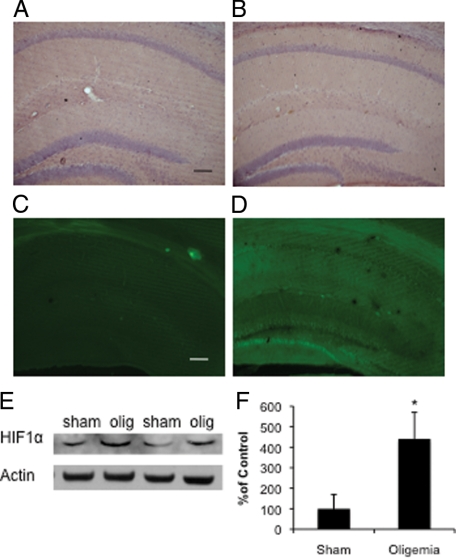
To confirm that an oligemic, and not an ischemic, insult was induced, we sacrificed the mice 48 hours postinjury to determine whether there was evidence of cell death or changes in neuronal morphology in the brain. No differences in cell morphology were evident between sham- and oligemia-treated mice 48-hours post surgery following staining with H&E (Figure 1A sham, B oligemic). Likewise, fluoro-jade labeling revealed no evidence of neurodegeneration in oligemic tissue (Figure 1C), as opposed to tissue collected from mice 48-hours after a 12-minute ischemic bilateral common carotid artery occlusion (Figure 1D). Immunohistological assessment of neuronal nuclei using the marker NeuN, also revealed no quantitative differences between sham- and oligemia-treated groups (data not shown). In contrast, we did find significant increases in steady state HIF1α levels by Western blot analysis of hippocampal lysates (P ≤ 0.05) (Figure 1, E and F). Under normoxic conditions, HIF1α is proteolytically degraded, however, under hypoxic conditions it accumulates and it able to bind to HIF1β and translocate to the nucleus where it acts as a potent transcription factor. The increase we observe in HIF1α levels indicates that our oligemic surgery successfully occluded blood flow, and therefore oxygen supply, to the brain. Together, these results demonstrate that bilaterally occluding the common carotid arteries for 4 minutes does not induce cell death or detectable changes in cell morphology, but does reproducibly alter cerebral blood flow and oxygenation, consistent with the induction of an oligemic, and not ischemic, insult.
Figure 1.
Induction of global oligemia in 3xTg-AD mice. A: Pathological analysis of H&E staining of sham and (B) oligemic 3xTg-AD mice failed to reveal any alteration in the gross or microscopic architecture of the brain (Scale bar = 250 μm). C: Fluoro-jade staining of the oligemic mice did not reveal any degenerating cells, consistent with the absence of cell death associated with an oligemic event. D: In contrast, following a global ischemic event, there was prominent cell loss in the dentate gyrus and hippocampal subfields (Scale bar = 250 μm). Western blot (E) and quantitative analysis (F) revealed a dramatic increase in HIF1α in tissue from oligemic mice.
Oligemia Alters APP Processing and Increases Aβ42
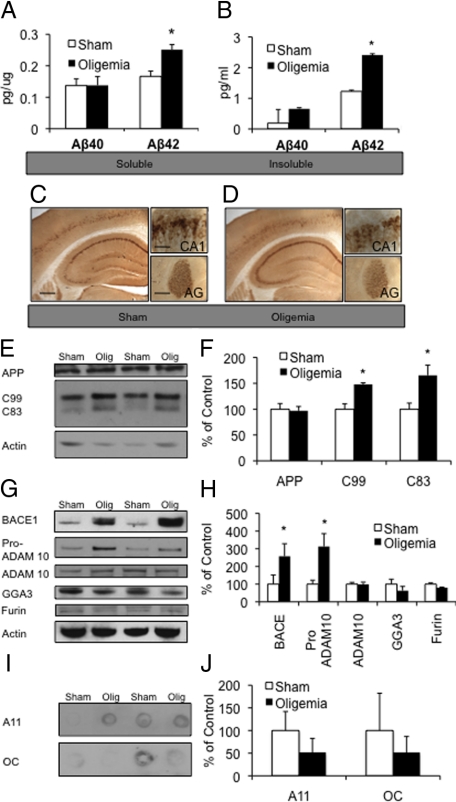
Three-month-old, presymptomatic, 3xTg-AD mice were bilaterally occluded at the carotid arteries for 4 minutes, to induce an oligemic insult. Mice were left to recover for 48-hours and then sacrificed (n = 10 oligemia 3xTg-AD and n = 8 sham 3xTg-AD mice). Half brains from each animal were collected for immunohistochemistry, while the other half was taken for biochemical analyses. Microdissection of the hippocampus was performed in five oligemia and four sham animals, to look at protein degradation, tau phosphorylation, and APP processing pathways. The remaining half-brains were homogenized for analysis of AD-pathological proteins, as expression is present throughout the hippocampus, cortex, and amygdala. To determine the acute impact of an oligemic event on AD pathology, both detergent soluble and insoluble Aβ levels were measured using a quantitative Aβ sandwich ELISA. These comparisons revealed that both soluble (P ≤ 0.05) and insoluble Aβ42 levels (P ≤ 0.01) were significantly increased by 50% and 96%, respectively, in oligemic versus sham-treated 3xTg-AD mice (Figure 2, A and B). Although Aβ42 levels were elevated as measured by ELISA, immunohistochemical labeling with the antibody 6E10 revealed no differences between oligemia and sham-treated groups (Figure 2, C and D). The discrepancy between the histochemical and ELISA measurements likely reflects the increased sensitivity and quantitative nature of the ELISA, and because 6E10 antibody detects both Aβ and APP by histochemistry, whereas the ELISA was performed using an Aβ42-neopepitope antibody. In agreement with this, levels of APP were unaltered between oligemic and sham-treated mice by Western blot analyses in hippocampal lysates (Figure 2, E and F).
Figure 2.
Oligemia led to higher Aβ42 levels and altered APP processing. A, B: ELISA measurements of soluble and insoluble Aβ levels 48 hours after oligemia, showed a significant increase in Aβ42 in both the soluble and insoluble fractions. C, D: Immunohistochemical analysis of sham and oligemic 3xTg-AD mice with antibody 6E10 did not reveal any profound alterations (hippocampus Scale bar = 500 μm). Insets show higher magnification of the CA1 (Scale bar = 62.5 μm) and amygdala (AG) (Scale bar = 125 μm). E, F: Western blot and quantitative analysis of APP, C99, and C83 showing increased levels of C99 and C83 in the oligemic samples. G, H: Western blot and quantitative analysis of proteins involved in APP processing. I, J: Measurement of Aβ oligomers via dot blot the antibodies A11 and OC failed to reveal any differences in relative density between sham and oligemic mice. All Western blots were normalized to actin and represented as a percentage of sham.
APP is sequentially cleaved, first by either α- or β-secretase and then by γ-secretase. α-secretase produces a large ectodomain fragment, sAPPα, and the C-terminal fragment C83, whereas β-secretase cleavage generates sAPPβ and a slightly larger C-terminal fragment termed C99. Importantly, α-secretase cleavage of APP occurs within the Aβ sequence, precluding Aβ formation. As the biochemical data revealed increased Aβ levels but no changes in APP expression, we next examined hippocampal lysates to determine whether levels of the C83 or C99 were altered following oligemia. As demonstrated, oligemia paradoxically leads to significantly higher levels of both C83 (P ≤ 0.01) and C99 (P ≤ 0.05) 48-hours post injury (Figure 2, E and F). An elevation in both C83 and C99 without a concomitant increase in holo-APP levels suggests that both α- and β-secretase activities are increased, or alternatively that the cleavage of C83 and C99 by γ-secretase is impaired.
To further assess the mechanism by which oligemia elevates Aβ levels, we examined levels of the putative constitutive α-secretase; ADAM 10. Levels of mature ADAM 10 were unaltered by oligemia, but levels of its immediate precursor; pro-ADAM10, were significantly increased (P ≤ 0.05) (Figure 2, G and H). This finding could suggest that there is a decrease in the proteinases that convert the pro-ADAM 10 to the catalytically active mature form, however we found no changes in furin, a proteinase known to cleave ADAM10 (Figure 2, G and H).29
BACE-1 is the primary β-secretase that mediates cleavage of APP to generate C99, thus allowing for the subsequent generation of Aβ. Interestingly, we found a dramatic increase in BACE-1 protein levels in oligemic versus sham hippocampal lysates (P ≤ 0.05) (Figure 2, G and H). Further analyses of BACE1 levels in immunostained tissue from sham and oligemia 3xTg-AD mice also showed increases in the oligemia induced tissue (data not shown). A previous report found that following severe focal ischemia, BACE-1 levels are increased via a reduction in lysosomal degradation of BACE-1 caused by insufficient GGA3 protein.15 GGA3 traffics BACE-1 to the lysosome under endogenous conditions but is depleted following ischemic stress.15 Although we examined levels of GGA3 following oligemia, we found no significant differences indicating that the mechanism by which Aβ is elevated following an oligemic insult likely differs from that observed following a more severe ischemic insult (Figure 2, G and H). BACE1 levels have also been shown to increase via HIF1α dependent transcription4—therefore, increased HIF1α (Figure 1, E and F) in these mice could account for the selective elevation in BACE1 steady state levels.
Soluble Aβ oligomers are strongly implicated in the development of AD.30 Hence, we investigated whether levels of Aβ oligomers are altered following oligemia. We used A11 and OC antibodies, to examine soluble oligomers and soluble fibrils respectively.31 No significant changes in soluble Aβ oligomers or fibrils were detected 48 hours following oligemia (Figure 2, I and J), suggesting that short-term oligemia does not modulate this particular aspect of AD pathology, despite increases in overall Aβ levels.
Oligemia Decreases Total Tau 48 Hours Postinsult
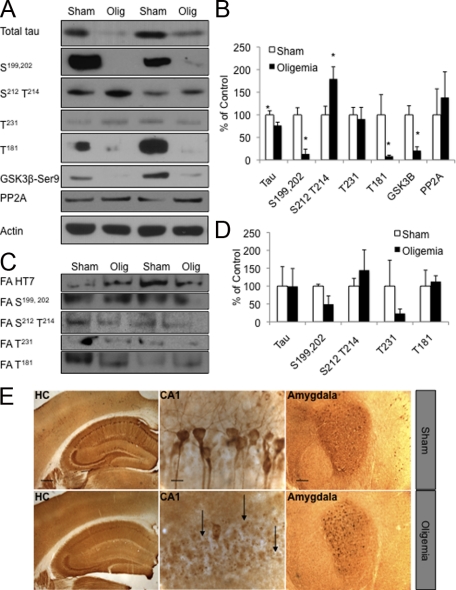
The 3xTg-AD model develops both Aβ and tau pathologies, allowing us to assess the impact of a transient oligemia on both of these hallmark pathologies of AD. Hence, we examined total tau protein levels in the oligemia versus sham-treated 3xTg-AD mice. Notably, we found a significant decrease in total tau levels (P ≤ 0.05) in brain homogenates from mice exposed to transient oligemia (Figure 3, A and B). The somatodendritic mislocalization of tau is an important component of AD-related pathology; consequently, we assessed the subcellular distribution of tau within hippocampal CA1 neurons following oligemia by light microscopy. We observed a marked reduction in the levels of total human tau in the hippocampus of oligemic mice compared with sham mice (Figure 3E, HC). Whereas sham-treated mice had extensive somatodendritic tau staining in the hippocampus, somatodendritic tau was all but absent in the oligemia-treated mice (Figure 3E). The tau that remained in the oligemic tissue was segregated into small puncta within the pyramidal cells of the hippocampus. We next examined the distribution of tau within the amygdala, an area that is unaffected by common carotid artery occlusion but that demonstrates high tau protein levels in the 3xTg-AD mice. Tau localization was unaltered within this region demonstrating the regionally specific influence of oligemia on tau distribution (Figure 3E; amygdala).
Figure 3.
Global oligemia led to a reduction in total tau levels. A, B: Western blot and quantitative analysis of total tau (HT7) and phosphorylated tau (labels indicate phosphorylation site recognized by the antibody) 48-hours after insult revealed a significant decrease in total tau, tau phosphorylated at S199,202 and T181 and GSK3ß phosphorylated at Ser9 protein levels. C, D: Analysis of total and phosphotau epitopes in the formic acid fraction by Western blot analysis revealed no alterations between sham and oligemic tissue. E: Immunohistochemical analysis of sham versus oligemic mice showed a prominent reduction of total tau in the CA1, but not in the amygdala. HC (hippocampus; Scale bar = 500 μm); CA1 subfield of the hippocampus (CA1; Scale bar = 62.5 μm) and amygdala (Scale bar = 125 μm). Arrows indicate areas of decreased staining. Western blots were normalized to actin and represented as a percentage of sham.
Given these specific effects on tau accumulation within the hippocampal region we used microdissected hippocampal lysates from sham and oligemia-induced 3xTg-AD mice to examine steady state levels of hyperphosphorylated tau via Western blot (Figure 3, A and B). No changes in tau phosphorylated at Thr214 were seen in the mice following the oligemic insult, however, significant decreases in tau phosphorylated at Ser199, 202 and Thr181 were detected (Figure 3, A and B; P ≤ 0.05). These reductions are likely due to the overall reduction in total human tau, which includes hyperphosphorylated tau, rather than specific changes in tau phosphorylation/dephosphorylation. Indeed, analyses of steady state levels of the major tau phosphatase, PP2A, revealed no changes following induction of oligemia (Figure 3, A and B). Notably, protein levels of tau phosphorylated at Ser212 and Thr214 were increased following oligemia, indicating that tau may be specifically phosphorylated post injury or that this phosphotau epitope may be more resistant to degradation (P ≤ 0.05). GSK3β is a kinase implicated in the phosphorylation of tau at this epitope,32 and its activity is negatively regulated by its phosphorylation at Ser9. Consistent with the increase in tau phosphorylated at Ser212/Thr,214 we found a significant decrease in inactivated GSK3β, indicating increased GSK3β activity (Figure 3A, B; P ≤ 0.05).
We next sought to account for the robust reduction in steady state levels of human tau following induction of oligemia. One possibility is that the oligemic insult was inducing tau aggregation that would cause it to move into the detergent-insoluble fraction. Given the stark clearance of somatodendritic tau from hippocampal neurons, as shown by immunohistochemistry (Figure 3E) which detects all tau regardless of aggregation state, movement of tau into the detergent insoluble fraction is unlikely. However, to rule out the possibility, we examined the levels of tau and phosphotau protein in the insoluble fraction. In agreement with our immunohistochemical data, we found no significant alteration of tau or phosphotau between sham and oligemia-treated animals within formic acid extracted samples (Figure 3C, D; P > 0.05).
Tau is Associated with Lysosomes in Oligemia-Induced 3xTg-AD Hippocampi
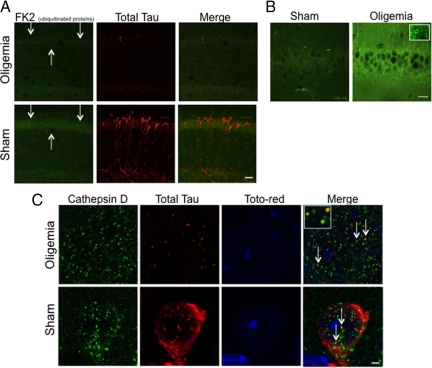
Given the reduction in steady state levels of tau and the change in staining pattern from extensive somatodendritic to small puncta following oligemia, we hypothesized that oligemia may induce increased tau degradation by lysosomal or ubiquitin-proteosomal targeting and degradation of tau. We first confirmed the reduction in total tau using immunofluorescence and found a marked decrease in HT7 staining 48 hours following oligemia (Figure 4A; red). We next examined ubiquitinated proteins using FK2 antibody (Figure 4A; green), which recognizes only ubiquitinated proteins (mono- and poly-) but not free ubiquitin. Confocal microscopy revealed a decrease in the overall level of ubiquitinated proteins within CA1 of the hippocampus (Figure 4A, arrows) suggesting that alterations in the ubiquitin-proteosome system may have occurred.
Figure 4.
Hippocampal tau and ubiquitinated protein immunoreactivity decreased following oligemia and tau colocalized with lysosomes. A: Confocal images reveal a decrease in the ubiquitinated protein marker, FK2, in oligemia treated animals (Scale bar = 40.5 μm; arrows indicate CA1) and confirmed the decrease in HT7 observed in Figure 3. B: Increased LC3 staining was found in the hippocampi of animals exposed to oligemia compared with sham animals, inset shows the granular nature of the staining (Scale bar = 20um). C: Confocal microscopy of the lysosomal marker, (cathepsin D; green), human tau, (HT7; red), and nuclear marker, toto-red (blue) demonstrated that tau immunoreactive puncta colocalized with intraneuronal lysosomes (arrows indicate colocalization, yellow puncta. Inset shows magnification of three puncta) (Scale bar = 2.5 μm).
Autophagy is a process whereby proteins and organelles are delivered to the lysosome for degradation. To determine whether alterations in autophagy might also be involved in the observed reduction in tau protein, we examined the autophagosome-associated protein, LC3, by confocal microscopy. LC3 is a protein that localizes to the membrane of autophagosomes and is involved in the induction of autophagosomes.33 LC3 exists in two forms—the first is a cytosolic form (LC3 I), which can then become lipidated and membrane associated (LC3 II) to form autophagosomes. We detected an increase in total LC3 staining within hippocampal neuronal cell bodies between sham- and oligemia-induced animals, which manifested as increased intensity as well as increased number of puncta (Figure 4B, inset represents higher magnification of the puncta).
Lysosomes are at the end stage of the autophagolysosomal pathway and have previously been shown to degrade tau on exposure to stressors.34,35 Consequently, we performed triple immunofluorescent analysis of subcellular tau localization using a nuclear marker (toto-red), an antibody against total human tau (HT7), and a lysosomal marker (cathepsin-D) (Figure 4C). Z-slice confocal microscopy imaging revealed many examples of HT7-positive tau puncta in oligemic CA1 neurons that either colocalized or were juxtaposed with cathepsin-D immunoreactivity (Figure 4C, arrows, inset represents higher magnification). In hippocampal sections from sham animals, the vast majority of human tau appeared diffuse and cytosolic, rather than solely in lysosome associated-puncta. However, some human tau was also associated with lysosomes suggesting that this association is not uniquely induced by oligemia, but that it is altered by oligemia. Hence, by immunofluorescence, changes in both macroautophagy and the ubiquitin-proteosome pathway occur following oligemia in areas of the brain that somatodendritic clearance of human tau was observed.
Oligemia Induces Markers of Degradation Pathways
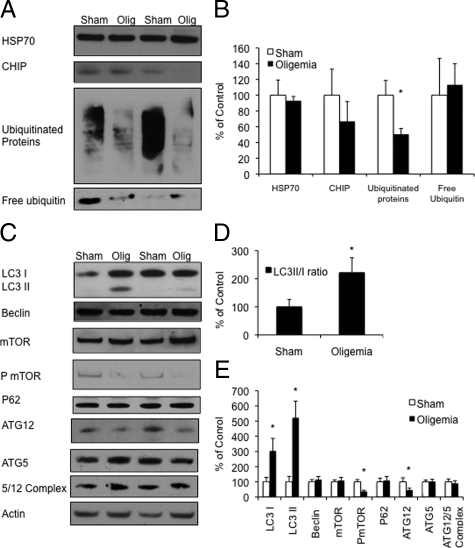
To further examine the putative role of lysosomal and ubiquitin-proteosomal-dependent pathways in oligemia-induced tau reduction, we examined both lysosomal and proteasomal-related co-chaperone and marker proteins. Degradation of tau by the proteasome is an established phenomena.36 Hence, we examined 3 markers of protein degradation involved in the ubiquitin-proteosomal pathway, ubiquitinated proteins, and known tau chaperones CHIP and HSP70. Due to the robust clearance of somatodendritic tau from hippocampal neurons we performed these Western blots with microdissected hippocampal tissue. No changes in steady state levels of CHIP or HSP70 were seen following oligemia (Figure 5, A and B). However, in accordance with the previously observed immunofluorescent reduction in ubiquitinated proteins, we found significant reductions in overall ubiquitinated proteins following oligemia (P ≤ 0.01 Figure 5, A and B). We repeated this experiment with a second antibody that detects both ubiquitinated proteins, as well as free ubiquitin. Again ubiquitinated proteins were markedly decreased in the oligemia-induced animals, but free ubiquitin was unaltered (Figure 5, A and B). Based on the decrease in ubiquitinated proteins, and the fact that free ubiquitin was not altered, these data suggest that there is an increase in ubiquitin-proteosome-related degradation and that tau could be cleared through this pathway.
Figure 5.
Oligemia increased autophagy and ubiquitinated protein markers. A: Western blot analysis comparing levels of proteosome-degradation related markers in sham versus oligemic mice. B: Quantification of Western blots normalized to actin and graphed as a percentage of control. C: Western blots of autophagy-related proteins. D: LC3II/I ratio demonstrates an increase in LC3II to LC3I levels. E: Quantification of Western blots normalized to actin and graphed as a percentage of control.
Based on the colocalization of total tau with lysosomal markers we next proceeded to determine the impact oligemia had on autophagy-related proteins. Given that much of the tau remaining in the hippocampus at 48 hours after oligemia colocalized with a marker within the autophagolysosomal pathway, we next determined the impact of oligemia on LC3. As we previously found an alteration in LC3 by immunohistochemistry, we next used Western blot to assess LC3 activity. LC3 can exist in multiple states; an 18kDa pro-form LC3I or LC3II a 16kDa membrane-bound active form associated with autophagosomes. Interestingly, Western blot analysis of LC3 revealed a significant increase in both the pro (P ≤ 0.05) and active forms (p<0.05) of this protein 48 hours postoligemia, again supporting the notion that autophagy is up-regulated following oligemia (Figure 5, C and E). Furthermore, we found a significantly higher ratio of LC3II to LC3I in the oligemic animals (P ≤ 0.05, Figure 5D), suggesting that autophagy may be increased in these samples. Phosphorylated mammalian target of rapamycin (PmTOR) is a negative inhibitor of autophagy and has also been widely used as a marker of autophagy. We observed a significant decrease in PmTOR levels (P ≤ 0.01) following oligemia, without an alteration in total mTOR protein levels, further indicating that autophagy pathways were activated (Figure 5, C and E). Lastly, we examined ATG12 and ATG5 levels. ATG12 covalently binds to ATG5 during the formation step of autophagosome elongation. We found a significant decrease in levels of ATG12 (P ≤ 0.05) but no alterations in ATG5 or the ATG5/12 complex (Figure 5, C and E). However, we did not find changes in two other proteins implicated in autophagy: Beclin1, a protein involved in the induction of autophagy and p62, a polyubiquitinated protein that is normally degraded by autophagy (Figure 5, C and E).37
Oligemia Continues to Alter AD-Related Proteins Three Weeks Postinjury
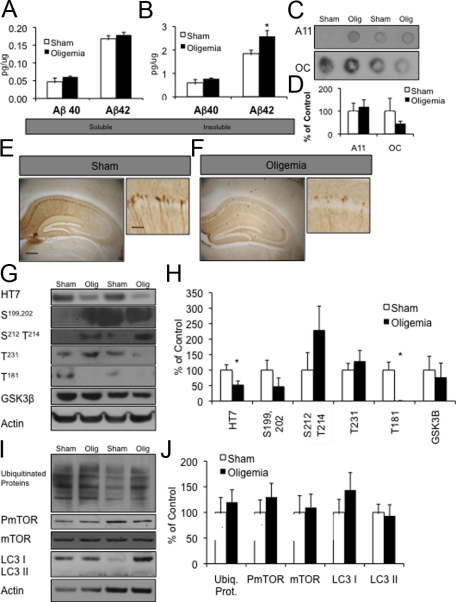
To determine the impact of oligemia on AD-related proteins after the acute response receded, we induced oligemia in six 3-month-old 3xTg-AD mice with six sham animals and survived them for 3 weeks post-surgery. We examined detergent soluble and insoluble Aβ levels by sandwich ELISA and found that soluble Aβ40 levels had returned to baseline, but that there was still a significant increase in Aβ42 levels in the insoluble fraction (P ≤ 0.05) (Figure 6, A and B). In accordance with the 48-hour survival time point, we did not find any changes in soluble oligomeric and fibrillar Aβ by dot blot (Figure 6, C and D). Notably, total tau levels also remained decreased 3 weeks after oligemia by immunohistochemistry (Figure 6, E and F). Western blot analysis confirmed these findings (P ≤ 0.01) (Figure 6, E, G, and H). We also determined that while tau phosphorylated at Ser181 was still decreased at this time point (P ≤ 0.05), all other phosphotau epitopes examined were no longer significantly altered (Figure 6, G and H). Autophagy and ubiquitin-proteosome markers were also no longer elevated above sham 3 weeks after injury (Figure 6, I and J), suggesting that short-term oligemia produces only a transient changes in ubiquitin-proteosome pathways and macroautophagy.
Figure 6.
At three weeks postoligemia, Aβ remained increased whereas tau continued to be decreased. A, B: ELISA measurements of soluble and insoluble Aβ levels three weeks after oligemia, showed a significant increase in Aβ42 in the insoluble fractions. C, D: Measurement of soluble Aβ oligomers, A11, and Aβ fibrils, OC, failed to reveal any alteration between sham and oligemic mice. E, F: Immunohistochemical stains of sham versus oligemic tissue showed a continued reduction of total tau in the hippocampus (Scale bar = 340 μm hip and 10 μm CA1). G, H: Western blot analysis revealed sustained alterations in total tau and one phosphotau protein levels. I, J: Western blot analysis revealed that autophagy-related markers were no longer different between sham and oligemic mice. Quantification of all Western blots was normalized to actin and graphed as a percentage of control.
AD-Related Proteins Return to Baseline Three Months Postoligemia
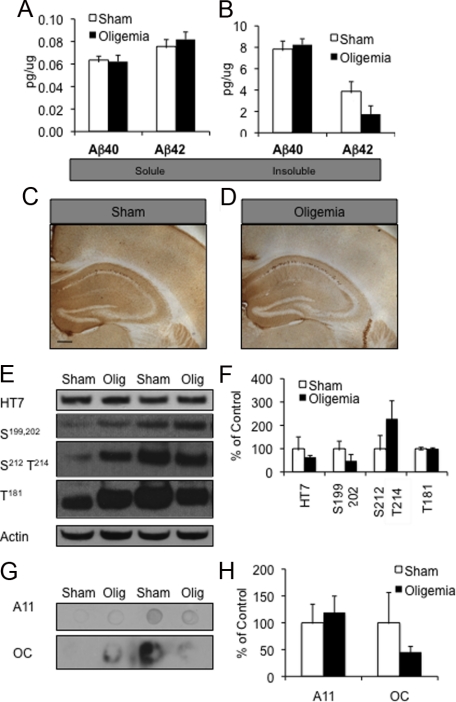
To determine the long-term impact of a single mild oligemic insult on AD-related proteins, we survived mice for 3 months following the 4-minute transient bilateral common carotid artery oligemic occlusion (n = 6 for oligemic mice and n = 6 for sham animals). We found no difference in Aβ levels as measured by ELISA between the sham- and oligemia-treated mice, (Figure 7, A and B). By 3 months postoligemic insult, we also no longer detected a change in tau or phosphotau levels by either immunohistochemistry (Figure 7, C and D) or Western blot (Figure 7, E and F), nor did we find any alterations in ubiquitinated proteins (data not shown). Furthermore, we found no changes in soluble oligomeric and fibrillar Aβ by dot blot (Figure 7, G and H). Hence, 3 months after a single oligemia insult, AD-related protein levels returned to baseline.
Figure 7.
At three months postoligemia Aβ and tau levels returned to baseline. A, B: ELISA measurements of Aβ no longer revealed any difference between sham and oligemic tissue. C–F: Tau immunohistochemistry and Western blot analyses also failed to reveal differences between sham and oligemic tissue at this time point hippocampus (Scale bar = 340 μm). Quantification of all Western blots was normalized to actin and graphed as a percentage of control. G, H: Measurement of soluble Aβ oligomers, A11, and Aβ fibrils, OC by dot blot failed to reveal any alteration between sham and oligemic mice.
Discussion
Understanding and defining the factors that lead to AD or impacts its progression is crucial for the development of strategies to prevent or delay disease onset in patients. Hypoperfusion injury is implicated both as a risk factor for AD, as well as a comorbidity that can accelerate cognitive decline.5,38,39 By modeling an oligemic insult in young male 3xTg-AD mice, we show that even a single, mild hypoperfusion event has profound and long-lasting effects on AD-related proteins, highlighting the close relationship between cerebral blood flow and tau and Aβ levels.
We show that Aβ42 levels rise following oligemia, and that elevated levels are sustained for at least 3 weeks following the single insult. This is the first time that a transient oligemic hypoperfusion injury has been systematically tested in a mouse model of AD. Furthermore because the 3xTg-AD mice develop both tau and Aβ pathologies the alteration in Aβ may more accurately reflect the human disease, in which both proteins accumulate. It should be noted that transgenic mouse models of AD are based on overexpression of human transgenes under artificial promoters meaning that any effects of hypoperfusion on human promoter–dependent APP and tau expression are not recapitulated. Therefore, we need to use epidemiological data from humans combined with pre-pathological transgenic mice to gain insights into how hypoperfusion insults can affect pathways leading to the accumulation of pathologies. For example, here we have shown robust increases in BACE1, which leads to increased production of C99 and subsequently Aβ, combined with striking reductions in tau accumulation.
Clinical data show strong links between hypoperfusion insults, through ischemia and strokes, and the development of AD. Hence, our data, with a single mild hypoperfusion event, recapitulates some of these clinical features. We cannot surmise that a single mild hypoperfusion event can increase the risk of developing AD, due to limitations in rodent models of sporadic AD, however we can show that such an event can profoundly alter AD-related proteins in a mouse which, although programmed to develop extensive pathology over its lifespan, has only very mild pathology at the time of the insult. Taken together, our data, and that of others, show a relationship between hypoperfusion insults, both mild and severe, and changes in AD-related proteins. Indeed, previous studies using severe and prolonged focal ischemia have also highlighted increases in BACE1 protein and Aβ following insult, which have been mediated by reduced clearance of BACE1 by the lysosome,15 and through increased HIF1α dependent transcription.4 Similar results have also been reported in rodents in a hypoxic environment,5 as well as chronically hypoperfused rats.38 The sum of these findings suggest that even mild changes in blood flow and oxygen availability, is a potent regulator of BACE levels and hence Aβ production, and this is further supported by numerous in vitro experiments showing hypoxic regulation of Aβ production.2,3,40 Furthermore, these findings are potentially important as mild hypoperfusion events are more common in the aging population than severe ischemic insults, and can occur from a number of age-related injuries including atherosclerosis, diabetes, migraine, chronic obstructive pulmonary disease, transient ischemic attack, and within the stroke penumbra. As oligemic insults do not produce cell loss and as such, do not manifest clinically in the same way as ischemic insults do, they are likely to be underreported and underdiagnosed, despite potentially playing a role in the predisposition to, and the development of AD.
Given the evidence that hypoperfusion injury enhances the risk for and progression of AD, it is somewhat counterintuitive to have lower Braak staging in AD patients who have suffered from hypoperfusion, than patients with “pure” AD.41,42,43 Braak staging reflects the distribution of neurofibrillary tangles. As such, while a decreased Braak score does not directly indicate lower tau, a lower Braak score often coincides with less total tau.44 Hence, altered Braak staging with cerebrovascular disease could indicate reduced tau levels, as our data would support, an overall altered distribution of tau, or that the presence of both tau pathology plus cerebrovascular pathology induces dementia at lower tau levels than “pure” AD. Notably, we do find an increase in tau phosphorylated at Ser212, Thr214, which coincides with increased activity in the tau kinase GSK3β. This phosphotau epitope is found mostly in AD tissue,45 and could play an important role in the long-term formation of neurofibrillary tangles. Furthermore, the changes in tau we observed last for at least 3 weeks postoligemia. This long lasting decrease may be due to the long-lived nature of microtubule-associated proteins such as tau necessitating a longer time to accumulate within the somatodendritic compartment.
We show that that the decrease in tau postoligemia is coincident with increases in specific macroautophagy markers, and that the remaining tau often colocalizes with lysosomal marker. We also found a decrease in ubiquitinated proteins following oligemia indicating that the ubiquitin-proteosome pathway may also be involved in the observed decrease in tau. These findings, taken together with the previous clinical and cell culture data, indicate that tau may be cleared via lysosomes and the ubiquitin-proteosome system following an oligemic hypoperfusion insult.34,35
In conclusion, this study reports the novel findings that even a single, mild hypoperfusion injury induces long lasting changes in AD-related proteins. This provides further insight into previous clinical data on comorbid AD and hypoperfusion injuries. Our data clearly demonstrate that oligemic insults can severely modulate tau and Aβ levels and may influence disease progression.
Acknowledgments
We thank Dr. Masashi Kitazawa for helpful discussion and Mr. Franklin G. Garcia, Mr. Kristoffer Myczek, and Mr. Saege Hancock for technical assistance.
Footnotes
Address reprint requests to Frank M. LaFerla, Ph.D., Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, 3212 Biological Sciences III, Irvine, CA 92697-4545, E-mail: laferla@uci.edu.
Supported in part by grants from the National Institute of Health AG-021982 to F.M.L. and National Institute of Health 1F31NS063650-01A1 and Southern California ARCS award to M.A.K., and National Institute of Health AG-029378 to M.B.J.
M.A.K. and K.N.G. contributed equally to this manuscript.
None of the authors declare any relevant financial relationships.
References
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Green KN, Boyle JP, Peers C. Hypoxia potentiates exocytosis and Ca2+ channels in PC12 cells via increased amyloid beta peptide formation and reactive oxygen species generation. J Physiol. 2002;541:1013–1023. doi: 10.1113/jphysiol.2002.017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Peers C. Amyloid beta peptides mediate hypoxic augmentation of Ca(2+) channels. J Neurochem. 2001;77:953–956. doi: 10.1046/j.1471-4159.2001.00338.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, Zhang YW. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Hwang EM, Hong HS, Boo JH, Mook-Jung I, Huh K. Effect of ischemic neuronal insults on amyloid precursor protein processing. Neurochem Res. 2006;31:821–827. doi: 10.1007/s11064-006-9086-y. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann NY Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET). Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihashi T, Inao S, Kajita Y, Kawai T, Sugimoto T, Niwa M, Kabeya R, Hata N, Hayashi S, Yoshida J. Expression and distribution of beta amyloid precursor protein and beta amyloid peptide in reactive astrocytes after transient middle cerebral artery occlusion. Acta Neurochir (Wien) 2001;143:287–295. doi: 10.1007/s007010170109. [DOI] [PubMed] [Google Scholar]
- van Groen T, Puurunen K, Maki HM, Sivenius J, Jolkkonen J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36:1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- Makinen S, van Groen T, Clarke J, Thornell A, Corbett D, Hiltunen M, Soininen H, Jolkkonen J. Coaccumulation of calcium and beta-amyloid in the thalamus after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2008;28:263–268. doi: 10.1038/sj.jcbfm.9600529. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Simpkins JW. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004;1022:30–38. doi: 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang SH, Liu R, Perez EJ, Brun-Zinkernagel AM, Koulen P, Simpkins JW. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta. 2007;1772:473–483. doi: 10.1016/j.bbadis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Gordon-Krajcer W, Kozniewska E, Lazarewicz JW, Ksiezak-Reding H. Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem Res. 2007;32:729–737. doi: 10.1007/s11064-006-9199-3. [DOI] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y, Mayeux R. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Loeb C, Gandolfo C, Croce R, Conti M. Dementia associated with lacunar infarction. Stroke. 1992;23:1225–1229. doi: 10.1161/01.str.23.9.1225. [DOI] [PubMed] [Google Scholar]
- Moroney JT, Bagiella E, Desmond DW, Paik MC, Stern Y, Tatemichi TK. Risk factors for incident dementia after stroke. Role of hypoxic and ischemic disorders. Stroke. 1996;27:1283–1289. doi: 10.1161/01.str.27.8.1283. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Paik M, Bagiella E, Desmond DW, Stern Y, Sano M, Hauser WA, Mayeux R. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology. 1994;44:1885–1891. doi: 10.1212/wnl.44.10.1885. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Paulus W, Wrocklage C, Litvan I. Effects of closed traumatic brain injury and genetic factors on the development of Alzheimer’s disease. Eur J Neurol. 2001;8:707–710. doi: 10.1046/j.1468-1331.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1). J Neural Transm. 2004;111:523–536. doi: 10.1007/s00702-003-0095-6. [DOI] [PubMed] [Google Scholar]
- Clinton J, Ambler MW, Roberts GW. Post-traumatic Alzheimer’s disease: preponderance of a single plaque type. Neuropathol Appl Neurobiol. 1991;17:69–74. doi: 10.1111/j.1365-2990.1991.tb00695.x. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Webster CM, Kelly S, Koike MA, Chock VY, Giffard RG, Yenari MA. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol Dis. 2009;33:301–312. doi: 10.1016/j.nbd.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15:1837–1839. doi: 10.1096/fj.01-0007fje. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Zheng-Fischhofer Q, Biernat J, Mandelkow EM, Illenberger S, Godemann R, Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur J Biochem. 1998;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Akiyama H, Arai T, Kondo H, Haga C, Tsuchiya K, Yamada S, Murayama S, Hori A. Neurons containing Alz-50-immunoreactive granules around the cerebral infarction: evidence for the lysosomal degradation of altered tau in human brain? Neurosci Lett. 2000;284:187–189. doi: 10.1016/s0304-3940(00)01009-0. [DOI] [PubMed] [Google Scholar]
- Tseng BP, Green KN, Chan JL, Blurton-Jones M, Laferla FM. Abeta inhibits the proteasome and enhances amyloid and tau accumulation, Neurobiol Aging. 2008;29:1607–1618. doi: 10.1016/j.neurobiolaging.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Zhiyou C, Yong Y, Shanquan S, Jun Z, Liangguo H, Ling Y, Jieying L. Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer’s disease. Neurochem Res. 2009;7:1226–1235. doi: 10.1007/s11064-008-9899-y. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. Vascular factors in Alzheimer’s disease. Int Psychogeriatr. 2003;15 Suppl 1:47–52. doi: 10.1017/S1041610203008950. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2007;30:1091–1098. doi: 10.1016/j.neurobiolaging.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Goulding JM, Signorini DF, Chatterjee S, Nicoll JA, Stewart J, Morris R, Lammie GA. Inverse relation between Braak stage and cerebrovascular pathology in Alzheimer predominant dementia. J Neurol Neurosurg Psychiatry. 1999;67:654–657. doi: 10.1136/jnnp.67.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekse RG, Leverenz JB, McCormick W, Bowen JD, Teri L, Nochlin D, Simpson K, Eugenio C, Larson EB, Tsuang D. Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J Am Geriatr Soc. 2004;52:1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ. Small concomitant vascular lesions do not influence rates of cognitive decline in patients with Alzheimer disease. Arch Neurol. 2000;57:1474–1479. doi: 10.1001/archneur.57.10.1474. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, He D, Gordon-Krajcer W, Kress Y, Lee S, Dickson DW. Induction of Alzheimer-specific Tau epitope AT100 in apoptotic human fetal astrocytes. Cell Motil Cytoskeleton. 2000;47:236–252. doi: 10.1002/1097-0169(200011)47:3<236::AID-CM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]