Abstract
We have developed a method to generate alloreactive regulatory T cells in vitro in the presence of interferon (IFN)-γ and donor antigen presenting cells (APCs). We hypothesized that these IFN-γ–conditioned T cells (Tcon) would reduce transplantation-associated arteriosclerosis. Tcon were generated from mouse (CBA.Ca, H-2k) CD4+ T cells cultured in the presence of IFN-γ for 14 days. These cultures were pulsed with bone marrow–derived B6 (H-2b) APC. 1 × 105 CD25−CD4+ effector T cells from naive H-2k mice were then cotransferred with 4 × 105 Tcon into CBA-rag−/− mice. One day later, these mice received a fully allogenic B6 CD31−/− abdominal aorta transplant. Transfer of CD25−CD4+ effectors resulted in 29.7 ± 14.5% luminal occlusion of allogeneic aortic grafts after 30 days. Cotransfer of Tcon reduced this occlusion to 11.7 ± 13.1%; P < 0.05. In addition, the CD31− donor endothelium was fully repopulated by CD31+ recipient endothelial cells in the absence of Tcon, but not in the presence of Tcon. In some experiments, we cotransplanted B6 skin with aortic grafts to ensure enhanced reactivation of the regulatory cells, which led to an additional reduction in vasculopathy (1.9 ± 3.0% luminal occlusion). In the presence of Tcon, CD4+ T cell infiltration into grafts was markedly reduced by a regulatory mechanism that included reduced priming and proliferation of CD25−CD4+ effectors. These data illustrate the potential of ex vivo generated regulatory T cells for the inhibition of transplant-associated vasculopathy.
Transplant arteriosclerosis is the main cause of allograft loss after cardiac transplantation1 and is critically dependent on an inflammatory process mediated by T lymphocytes,2,3 especially CD4+ T cells.4,5 We have previously shown that CD4+ T cell–mediated rejection of skin allografts can be successfully inhibited in a mouse adoptive transfer model by CD25+CD4+ regulatory T cells generated in vivo by donor-specific blood transfusion under the cover of an anti-CD4 antibody.6,7 This pretreatment protocol is also successful in inducing tolerance to heterotopic cardiac allografts in primary immunocompetent recipients.8 Further, we have shown that CD25+CD4+ regulatory T cells generated to alloantigen in vivo using donor-specific blood transfusion and anti-CD4 antibody regulate transplant arteriosclerosis of allogeneic mouse abdominal aorta transplants, both in adoptive transfer and primary recipient systems.9 However, the development of protocols to generate regulatory T cells in vivo may be much more difficult in the clinical situation than in rodent models. An alternative approach emerging as an attractive way of exploiting T cell regulation in man is the potential transfer of in vitro generated or expanded recipient-derived regulatory T cell populations as a cellular therapy. Several different methods for in vitro expansion/generation of Tregs have been described, including polyclonal expansion of naturally occurring Tregs,10 generation of Tregs using allogeneic antigen presenting cells (APCs), interleukin-2, and tumor growth factor (TGF)-b,11,12 ectopic expression of the key transcription factor Foxp3,13,14,15 and selection of Tregs using T cell receptor (TCR) stimulation in the presence of rapamycin.16
We have developed an additional novel method to generate alloreactive regulatory T cells in vitro in which naïve recipient CD4+ T cells are stimulated with bone marrow–derived donor APC in the presence of interferon (IFN)-γ. This conditioning protocol results in the emergence of a dominant CD25+CD62L+FoxP3+ regulatory T cell population (conditioned T cells, Tcon) by initiating apoptosis of potential effectors, inhibiting Th17 responses, and promoting Tregs development by expansion of naturally occurring Tregs and conversion of FoxP3− precursors.17,18 The resultant population inhibits the rejection of donor-specific skin grafts mediated by naïve CD25−CD4+ effector T cells in a sensitive adoptive transfer mouse allograft model.17 The emergence of this population appears to be independent of endogenous interleukin-10 as none is detected in the cultures but is critically dependent on IFN-γ because cells driven under identical conditions in the absence of exogenous cytokine lack regulatory activity and contribute directly to allograft rejection.17,18 Here we demonstrate that these Tregs also have the ability to impact the development of transplant associated vasculopathy and explore some of the mechanisms involved.
Materials and Methods
Mice
CBA.Ca (CBA, H2k), CBA.Ca rag1−/− (CBA-rag−/−, H2k), CBA.Ca CP-1 (CP-1, H2k), C57BL/6 (B6, H2b), and C57BL/6 CD31−/− (B6 CD31−/−, H2b) mice were obtained from and housed in the Biomedical Services Unit of the John Radcliffe Hospital (Oxford, UK). CBA-rag−/− mice were originally kindly provided by Dr. D. Kioussis (National Institute for Medical Research, Mill Hill, London, U.K.). CP-1 mice are transgeneic for human CD52, a pan-leukocyte surface antigen, and were originally kindly provided by H. Waldmann.19 B6 CD31−/− mice were generously provided by Drs. G. Duncan and T. Mak (Amgen Institute, Toronto, Canada). All animals were housed and treated in accordance with the Animals (Scientific Procedures) Act 1986. Sex-matched mice between 6 and 12 weeks of age at the time of the first experimental procedure were used in all experiments.
In Vitro Conditioning of CD4+ T Cells
CD4+ T cells were isolated from naive CBA spleens using Miltenyi CD4 (L3T4) MicroBeads. On reanalysis the CD4+ population was more than 95% pure. Bone marrow–derived dendritic cells (DCs) were generated from B6 donors using a modification of published methods.20,21 Briefly, bone marrow was harvested from mouse femurs, RBCs were lysed, and B cells, T cells, and MHC class II–positive cells depleted using RA3.6B2 (rat-anti-mouse B220; hybridoma obtained from ATCC, Manassas, VA), YTA 3.1 (rat-anti-mouse CD4), and YTS 169 (rat-anti-mouse CD8; hybridomas kindly provided by Prof. H. Waldmann, Sir William Dunn School of Pathology, University of Oxford, Oxford, U.K.), TIB120 (rat-anti-mouse MHC class II; ATCC), followed by negative selection using sheep-anti-rat IgG-coated Dynabeads (Dynal Biotech, Oslo, Norway). 1 × 106 enriched DCs precursor cells were cultured with 2 ng/ml each of rmGM-CSF and rhTGF-β 1 (PeproTech, London, UK), and at day 6, conditioned DCs were harvested, washed, and counted before use. 5 × 105 purified naive CBA CD4+ T cells were cocultured with 5 × 106 conditioned B6 DCs for 7 days in the presence of 5 ng/ml IFN-γ (IFN-γ conditioning). On day 7 the T cells were restimulated with conditioned B6 DCs under identical conditions and harvested after a further 7 days for adoptive transfer into immune deficient mice.
Cell Harvest, Isolation of CD25−CD4+ T Cells, and Adoptive Transfer
CD25−CD4+ T cells for use as an effector population were purified from naïve wild-type CBA or CP1-CBA spleens. CD4+ T cells were enriched using TIB 120, M1/70 (rat-anti-mouse monocyte/macrophage, ATCC), RA3–6B2, and YTS 169 followed by incubation with anti-rat Dynabeads and magnetic separation. The enriched cells (>85% CD4+) were incubated with anti-CD25-PE antibody followed by anti-PE microbeads and CD25− cells were recovered using the MACS-system (Miltenyi Biotech Ltd., Bisley, UK). CBA-rag−/− mice were injected intravenously with 1 × 105 CD25−CD4+ T cells with or without coinjection of 4 × 105 conditioned T cells. Each experiment contained animals reconstituted with CD25−CD4+ T cells only to validate the efficacy of the effector population.
Aorta Transplantation
One day after adoptive transfer, transplantation of a section of abdominal aorta from B6 CD31−/− donors was performed using a technique described by Koulack and colleagues.22
Skin Transplantation
In some mice, full-thickness B6 tail skin allografts were transplanted onto graft beds on the flanks of reconstituted mice immediately following aorta transplantation.
Flow-Sorting
Tcon generated from total CD4+ T cells from B6 (H2b) Foxp3-GFP reporter mice were stained for CD4 and TCR-β then flow sorted using a BD FACSAria instrument. GFP+ and GFP− cells were then transferred independently into B6.Rag mice together with syngeneic CD25− cells as an effector population. One day later, these reconstituted mice were transplanted with CBA (H2k) aorta allografts.
Flow Cytometric Analysis
Purity of enriched T cell populations was evaluated by flow cytometry. Cells were stained for 30 minutes at 4°C with the flourochrome-coupled antibodies 145-2C11 (anti-CD3-FITC), RM4-5 (anti-CD4-PerCP), and 7D4 (anti-CD25-PE; all BD Biosciences). For analyses from mouse spleens, single cell suspensions were prepared, RBCs were osmotically lysed, and the cells incubated with anti-CD4 FITC (clone GK1.5) and anti-TCR-β PE (clone H57-597; both BD Pharmingen, Oxford, UK). Staining of intracellular Foxp3 was performed according to the manufacturer’s instructions (clone FJK-16s, eBioscience, San Diego, CA). In other assays, cells were incubated with anti-human CD52, anti-CD4-PerCP, anti-CD25-PE, and then stained for intracellular Foxp3. Data were acquired using a FACSort flow cytometer (Becton Dickinson, San Jose, CA) and analyzed using Cellquest software.
IFN-γ ELISpot Assay
96-well MultiScreenHTS-IP filter plates (Millipore, Billerica, MA) were coated with anti-mouse IFN-γ capture antibody (AN-18, Mabtech AB, Sweden). B6 T cell depleted stimulators were prepared using rat anti-mouse CD4 (YTA3.1) and rat anti-mouse CD8 (YTS169) followed by Dynabeads and magnetic separation. Responder cells were titrated from 1 × 105 cells per well and incubated with or without 3 × 105 stimulators at 37°C and 5% CO2 for 14 hours. Spots were developed by sequential incubations with biotinylated anti-mouse IFN-γ detection antibody (R4-6A2), streptavidin-alkaline phosphatase, and BCIP/NCT substrate (all Mabtech AB, Sweden) and plates read and analyzed on an AID Bio-Tek reader (AID, Winooski, VT). The frequency of donor-reactive IFN-γ secreting CD4+ cells was calculated using the following formula:
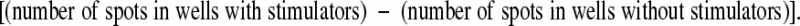 |
Analysis of the Aortic Graft
Aortic grafts were harvested 30 days after transplantation, flushed with saline then OCT-compound (Tissue-Tek, Sakura, The Netherlands) and snap-frozen embedded in OCT-compound in liquid nitrogen. Transverse sections of 5 to 6 μm thickness were cut and stained with Hematoxylin and Eosin (H&E) or Elastin van Giesson (EvG). Morphometric analysis of transplant arteriosclerosis was performed on EvG-stained sections. Digital photographs of three EvG-stained sections from each graft were taken at ×40 original magnification using a light microscope (Nikon, Tokyo, Japan) and a Coolpix digital camera (Nikon). Digitized images were then analyzed using Photoshop software (version 6.0, Adobe, San Jose, CA). The areas within the lumen of the vessel and within the internal elastic lamina were circumscribed and the respective absolute pixel counts recorded. From these measurements, the quotient for the thickness of the intima was calculated (Qint). The Qint indicates relative thickness (%) of the intima or % luminal occlusion [Qint = intima/ (lumen + intima) × 100].
Immunohistochemistry
Biotinylated or unconjugated antibodies against CD31, CD3, CD4, CD8, CD11b, and GR1 (BD Biosciences, Oxford, UK) were used for immunohistochemistry. Transverse 6-μm sections were air-dried, fixed, and stored at −80°C until further analyses. Primary antibodies were applied for 1 hour at room temperature or overnight at 4°C, followed by secondary streptavidin-HRP (Vector Laboratories, Burlingame, CA) for biotinylated primary antibodies or by mouse-anti-rat-IgG-HRP (Jackson Immunoresearch, West Grove, PA) for unconjugated primary antibodies for 45 minutes at room temperature. Staining was visualized using dimethylaminoazobenzene (Sigma, St. Louis, MO) and hematoxylin counterstain. For enumeration of CD4+ T cell infiltration, five random high-power fields were examined by area-counting in sections taken from three transverse planes from each individual aorta.
Statistical Analysis
Data were compared between groups by the Mann–Whitney U test or by analysis of variance followed by Bonferroni post hoc analyses if multiple groups were compared. P values <0.05 were considered statistically significant.
Results
CD25−CD4+ T Cells Reconstitute Transplant Arteriosclerosis in Rag-Mice
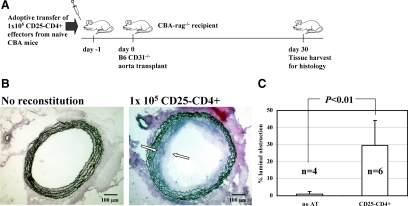
We used an adoptive transfer model using T cell– and B cell–deficient rag−/− mice to examine the effect of T cell regulation in vivo. In this model nonreconstituted CBA-rag−/− recipients of fully allogeneic B6 CD31−/− aorta transplants do not develop transplant arteriosclerosis (Figure 1, A–C). However, transfer of 1 × 105 naïve CD25−CD4+ T cells resulted in pronounced transplant arteriosclerosis (mean intimal obstruction: 29.3 ± 19.4%, n = 6 mice) 30 days after implantation indicating successful effector cell reconstitution.
Figure 1.
CD25−CD4+ T cells reconstitute transplant arteriosclerosis in rag−/−-mice. A: Design of adoptive transfer experiments. Naïve CD25−CD4+ effector T cells were prepared from spleens harvested from untreated wild-type CBA mice. 1 × 105 CD25−CD4+ effector T cells were injected intravenously into untreated CBA-rag−/− hosts (day −1). One day later these animals were transplanted with a fully allogeneic B6 CD31−/− aorta as an abdominal interposition allograft. The grafts were harvested after 30 days for histology. B: Elastin-van-Giesson staining was performed to enable quantification of luminal obstruction due to transplant arteriosclerosis. Transplant arteriosclerosis did not develop in the absence of adoptively transferred lymphocytes in CBA-rag−/− animals bearing B6 CD31−/− aorta allografts (left panel; harvested on day 30). In the presence of 1 × 105 CD25−CD4+ effector T cells, transplant arteriosclerosis consistently developed in aorta allografts (right panel; marked with white arrows). C: Quantification of luminal obstruction caused by transplant arteriosclerosis was perfomed by morphometric measurement of intimal hyperplasia, and mean values with SD are given. Transfer of CD25−CD4+ effector T cells elicited transplant arteriosclerosis as compared with non-reconstituted animals (P < 0.001; Mann–Whitney U test).
Cotransfer of in Vitro IFN-γ-Conditioned CD4+ T Cells Reduces Transplant Arteriosclerosis
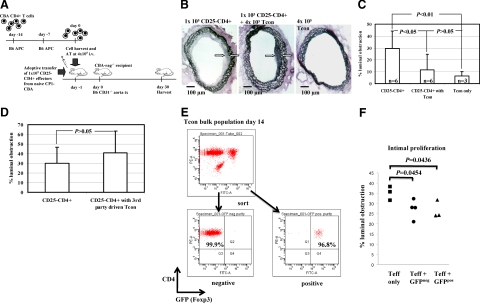
Based on the observation that adoptive transfer of regulatory T cells generated in vivo suppress the development of transplant arteriosclerosis in allogeneic aorta transplants,9 we hypothesized that T cells conditioned with IFN-γ in vitro18 would similarly regulate the development of transplant arteriosclerosis in allogeneic aorta transplants. CBA-rag−/− recipients of fully allogeneic B6 CD31−/− aorta transplants were coreconstituted with 1 × 105 naïve CBA CD25−CD4+ T cells plus 4 × 105 IFN-γ–conditioned T cells (Figure 2A). This resulted in a substantial reduction in luminal occlusion (29.3 ± 14.5% and 11.7 ± 13.1%, effectors only and effectors + Tcon respectively; Figure 2, B and C), demonstrating the potential of such populations to influence the development of transplant arteriosclerosis. This effect is donor alloantigen specific because CBA T cells driven under identical conditions but with third-party B10.S (H2s) bone marrow–derived DCs were unable to prevent vasculopathy in B6 H2b CD31−/− aortic grafts in the same adoptive transfer mouse model (Figure 2D), a phenomenon also seen with skin allografts.18
Figure 2.
Cotransfer of in vitro IFN-γ–conditioned CD4+ T cells reduces transplant arteriosclerosis. A: Tcon were generated from purified naïve CBA total CD4+ T cells cocultered with irradiated B6 APC and recombinant mouse-IFN-γ for 14 days. Tcon were harvested and without further purification or manipulation transferred intravenously into CBA-rag−/− hosts at 4 × 105 per animal. 1 × 105 naïve CD25−CD4+ T cells were cotransferred as an effector population (day −1). The following day, a fully allogeneic B6 CD31−/− aorta was transplanted. The grafts were harvested after 30 days for histology. B and C: Representative histology of aortic sections harvested 30 days after transplantation into CBA-rag−/− hosts. Grafts harvested from animals reconstituted with effector T cells only (left panel) showed transplant arteriosclerosis with intimal hyperplasia (marked with white arrows) reducing the vessel lumen by about 30%. Grafts from experimental animals after cotransfer of effector T cells and Tcon (center panel) showed a threefold reduction of intimal hyperplasia (11.7%, marked with white arrows), whereas control grafts from animals reconstituted with Tcon only developed intimal hyperplasia occluding 6.3% of the vessel lumen. Differences among groups were statistically significant (P = 0.0039; analysis of variance with Bonferroni post hoc analysis). D: CBA Tcon were generated as described above but with third-party B10.S (H2s) APC. On day 14, the resultant population was harvested and CBA.rag−/− mice reconstituted and transplanted with B6 CD31−/− aorta grafts as described above. These ‘third-party’ Tcon had no impact on the development of vasculopathy. E: Tcon were generated from B6 Foxp3-GFP reporter CD4+ T cells driven by CBA (H2k) APC. On day 14, the resultant cells were flow sorted into GFP-negative and GFP-positive populations. Figures in the respective quadrants show percent purity when gated on TCR-β. These populations (4 × 105) were then transferred into B6 immunodeficient mice together with (1 × 105) syngeneic CD25−CD4+ T cells as an effector population. One day later, the reconstituted mice were transplanted with CBA (H2k) aorta allografts. Aortas were harvested on day 30 and evaluated for luminal obstruction (F).
Although imperfect, Foxp3 is widely seen as the most useful single marker of regulatory T cells. We therefore asked whether in this system, regulation is Foxp3-dependent. Because it is not possible to sort cells directly on the basis of Foxp3 expression, we took advantage of B6 (H2b) mice in which GFP has been knocked-in to the Foxp3 locus.23 Total CD4+GFP+ T cells were stimulated with CBA (H2k) BM-DCs for 14 days in the IFN-γ conditioning protocol then resorted into GFP+ and GFP− populations which were adoptively transferred into immunodeficient B6 mice together with B6 CD25−CD4+ cells as an effector population. One day later, the reconstituted mice were transplanted with CBA (H2k) aortic allografts. As shown in Figure 2E, stimulation of cells from these reporter mice with alloantigen in the presence of exogenous IFN-γ results in enrichment for Foxp3+ cells, consistent with our previous observations with these cells.18 Reconstitution of immunodeficient B6 mice with CD25−CD4+ cells alone resulted in significant intimal proliferation, and this was reduced substantially by cotransfer of sorted Foxp3+GFP+ Tcon. (Figure 2F). Surprisingly, the reciprocal Foxp3−GFP− population controlled intimal expansion to the same extent, suggesting that in this system, regulation does not appear to be Foxp3-dependent. Although the reasons for this unexpected outcome are not clear at present, recent analyses have shown that while naturally occurring regulatory T cells (nTregs) have stable epigenetic modifications in the Foxp3 locus and thus, stable Foxp3 expression, these changes and Foxp3 expression can be transient in adaptive Tregs.24 Thus, it is possible that in some situations, loss of Foxp3 expression may either precede loss of regulatory function25 or that the suppressive effect of ex vivo generated Tregs can be Foxp3-independent.
Cotransfer of in Vitro IFN-γ-Conditioned CD4+ T Cells Inhibits Endothelial Repopulation
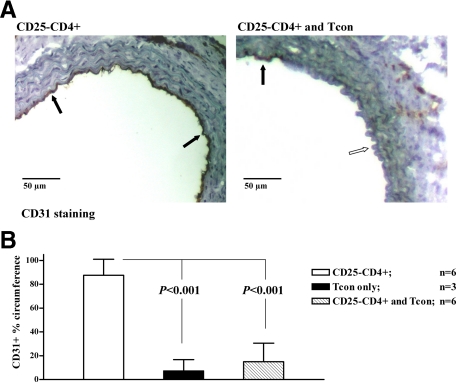
The pathogenesis of fibrotic lesions that eventually led to transplant arteriosclerosis involves repopulation with host-derived endothelial cells.26,27 Thus, endothelial repopulation represents an additional parameter with which to assess graft damage. To track the origin of endothelial cells in the graft, we used CD31−/− donors whose endothelial cells do not stain for CD31 on immunohistochemistry.28 In CBA.rag−/− mice transplanted without cell transfer, B6 CD31−/− aorta grafts showed no repopulation and the endothelial cells remained CD31 negative (data not shown). In contrast, transplanted animals reconstituted with naïve CD25−CD4+ effector T cells alone showed an almost complete turnover of endothelial cells such that 30 days posttransplant, the endothelial lining was >80% recipient-derived (Figure 3, A and B). However, this effect was almost completely inhibited by the cotransfer of IFN-γ–conditioned T cells where the vast majority of the endothelium remained donor-derived, demonstrating clearly the efficiency of regulation in this system.
Figure 3.
Cotransfer of in vitro IFN-γ–conditioned CD4+ T cells inhibits endothelial repopulation. A: B6 CD31−/− donors were used to enable differentiation of the origin of endothelial cells. On staining with anti-CD31 Ab endothelial cells of donor origin remain unstained, whereas endothelial cells of recipient (CD31 wild-type) origin stain brown. Reconstitution with 1 × 105 CD25−CD4+ effector T cells resulted in nearly circumferential recipient endothelial cell repopulation (left, CD31+ cells indicated by black arrows). Cotransfer of 4 × 105 conditioned T cells significantly reduced endothelial cell repopulation (right, white arrow). B: Differences among the effector only (CD25−CD4+) group and the groups having received a Tcon cotransfer or Tcon only (histology not shown) were statistically significant (P < 0.0001; analysis of variance with Bonferroni post hoc analysis).
IFN-γ-Conditioned Tregs Inhibit Accumulation of Intragraft CD4+T Cells
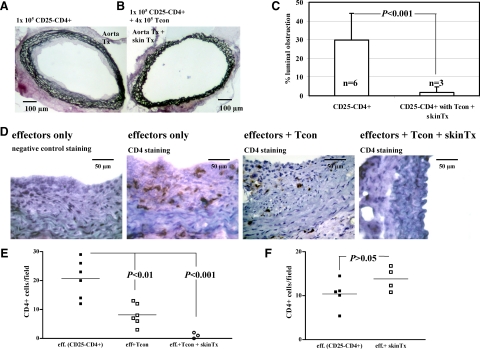
To maximize the chances of detecting informative phenotypic changes in tissue histology, we sought to optimize regulation in this model given the fact that cotransfer reduced rather than abolished transplant arteriosclerosis. It has been shown previously that regulation mediated by in vivo generated regulatory cells can be enhanced by antigen-specific reactivation.29,30 We took advantage of the observation that allogeneic skin allografts are accepted in the presence of conditioned T cells in rag−/− hosts,17,18 and hypothesized that skin but not aorta grafts contain sufficient donor antigen presenting cells for full reactivation of the IFN-γ conditioned Tregs. Therefore, mice were reconstituted with naïve CD25−CD4+ cells plus IFN-γ–conditioned cells and simultaneously transplanted with skin and aorta grafts. This resulted in a further reduction in transplant arteriosclerosis (1.9 ± 3.0% luminal occlusion), indicating improved suppressive function mediated by the IFN-γ–conditioned Tregs, consistent with enhanced reactivation in vivo (Figure 4, A–C versus Figure 2C). Importantly, the presence of an additional skin graft resulted in luminal obstruction that was virtually identical to that seen in mice transplanted with aortic allografts but without cell reconstitution (Figure 1C, P = 0.55). Thus, additional alloantigen challenge reduced vasculopathy to a level that resulted from the surgery itself.
Figure 4.
Tcon inhibit the accumulation of intragraft CD4+ T cells. Tcon were transferred intravenously into CBA-rag−/− hosts at 4 × 105 per animal. 1 × 105 naïve CD25−CD4+ T cells were cotransferred as an effector population (day −1). The following day, a fully allogeneic B6 CD31−/− aorta and full-thickness skin were transplanted. The skin cotransplant was aimed at improving alloantigen-specific reactivation of Tcon in vivo. Aorta grafts were harvested after 30 days for histology. A and B: Transplant arteriosclerosis was virtually absent in recipients of a Tcon cotransfer that also had received a B6 skin allograft at the time of aorta transplantation. C: On comparison with animals that had received effector cells only, the difference was statistically significant (P < 0.001; Mann-Whitney U test). D: Representative immunocytochemistry showing CD4+ T cell infiltration into aorta allografts in the situations described above each panel. E: Enumeration of CD4+ T cell infiltration. Each point represents a single animal. Statistical differences between the groups are shown (analysis of variance with Bonferroni post hoc analysis). F: CBA-rag−/− mice were reconstituted with naïve CD25−CD4+ T cells only and transplanted one day later with an aorta allograft alone or with an aorta plus skin allograft. On day 30, the number of CD4+ T cells infiltrating the aorta grafts in both groups was enumerated by immunohistochemistry. The presence of an additional skin graft did not influence the number of CD4+ cells infiltrating the aorta.
A clear impact of the additional skin graft was also seen in terms of the number of CD4+ T cells found within the aorta itself. As shown in representative photomicrographs (Figure 4D), CD4+ T cells were readily detected in aorta allografts of mice reconstituted with effectors only and in mice reconstituted with effectors plus Tcon and correlated closely with CD3 staining (data not shown). However, in aorta allograft recipients reconstituted with T effectors plus Tcon and cotransplanted with an additional skin graft, CD4+ T cells were virtually absent. Enumeration showed that this effect is statistically significant (Figure 4E) indicating that at least part of the mechanism of regulation mediated by reactivated Tregs involves an inhibition of effector cell recruitment to the graft itself. One trivial but plausible explanation for the reduced numbers of CD4+ T cells in aortas of mice transplanted with an additional skin allograft is that this is due simply to sequestration of effector T cells within the skin graft itself rather than enhanced regulatory T cell activation. To test this possibility, CBA.rag−/− mice were reconstituted with CD25−CD4+ cells only then transplanted with aorta plus skin allografts or with aorta allografts alone. Thirty days later, the aortas were harvested and CD4+ T cell infiltration enumerated by immunohistochemistry and direct counting. As shown in Figure 4F, there was no difference in the number of CD4+ T cells within the aorta grafts in the two groups providing further support for the hypothesis that the reduction in vasculopathy mediated by Tcon in the presence of an additional skin graft (Figure 4E) is indeed due to enhanced activation of the regulatory T cell population.
Tcon Reduce the Number of Circulating CD4+ T Cells and Inhibit Effector T Cell Priming
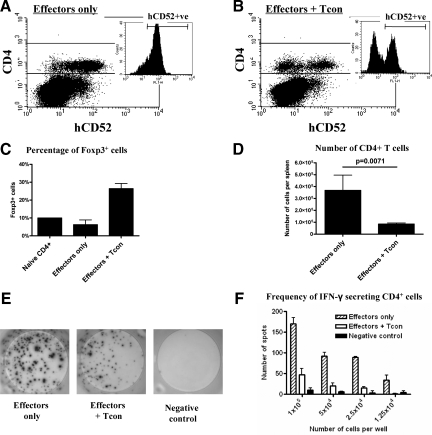
To rule out the possibility that cotransfer of IFN-γ–conditioned cells simply prevented successful CD25−CD4+ cell reconstitution, 1 × 105 CD25−CD4+ cells from CBA mice transgenic for human CD52+ (CP-1 mice) were transferred to CBA.rag−/− mice with and without 4 × 105 IFN-γ–conditioned cells. In spleens harvested 31 days after cell transfer and 30 days after aorta transplantation, CD4+hCD52+ cells were readily detected in mice reconstituted with CD25−CD4+ cells only (Figure 5A) and importantly, the same population was detected in the presence of Tcon (Figure 5B). These data rule out ineffective effector cell reconstitution as a trivial explanation for the effect of Tcon in vivo. To investigate the mechanisms by which conditioned T cells prevent transplant arteriosclerosis, we reconstituted additional CBA-rag−/− mice with 1 × 105 CD25−CD4+ effector cells (n = 2) ± 4 × 105 Tcon (n = 4). The following day a B6 aortic allograft was implanted, and 14 days later spleens were harvested and splenocytes stained for FACS analysis. As shown in Figure 5C, FoxP3+ cells were readily detected in mice that received a cotransfer of conditioned T cells showing effective Tregs engraftment and stable expression of this Tregs-associated transcription factor. Significantly, mice that received a cotransfer of conditioned T cells had substantially fewer CD4+ T cells per spleen when compared with effector only controls (3.7 × 105 versus 8.5 × 104, effectors only and effectors + Tcon respectively, P = 0.0071, Figure 5D) indicating that at least one mechanism by which Tcon inhibit transplant arteriosclerosis is limiting effector T cell expansion. To ask whether IFN-γ–conditioned T cells can also influence T cell priming, CD4+ T cells were isolated from the spleens of reconstituted aortic allograft recipients and recall anti-donor responses determined in an IFN-γ ELISpot assay. Earlier studies had shown that arteriosclerosis in this model begins at approximately day 10 post transplant and thus day 14 rather than day 30 was chosen for this analysis to avoid the possibility of a decline in T cell responses. Representative ELISPOT wells are shown in Figure 5E and analyzed data in Figure 5F. An abundant IFN-γ response was detected from CD4+ T cells in transplanted mice reconstituted with effector cells only. Significantly, this was reduced by about 75% in mice cotransferred with IFN-γ–conditioned cells (Figure 5F) indicating that these in vitro generated Tregs have a profound effect on T cell priming.
Figure 5.
Tcon reduce the number of circulating CD4+ T cells and inhibit effector priming. Spleens were harvested from CBA-rag−/− hosts 31 days after adoptive transfer of CBA-CP1 (hCD52 transgeneic) effectors with (n = 6) or without (n = 6) CBA wild-type Tcon cotransfer and 30 days after an allogeneic B6 CD31−/− aorta transplant. Splenocytes were stained with the respective flourochrome-coupled antibodies. Representative FACS dot plots are shown. A and B: Dot plots gated on live lymphocytes stained with anti-CD4-PerCP and anti-human CD52-FITC, small insets: Histograms gated on live CD4+ lymphocytes discriminating hCD52− T cells derived from the Tcon population from hCD52+ T cells derived from the effector (CD25−CD4+) population (B). In other experiments CBA.rag−/− mice were reconstituted with 1 × 105 CD25−CD4+ effector cells (n = 2) or with effector cells and 4 × 105 Tcon (n = 4). The following day, a B6 aortic allograft was performed. Spleens were harvested on day 14 posttransplant. Splenocytes were stained with TCRβ-PE, CD4-PerCP and intracellular Foxp3-APC for FACS analysis. Alternatively, CD4+ cells were purified by magnetic positive selection from pooled splenocytes and challenged with B6 stimulators in an IFN-γ ELISpot assay. C: Percentage of CD4+TCRβ+ cells that express Foxp3. D: Absolute number of CD4+TCRβ+ cells. E: Representative ELISpot images (1 × 105 cells per well). F: Frequency of donor-reactive IFN-γ secreting CD4+ cells.
Discussion
In this study, we show that CD4+ T cells conditioned in vitro with IFN-γ and bone marrow–derived donor APCs reduce transplant arteriosclerosis mediated by naïve CD25−CD4+ T cells. In this model the induction of transplant arteriosclerosis is entirely dependent on transferred CD25−CD4+ T cells, and leads to 20% to 30% occlusion of the vessel lumen 30 days after cell reconstitution. This is consistent with reports from other models and underlines the importance of CD4+ T cells in vasculopathy.5 In the setting of transplantation, CD4+ T cells can be stimulated by both the direct and indirect pathways of allorecognition where recipient T cells recognize allogeneic APC and self APC presenting allogeneic peptides, respectively.31 This is relevant for the model used in this study, because the IFN-γ–conditioned (H2k) regulatory T cells arise from total CD4+ T cells stimulated only via the direct pathway by allogeneic (H2b) APC. Thus, a potential concern for effective regulation of transplant arteriosclerosis in this situation is that the naïve effector T cell population is probably stimulated predominantly by the indirect pathway following aorta transplantation because endothelial cells are relatively poor antigen presenting cells,32 and the vessel graft itself might contain only few ‘professional’ donor APC capable of direct pathway stimulation. While this potential lack of APC may reduce direct pathway stimulation of the effector population, it could also limit direct pathway reactivation of the IFN-γ–conditioned Tregs. The fact that arteriosclerosis was reduced to a greater extent in mice given a skin and aorta transplant compared with that in mice transplanted with an aorta graft alone supports this contention and could thus emphasize the importance of reactivation to elaborate full Tregs function in vivo. Although additional strategies to ensure direct pathway reactivation may be a requirement of the specific transplant model used in the current study and is not likely to be a feature of whole-organ transplantation where the donor organ contains abundant donor APC, the overall phenomenon may be of particular significance for example in islet transplantation and in transplantation of tissues derived from stem cells where the indirect pathway may be the predominant route of allosensitization.
Reconstituted immunodeficient mice were used in this study so that the numbers of Tregs and potential effector cells could be controlled independently, but whether IFN-γ–conditioned Tcon can influence alloreactive responses in nonlymphopenic mice remains an active area of investigation in this laboratory. We have shown previously that in vivo generated Tregs cannot only prevent allograft rejection in reconstituted immunodeficient mice but are also responsible for preventing rejection in primary transplant recipients with an intact immune repertoire.33 Such observations lead to cautious optimism that when delivered in sufficient numbers, probably in conjunction with short-term adjunctive immunotherapy, IFN-γ–conditioned Tcon may be capable of affecting graft outcome in immunocompetent recipients.
The data presented in this study not only emphasize the potential of in vitro generated Tregs but also indicate the dual role of IFN-γ in shaping immune responses whereby IFN-γ can act as a cytotoxic effector molecule or as a cytokine involved in regulation depending on the specific microenvironment and context. Although the precise mechanism by which arteriosclerosis is prevented by IFN-γ–conditioned regulatory T cells is unclear, the presence of these cells results in a 3.5-fold reduction in the number of effector T cells capable of secreting IFN-γ (Figure 5F). Given the fact that IFN-γ is recognized as one of the main effector mechanisms responsible for tissue damage and the development of transplant arteriosclerosis,34,35,36 an inhibition of IFN-γ production seems to be a likely explanation. In the context of endothelial cells, IFN-γ can be proapoptotic by enhancing the expression of both Fas and pro-caspase 8.37 Furthermore, it has recently been shown that IFN-γ and the Fas/FasL pathways cooperate in the development of transplant associated vasculopathy.38 Thus, an inhibition of IFN-γ production does appear to be consistent with the protective effects mediated by this population of alloantigen-driven adaptive Tregs.
Taken together, our data show that IFN-γ–conditioned T cells can ameliorate transplant arteriosclerosis by reducing effector cell priming, expansion, and graft infiltration. These cells could be beneficial in clinical solid organ transplantation for induction therapies in settings where the HLA typing of the donor is known before the transplant, such as living donor organ transplantation, and for improving maintenance therapy by boosting T cell regulation after transplantation. The latter could even be important in the context of chronic rejection, given the potential of these in vitro generated cells for reducing transplant arteriosclerosis.
Footnotes
Address reprint requests to Gregor Warnecke, M.D., Transplantation Research Immunology Group, Nuffield Department of Surgery, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom. E-mail: warnecke.gregor@mh-hannover.de.
Supported by the DFG (Deutsche Forschungsgemeinschaft, ENP grant Wa 1700/1-1 to G.W.). S.N.N. was supported by the American Society of Transplantation. K.J.W. holds a Royal Society Wolfson Research Merit Award.
G.W. and G.F. contributed equally to this study. K.J.W. and A.B. are joint senior authors.
References
- Billingham ME. Cardiac transplant atherosclerosis. Transplant Proc. 1987;19:19–25. [PubMed] [Google Scholar]
- Hruban RH, Beschorner WE, Baumgartner WA, Augustine SM, Ren H, Reitz BA, Hutchins GM. Accelerated arteriosclerosis in heart transplant recipients is associated with a T-lymphocyte-mediated endothelialitis. Am J Pathol. 1990;137:871–882. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Burns WR, Tang PC, Yi T, Schechner JS, Zerwes HG, Sessa WC, Lorber MI, Pober JS, Tellides G. Interferon-gamma plays a nonredundant role in mediating T cell-dependent outward vascular remodeling of allogeneic human coronary arteries. FASEB J. 2004;18:606–608. doi: 10.1096/fj.03-0840fje. [DOI] [PubMed] [Google Scholar]
- Shi C, Lee WS, He Q, Zhang D, Fletcher DL, Jr, Newell JB, Haber E. Immunologic basis of transplant-associated arteriosclerosis. Proc Natl Acad Sci U S A. 1996;93:4051–4056. doi: 10.1073/pnas.93.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger SM, Spriewald BM, Witzke O, Pajaro OE, Yacoub MH, Morris PJ, Rose ML, Wood KJ. Indirect allorecognition can play an important role in the development of transplant arteriosclerosis. Transplantation. 2002;73:279–286. doi: 10.1097/00007890-200201270-00022. [DOI] [PubMed] [Google Scholar]
- Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: cTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- Pearson TC, Madsen JC, Larsen CP, Morris PJ, Wood KJ. Induction of transplantation tolerance in adults using donor antigen and anti-CD4 monoclonal antibody. Transplantation. 1992;54:475–483. doi: 10.1097/00007890-199209000-00018. [DOI] [PubMed] [Google Scholar]
- Warnecke G, Bushell A, Nadig SN, Wood KJ. Regulation of transplant arteriosclerosis by CD25+CD4+ T cells generated to alloantigen in vivo. Transplantation. 2007;83:1459–1465. doi: 10.1097/01.tp.0000265446.61754.d2. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- Feng G, Wood KJ, Bushell A. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008;86:578–589. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- Feng G, Gao W, Strom TB, Oukka M, Francis RS, Wood KJ, Bushell A. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur J Immunol. 2008;38:2512–2527. doi: 10.1002/eji.200838411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland LK, Walsh LA, Frewin MR, Wise MP, Tone M, Hale G, Kioussis D, Waldmann H. Elimination of the immunogenicity of therapeutic antibodies. J Immunol. 1999;162:3663–3671. [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-beta 1 and TNF-alpha on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells. 1997;15:144–153. doi: 10.1002/stem.150144. [DOI] [PubMed] [Google Scholar]
- Koulack J, McAlister VC, Giacomantonio CA, Bitter-Suermann H, MacDonald AS, Lee TD. Development of a mouse aortic transplant model of chronic rejection. Microsurgery. 1995;16:110–113. doi: 10.1002/micr.1920160213. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simper D, Wang S, Deb A, Holmes D, McGregor C, Frantz R, Kushwaha SS, Caplice NM. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108:143–149. doi: 10.1161/01.CIR.0000081703.34526.5D. [DOI] [PubMed] [Google Scholar]
- Hillebrands J, van den Hurk BM, Klatter FA, Popa ER, Nieuwenhuis P, Rozing J. Recipient origin of neointimal vascular smooth muscle cells in cardiac allografts with transplant arteriosclerosis. J Heart Lung Transplant. 2000;19:1183–1192. doi: 10.1016/s1053-2498(00)00209-6. [DOI] [PubMed] [Google Scholar]
- Ensminger SM, Spriewald BM, Steger U, Morris PJ, Mak TW, Wood KJ. Platelet-endothelial cell adhesion molecule-1 (CD31) expression on donor endothelial cells attenuates the development of transplant arteriosclerosis. Transplantation. 2002;74:1267–1273. doi: 10.1097/00007890-200211150-00012. [DOI] [PubMed] [Google Scholar]
- Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10:101–108. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Kreisel D, Krasinskas AM, Krupnick AS, Gelman AE, Balsara KR, Popma SH, Riha M, Rosengard AM, Turka LA, Rosengard BR. Vascular endothelium does not activate CD4+ direct allorecognition in graft rejection. J Immunol. 2004;173:3027–3034. doi: 10.4049/jimmunol.173.5.3027. [DOI] [PubMed] [Google Scholar]
- Bushell A, Wood K. GITR ligation blocks allograft protection by induced CD25+CD4+ regulatory T cells without enhancing effector T-cell function. Am J Transplant. 2007;7:759–768. doi: 10.1111/j.1600-6143.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Cole SE, Shah RV, Fukumoto Y, Libby P, Mitchell RN. Wild-type but not interferon-gamma-deficient T cells induce graft arterial disease in the absence of B cells. Cardiovasc Res. 2004;63:347–356. doi: 10.1016/j.cardiores.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Dimayuga PC, Li H, Chyu KY, Fredrikson GN, Nilsson J, Fishbein MC, Shah PK, Cercek B. T cell modulation of intimal thickening after vascular injury: the bimodal role of IFN-gamma in immune deficiency. Arterioscler Thromb Vasc Biol. 2005;25:2528–2534. doi: 10.1161/01.ATV.0000190606.41121.00. [DOI] [PubMed] [Google Scholar]
- Li JH, Kluger MS, Madge LA, Zheng L, Bothwell AL, Pober JS. Interferon-gamma augments CD95(APO-1/Fas) and pro-caspase-8 expression and sensitizes human vascular endothelial cells to CD95-mediated apoptosis. Am J Pathol. 2002;161:1485–1495. doi: 10.1016/s0002-9440(10)64424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart-Matyas M, Nejat S, Jordan JL, Hirsch GM, Lee TD. IFN-gamma and Fas/FasL pathways cooperate to induce medial cell loss and neointimal lesion formation in allograft vasculopathy. Transpl Immunol. 2010;22:157–164. doi: 10.1016/j.trim.2009.10.004. [DOI] [PubMed] [Google Scholar]