Abstract
Chondrogenic differentiation is pivotal in the active regulation of artery calcification. We investigated the cellular origin of chondrocyte-like cells in atherosclerotic intimal calcification of C57BL/6 LDLr−/− mice using bone marrow transplantation to trace ROSA26-LacZ-labeled cells. Immunohistochemical costaining of collagen type II with LacZ and leukocyte defining surface antigens was performed and analyzed by high-resolution confocal microscopy. Chondrocyte-like cells were detected in medium and advanced atherosclerotic plaques accounting for 7.1 ± 1.6% and 14.1 ± 1.7% of the total plaque cellularity, respectively. Chimera analysis exhibited a mean of 89.8% LacZ+ cells in peripheral blood and collagen type II costaining with LcZ revealed an average 88.8 ± 7.6% cytoplasmatic LacZ+ evidence within the chondrocyte-like cells. To examine whether hematopoietic stem cells contribute to the phenotype, stem cell marker CD34 and myeloid progenitor-associated antigen CD13 were analyzed. CD34+ was detectable in 86.9 ± 8.1% and CD13+ evidence in 54.2 ± 7.6% of chondrocyte-like cells, attributable most likely because of loss of surface markers during transdifferentiation. Chondrocyte differentiation factor Sox-9 was detected in association with chondrocyte-like cells, whereas Sm22α, a marker for smooth muscle cells, could not be demonstrated. The results show that the majority of chondrocyte-like cells were of bone marrow origin, whereas CD34+/CD13+ myeloid precursors appeared to infiltrate the plaque actively and transdifferentiated into chondrocytes-like cells in the progression of atherosclerosis.
Vascular calcification continues to be a major cause of death and disability in the developed nations.1 The extent of the calcification process is associated with atherosclerotic plaque burden and a heightened risk of myocardial infarction2 resulting in significant morbidity and mortality. Long considered as a passive degenerative process, converging evidence from both in vitro and in vivo analysis provide considerable evidence that vascular calcification shares intriguing similarities with bone formation.3 Chondrocyte and osteoblastic metaplasia within sites of arterial calcification has been reported in humans and mice.4 Evidence is overwhelming that osteoblasts and chondrocyte-like cells actively promote the calcification process analogous to endochondral bone formation.5 The origin of cells differentiating into chondrocytes or osteoblasts has long remained unknown. Three hypotheses have been postulated: local pericytes from the tunica adventitia, vascular smooth muscle cells (VSMC) from the tunica media, or progenitor cells derived from bone marrow.6
Media calcification is found mainly in patients with diabetes mellitus type 2 and chronic kidney disease7 and is associated with an increased risk of amputation and higher cardiovascular mortality.8 In an elegant work Speer et al9 recently demonstrated that chondrocyte-like cells in MGP−/− mice, a mouse model of medial artery calcification were derived from transdifferentiation of mature smooth muscle cells.
Intimal arterial calcification is the most common form of calcific vasculopathy with chronic inflammation as fundamental pathophysiological mechanism of the disease.10 The high-fat fed LDLR−/− mouse develops in a sequential fashion both medial and atherosclerotic calcification, the latter pronounced after 2 months and worsened by uremia.11 Recently, Duer et al12 examined the nanostructure of calcium deposits within calcified human atherosclerotic plaques and demonstrated marked morphological similarities compared with skeletal bone emphasizing the active regulation of the process. Morony et al13 demonstrated that atherosclerotic intimal calcification could significantly be reduced in C57BL/6 LDLr−/− mice by treatment with recombinant osteoprotegerin (Fc-OPG). Although leaving the atherosclerosis progression untouched, vascular intimal calcification as induced by chondrocyte-like cells was decreased, suggesting that the process of atherosclerosis and vascular calcification can be uncoupled. The authors hypothesized that the attenuation of vascular calcification was achieved by systemic long-term receptor activator of NF-κB ligand (RANKL) inhibition via OPG treatment. OPG is a known inhibitor of bone resorption and OPG serum levels displayed a positive correlation with the progression of atherosclerosis, coronary artery disease, stroke, and cardiovascular morbidity and mortality.14 OPG is a decoy receptor for RANKL, which is known to be a considerable mediator in osteoclastogenesis.15 Moreover, RANKL involvement is needed in lymph node genesis, activation, and survival of leukocytes from myeloid origin as well as differentiation of leukocytes.16
An in vitro study with adult pluripotent cells derived from human peripheral blood monocytes showed transdifferentiation from a monocyte-like structure to a chondrocyte-like structure, indicating that these myeloid cells have the potential to differentiate into collagen type II synthesizing chondrocytes.17 Moreover, Shafer et al18 recently demonstrated that early chondrocyte progenitors were of myeloid origin in a mouse model of chondrocyte differentiation.
Here we report that contrary to medial artery calcification in MGP−/− mice, where chondrocyte metaplasia is based on smooth muscle cell transdifferentiation, chondrocyte-like cells emerging in this mouse model examining atherosclerotic intimal calcification are of bone marrow- derived myeloid origin. Moreover, we provide evidence that the differentiation process is mediated by induction of the NF-κB ligand RANKL.
Materials and Methods
Animal Housing and Diets
Experimental procedures were conducted in accordance with the German Animal Studies Committee of Schleswig-Holstein (reference number 1/1a/06). At the age of 12 weeks and subsequent to bone marrow transplantation (BMT), female mice were fed an atherogenic high-fat diet (Harlan Teklad Bioservice for Science, Walsrode, Germany), containing 15% (w/w) fat, 1.25% cholesterol, and 0.5% sodium cholate. Mice (n = 5 per group) were anesthetized and euthanized by cervical dislocation at the indicated time points 8, 10, and 16 weeks later before proximal aorta, and heart tissues were quickly removed, rinsed with PBS, and analyzed as indicated below.
BMT and Chimera Analysis
BMT was performed as described elsewhere.19 Briefly, at the age of 12 weeks, recipient female C57BL/6 LDLr−/− mice were lethally irradiated. Bone marrow was harvested from female C57BL/6 ROSA26 mice (ubiquitously expressing the bacterial enzyme β-galactosidase) by flushing femurs and tibias with PBS. A volume of 0.2 ml containing 20 × 106 bone marrow cells was injected to each of the recipient mice via tail veins. Blood samples were taken at the indicated time points, and a chimera analysis was performed to evaluate the BMT. Briefly, cytospins from blood samples were stained with β-galactosidase (LacZ) chicken anti-mouse antibody (ab9361l Abcam, Oxford, UK) and counterstained with Hoechst 33258 (Sigma-Aldrich, Germany), while a total of 1000 cells per spin were evaluated for LacZ+ evidence as described previously.20
Tissue Processing and Atherosclerotic Lesion Analysis
Serial 7-μm cryosections were prepared starting from where the atrioventricular valves were visible and collected on poly-d-lysine-coated slides using a Jung 3000CM cryotome (Leica, Wetzlar, Germany). The slides were then examined with a Zeiss microscope (Zeiss Axiophot, Carl Zeiss, Göttingen, Germany), and immunohistochemistry/fluorescence was detected with a high-resolution confocal laser scanning microscope LSM 510 Meta (Zeiss). Detection of calcium phosphate crystals was attained by calcein staining and nuclear counterstaining with Hoechst 33258 (Sigma-Aldrich). Atherosclerotic lesions were examined by analysis of 60 accidentally chosen grids (each 10,000 μm2) per lesion and subsequent quantification of total cell number and total number of LacZ+ and chondrocyte-like cells and collagen II double staining.4
Immunohistochemical Analysis
To determine the pedigree of chondrocyte-like cells in atherosclerotic intimal calcification, immunohistochemical costaining of collagen type II with both LacZ and leukocyte defining surface antigens was performed. Cells were accounted when completely surrounded by collagen type II extracellular matrix. All antibodies used for immunohistochemistry were initially titrated and used at saturated concentrations and controls were used as described previously.21 Occurrence of collagen type II was determined with a rabbit anti-human polyclonal antibody as described previously4 (NCL-COLL-IIp; Novocastra, Newcastle, UK). LacZ was determined with a chicken anti-mouse β-galactosidase antibody (ab9361l Abcam). For macrophage detection, monoclonal rat anti-mouse MOMA-2 antibody was used (17PO0503; Dianova, Augst, Switzerland) for detection of circulating monocytes monoclonal rat anti-mouse antibody CD115 (MCA1898GA; AbDSerotec, Oxford, UK) for detection of early leukocyte differentiation rat anti-mouse CD13/aminopeptidase N (MCA2395A488; AbDSerotec) and for detection of hematopoietic stem cells rat anti-mouse CD34 (MCA1825; AbDSerotec). Detection of Sox-9 was performed by rabbit anti-mouse Sox-9 (SC20095; Santa Cruz Biotechnology, Heidelberg, Germany) and Sm22α by goat anti-mouse antibody (ab10135; Abcam). Moreover, to determine the expression of NF-κB ligand RANKL in LDL−/− mice, samples were immunostained with biotinylated rabbit anti-mouse CD254/sRANKL (AAM56B; AbDSerotec). After incubation with the primary AB, specimens were incubated with Alexa Fluor 555 or Alexa Fluor 488 (Invitrogen, Paisley, UK) corresponding antibodies.
Statistics
Laboratory data were analyzed with a multivariate analysis and nonparametric data with the Mann-Whitney U test, and Bonferroni correction was used for posthoc tests. All data were calculated with SPSS 13.0 (SPSS, Chicago, IL).
Results
BMT and Chimera Analysis
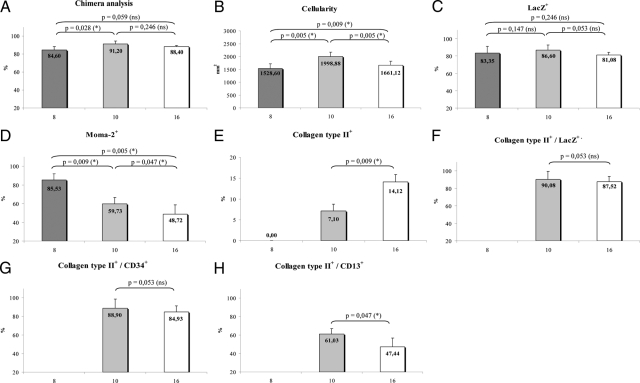
Blood samples were taken at the indicated time points, and a chimera analysis was performed to evaluate the degree of hematopoietic chimerism. The fraction of LacZ+ cells in peripheral blood of bone marrow transplanted C57BL/6 LDLr−/− mice was 84.6% ± 3.6% (mean ± SD, n = 5) 8 weeks after BMT, 91.2 ± 3.4% (n = 5) 10 weeks after BMT, and 88.4% ± 1.1% (n = 5) 16 weeks after BMT, respectively (Figure 1A). The sustained presence of LacZ+ leukocytes documented the replacement of hematopoietic stem cells, which are the only long-term self-renewing cells in the hematopoietic system.22
Figure 1.
Graphs displaying results with x-axis showing the duration of high-fat diet (weeks). A: Percentage of LacZ+ cells in peripheral blood. B: Total plaque cellularity, cells per square millimeter of lesion area. C: Percentage of LacZ+ cells of total plaque cellularity. D: Percentage of MOMA-2+ cells. E: Percentage of collagen type II+ cells. F: Percentage of collagen type II+ cells additionally expressing LacZ. G: Percentage of collagen type II+ cells additionally expressing CD13. H: Percentage of collagen type II+ cells additionally expressing CD34. *P < 0.05; ns, P ≥ 0.05.
Vascular Pathology in Bone Marrow Chimeras
In animals euthanized 8 weeks after BMT, earlier stages of atherosclerosis were detectable with rather homogenous plaque structure, an even contribution of macrophages and a diffuse punctate deposition of hydroxyapatite (data not shown). The lesions exhibited a medium degree of cellularity (1528.6 ± 184.1 cells per mm2) (Figure 1B) with an average of 85.5% macrophages as indicated by MOMA-2 and 83.4% LacZ+ cells (Figure 1, C and D). Medium advanced stages of atherosclerotic alteration of the vessel wall were visible in 22-week-old mice (10 weeks high-fat diet). Cellularity reached a maximum with 1998.9 ± 167.7 cells per mm2 with decreasing macrophage contingent within the plaques (59.7 ± 6.9%) compared with earlier stages of atherosclerosis and evidence of 86.6 ± 6.1% LacZ+ cells (Figure 1B). Mice fed the high-fat diet for 16 weeks exhibited a rather inhomogeneous plaque structure with a necrotic core, a distinct fibrous cap (Figure 2, A and B), extensive calcification as detected by calcein staining (Figure 2C) and lowered total cellularity (1661.1 ± 157.9 per mm2). Macrophage fraction as detected by MOMA-2 accounted for 48.7 ± 7.5% (Figure 1D) and LacZ+ evidence for 81.1 ± 2.9% of total cell number, respectively.
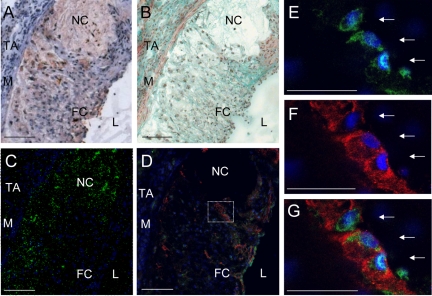
Figure 2.
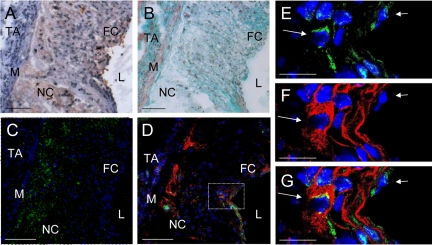
Chondrocyte-like cells within atherosclerotic plaques of C57BL/6 LDL−/− mice (16 weeks high-fat diet). A: Oil-Red O staining (fat = red, nuclei = purple). B: Masson-Goldner trichrome staining (nuclei = blue-black, cytoplasm = red, collagen = green, muscle tissue = bright red). C: Calcein staining (calcium phosphate = green fluorescence, nuclei = blue fluorescence as produced by the DNA-binding dye Hoechst 33258). D: Collagen type II costaining with Hoechst 33258 and β-galactosidase (LacZ) of chondrocyte-like cells. Collagen type II staining (red immunofluorescence), LacZ staining (green immunofluorescence), and blue fluorescence by Hoechst 33258. L indicates lumen; FC, fibrous cap; NC, necrotic core; TA, tunica adventitia; M, tunica media. Scale bar = 100 μm (A–D). E–G: Higher magnification of the area demarcated in D. E: Green channel; F: red channel; and G: red and green channel. Arrows indicate LacZ+ and collagen type II+ stained chondrocyte-like cells. Scale bar = 50 μm (E and F).
Myeloid Precursors Give Rise to Chondrocyte-Like Cells in Atherosclerotic Intima Calcification
Chondrocyte-like cells as detected immunohistochemically by collagen type II staining were not visible in mice 20 weeks of age (8 weeks high-fat diet). In orthotopic chondrocyte differentiation, the extracellular matrix production of collagen type II marks an intermediate stage where chondrocytes become encased in their extracellular matrix and acquire a characteristic rounded morphology.23 Twenty-two-week-old mice (10-week high-fat diet) displayed first evidence of chondrocyte-like cells (Figure 1E). Cells displayed a round nucleus and were surrounded by a typical dense ring of connective tissue that stained positively for collagen type II (Figure 2, D–G). Within these medium advanced plaques, chondrocyte-like cells accounted for 7.1 ± 1.6% of total cellularity. Immunohistochemical double staining revealed a mean of 90.1 ± 9.3% cytoplasmatic LacZ+ evidence within the chondrocyte-like cells (Figure 1F).
Advanced atherosclerotic plaques in mice 28 weeks of age (16 weeks high-fat diet) exhibited an increasing number of collagen type II expressing cells; 14.1 ± 1.7% of the total cell number within the plaque displayed typical collagen II evidence, whereof 87.52 ± 5.90% proved to be LacZ+ positive. In these advanced plaques, chondrocyte-like cells were mainly located in the fibrous cap and associated with different stages of calcium deposition (Figure 2, C and D).
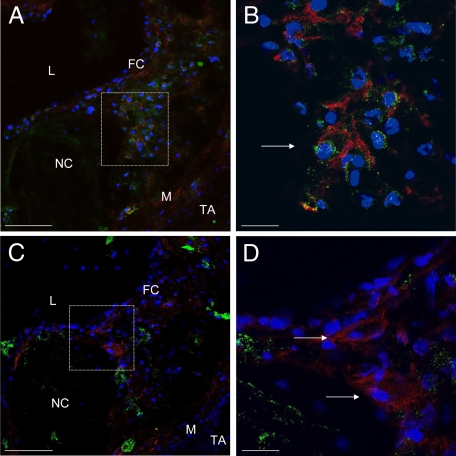
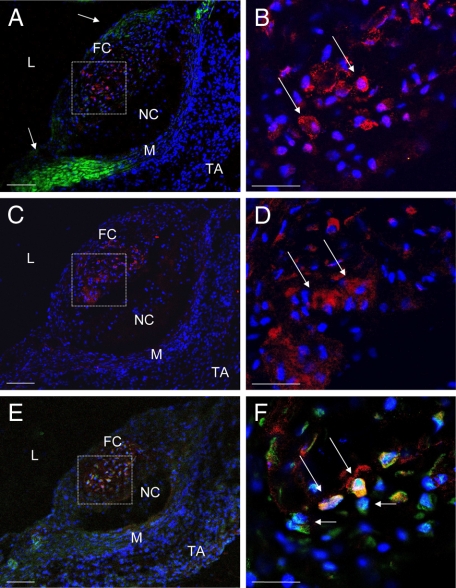
To determine the origin of chondrocyte-like cells in atherosclerotic calcification, immunohistochemical costaining of collagen type II with leukocyte defining surface antigens was performed. MOMA-2-stained macrophages were detectable as most frequent cell type located evenly through the atherosclerotic plaques. Costaining of collagen II and MOMA-2 showed no coexpression, thus excluding macrophages as a potential source of chondrocyte-like cells (Figure 3, A–D). To examine whether cells of the monocytic lineage and early progenitors of monocytic differentiation were involved, the specific surface marker CD115 was also costained. Indeed, no immunohistochemical evidence for marker CD115 could be detected in chondrocyte-like cells (Figure 4, C and D).
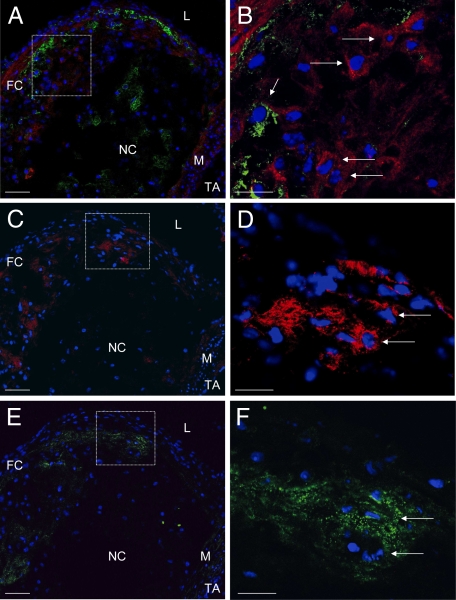
Figure 3.
Collagen type II costaining with Hoechst 33258 and MOMA-2 (A and B), collagen type II staining with Hoechst 33258 (C and D), and RANKL costaining with Hoechst 33258 (E and F) of serial sections of aortae from C57BL/6 LDL−/− mice (16 weeks high-fat diet). A, C, and E: L indicates lumen; FC, fibrous cap; NC, necrotic core; M, tunica media; A, tunica adventitia. A: Collagen type II staining (red immunofluorescence), MOMA-2 staining (green immunofluorescence), and blue fluorescence Hoechst 33258. B: Higher magnification of the area demarcated in A. Long arrows indicate collagen type II+ chondrocyte-like cells without evidence of MOMA-2. Short arrow indicates MOMA-2+ cell. C: Collagen type II staining (red immunofluorescence) and blue fluorescence by Hoechst 33258. D: Higher magnification of the area demarcated in C. Long arrows indicates collagen type II+ chondrocyte-like cells. E: RANKL staining (green immunofluorescence) and blue fluorescence by Hoechst 33258. F: Higher magnification of the area demarcated in E. Long arrows indicates RANKL+ cells. Collagen type II+ cells are located within the RANKL+ area. Scale bar = 50 μm (A, C, and E); 20 μm (B, D, and F).
Figure 4.
Collagen type II co-staining with Hoechst 33258 and CD13 (A and B) or CD115 (C and D) of aortae from C57BL/6 LDL−/− mice (16 weeks high-fat diet). A: Collagen type II staining (red immunofluorescence), CD13 staining (green immunofluorescence), and blue fluorescence as produced by the DNA-binding dye Hoechst 33258. B: Higher magnification of the area demarcated in A. Arrow indicates collagen type II-positive stained cell with colocalization of CD13. C: Collagen type II staining (red immunofluorescence), CD115 staining (green immunofluorescence), and blue fluorescence Hoechst 33258. D: Higher magnification of the area demarcated in C. Arrow indicates collagen type II+ cell with no colocalization of CD 115. A and C: L indicates lumen; FC, fibrous cap; NC, necrotic core; M, tunica media; TA, tunica adventitia. Scale bar = 100 μm (A and C); 25 μm (B and D).
CD34, a cell surface antigen expressed by hematopoietic stem and endothelial cells, was examined to prove whether hematopoietic stem or myeloid progenitor cells were involved in the transdifferentiation process. Immunohistological costaining revealed that in 22-week-old mice (10-week high-fat diet) 9.1 ± 3.1% of total cell number displayed CD34 evidence; 88.9 ± 9.8% of the chondrocyte-like cells were CD34 positive (Figure 1G). Atherosclerotic plaques in animals 16 weeks on the high-fat diet showed detectable CD34 evidence in 17.7 ± 3.6% of total cellularity; 84.9 ± 6.5% of chondrocyte-like cells were stained positively for CD34 (Figure 5).
Figure 5.
Chondrocyte-like cells within atherosclerotic plaques of aortas from C57BL/6 LDL−/− mice (16 weeks high-fat diet). A: Oil-Red O staining (fat = red, nuclei = purple). B: Masson-Goldner trichrome staining (nuclei = blue-black, cytoplasm = red, collagen = green, muscle-tissue = bright red). C: Calcein staining (calcium phosphate = green fluorescence, nuclei = blue fluorescence as produced by the DNA-binding dye Hoechst 33258). D: Collagen type II costaining with Hoechst 33258 and CD34 of chondrocyte-like cells. Collagen type II staining (red immunofluorescence), CD34 staining (green immunofluorescence), and blue fluorescence Hoechst 33258. L indicates lumen; FC, fibrous cap; NC, necrotic core; TA, tunica adventitia; M, tunica media. Scale bar = 100 μm (A–D). E–G: Higher magnification of the area demarcated in D. E: Green channel; F: red channel; and G: red and green channel. Long arrow indicates collagen type II+/CD34+ chondrocyte-like cell. Short arrow indicates CD34+ endothelial cell. Scale bar = 20 μm (E and F).
For more precise definition of the progenitor cells transdifferentiating into chondrocyte-like cells, myeloid progenitor-associated antigen CD13 was used. In medium-advanced plaques (22-week-old mice), 61.0 ± 6.0% of chondrocyte-like cells expressed CD13+ on their surface and in more advanced plaques 47.4 ± 9.2% (Figures 1H and 4, A and B).
To exclude the possibility that chondrocyte-like cells in this context were derived from smooth muscle cells, Sm22α a calponin-related protein that is expressed specifically in smooth muscle cells was examined. Sm22α was detected in VSMCs in nonaffected aortic media and within the fibrous cap but not within chondrocyte-like cells (Figure 6, A and B).
Figure 6.
Sox-9 costaining with Hoechst 33258 and SM-22α (A and B), collagen type II staining with Hoechst 33258 (C and D), and Sox-9 costaining with Hoechst 33258 and β-galactosidase (LacZ) (E and F) aorta of C57BL/6 LDL−/− mice (16 weeks high-fat diet). A, C, and E: L indicates lumen; FC, fibrous cap; NC, necrotic core; M, tunica media; TA, tunica adventitia. A: Sox-9 staining (red immunofluorescence), SM-22α staining (green immunofluorescence), and blue fluorescence by Hoechst 33258. Short arrows indicate SM-22α+ intact smooth muscle cells. B: Higher magnification of the area demarcated in A. Long arrows indicates Sox-9+ chondrocyte-like cells without evidence of SM-22α. C: Collagen type II staining (red immunofluorescence) and blue fluorescence by Hoechst 33258. D: Higher magnification of the area demarcated in C. Long arrows indicate collagen type II-positive stained chondrocyte-like cells. E: Sox-9 staining (red immunofluorescence), β-galactosidase (LacZ) staining (green immunofluorescence), and blue fluorescence by Hoechst 33258. F: Higher magnification of the area demarcated in E. Long arrows indicate Sox-9+/LacZ+ chondrocyte-like cells. Short arrows indicate LacZ+/Sox-9− bone marrow-derived cells. Scale bar = 100 μm (A, C, and E); 50 μm (B, D, and F).
For additional confirmation of the chondrocyte-like cells, staining for Sox-9, a specific intracellular marker for early chondrocyte differentiation was performed. Sox-9 staining was detectable within the area of collagen type II-positive cells as demonstrated in Figure 6, A–D. Costaining of Sox-9 and LacZ showed a double positive staining within the chondrocyte-like cells (Figure 6, E and F).
RANKL Expression in Chondrocyte-Like Cells
To determine the expression of NF-κB ligand RANKL in LDLr−/− mice, samples were immunostained with CD254/sRANKL antibody. Within the atherosclerotic plaques, RANKL-positive stained areas were located in close proximity to clusters of collagen type II-positive stained cells (Figure 3, E and F) and in vicinity to MOMA-2-positive macrophages (Figure 3, A and B, E and F).
Discussion
In the present study, we investigated the cellular origin of chondrocyte-like cells in atherosclerotic intimal calcification using BMT in C57BL/6 LDLr−/− mice. Specific determination of chondrocyte-like cells was performed by high-resolution confocal microscopy of immunohistochemical collagen type II costaining with β-galactosidase (LacZ) and leukocyte-defining surface antigens. Mechanisms and provenance of ectopic cartilage and osteoblastic metaplasia in vasculature have long been unknown. Recently, Speer et al24 demonstrated for the first time that chondrocyte-like cells in medial artery calcification were derived from transdifferentiation of mature smooth muscle cells (VSMC). Although the source of chondrocyte-like cells in this animal model of medial calcification was demonstrated, the origin of osteochondrogenic precursors in atherosclerotic intimal calcification had still to be discovered.25 Medial and intimal vascular calcification represents two entities of vascular disease yet with distinct pathological mechanisms of initiation, progression, and involvement of participating cells.6
Here bone marrow derived precursors from C57BL/6 ROSA-26 mice were detected immunohistochemically. Chimera analysis exhibited a mean of 89.8% LacZ+ cells in peripheral blood and both collagen type II and Sox-9 costaining with LacZ revealed an average 88.8% cytoplasmatic LacZ+ evidence within the chondrocyte-like cells. The results provide evidence that the majority of chondrocyte-like cells were of bone marrow origin in this mouse model of atherosclerotic intimal calcification. Immunhohistochemical staining for Sm22α was performed to exclude a potential involvement or transdifferentiation of local VSMC. More advanced plaques yielded an average of 14% of the cells being chondrocyte-like cells, mainly located within the fibrous cap and colocalized with calcification, which is in congruence with previous findings described in apoE−/−4 and LDLr−/− mice.13 Atherosclerotic plaques displayed a diffuse punctate calcification in female LDLr−/− mice congruent to previous studies using male mice. Our experiments were not designed to demonstrate gender differences of atherosclerotic development and calcification extent.
Previous studies have shown that adult pluripotent cells derived from human peripheral blood monocytes showed transdifferentiation from a monocyte-like structure to a chondrocyte-like structure,17 indeed we excluded both macrophage and monocyte lineage precursors as a source of chondrocyte-like cells in intimal calcification by collagen II costaining with MOMA-2 and CD115, respectively. Shafer et al demonstrated that early chondrocyte progenitors were of myeloid origin in a mouse model of chondrocyte differentiation.17 Moreover, a recent study demonstrated that patients with coronary atherosclerosis displayed a significant increase in the percentage of circulating CD34+ and CD133+ progenitor cells in peripheral blood.26 We examined whether hematopoietic stem cells or early stages of leukocyte differentiation as detected by stem cell marker CD34 and myeloid progenitor-associated antigen CD13 contribute to the phenotype. CD34+ was detectable in 87% of the chondrocyte-like cells and CD13+ evidence could be substantiated in 55% of chondrocyte-like cells, attributable most likely due to loss of the surface markers during transdifferentiation. The results suggest that bone marrow derived myeloid CD34+/CD13+ precursors actively infiltrate the plaque where they are capable of transdifferentiating into chondrocytes-like cells in the progression of atherosclerosis.
Accumulating evidence suggests that chondrocytes whether of VSMC origin in medial calcification or of hematopoietic origin in intimal calcification actively promote the development of vascular calcification. Because chondrocytes and osteoblasts are responsible for orthotopic calcification in bone and cartilage, it seems obvious that they fulfill similar functions within the arterial wall. A recent report examined the nanostructure of calcium deposits within calcified human atherosclerotic plaques and demonstrated marked morphological similarities compared with skeletal bone emphasizing the active regulation of the process.12 The body appears to have a distinct intention to produce mineralization within the plaque.27 The aim of the immune response is likely to isolate a chronic inflammation process as in tuberculous infection where a chronic, noncontrollable infection is isolated by a dense calcified ring. Although the atherosclerotic alteration of the vessel wall progresses, hyperlipidemia leads to a chronic exposure with oxidized low-density lipoprotein and other lipids causing the well documented inflammation with activation of leukocyte subsets.28 Taken together converging evidence, atherosclerotic calcification may simply be another attempt to wall-off a soft tissue focus of chronic inflammation, representing an immune response of last resort.27 In addition, a very recent work has gathered evidence that atherosclerotic plaque calcification within human calcified carotid plaques may be a structural marker of plaque stability.29
Although tuberculous infection exhibits distinct calcification, it is obvious that no chondrocyte metaplasia can be detected in this context suggesting that others forces are involved in the induction of the vascular phenotype. Cartilage of the hyaline type resists to tensile forces and compression30 because of the net-like organized structure of collage type II fibers combined with a high concentration of proteoglycans. It is a well-known fact that chondrocyte generation/differentiation both in vivo and in vitro requires biomechanical stimulation such as hydrostatic pressure, compression, shear stress or a lowered oxygen supply.31 This fact might explain the occurrence of chondrocyte-like cells in atherosclerotic intimal calcification in contrast to tuberculosis infection because atherosclerotic plaques suffer from both local hypoxia and exposure to mechanical and shear stress within pathological vasculature.
In case of a microtrauma in cartilage, chondrocytes begin to proliferate, form chondrocyte clusters, and produce extracellular matrix with increased collagen type II synthesis.30 Deep cartilage defects are repaired by precursor or stem cells of mesenchymal origin from the subchondral bone marrow.32 Multipotent mesenchymal stem cells from bone marrow migrate into the defect and differentiate into chondrocyte-like cells33 with enormous synthesis of proteoglycans and collagen II. There is, thus far, no uniformly accepted clear and specific definitive phenotype or surface marker for the identification of mesenchymal stem cells although lack of the expression of markers including CD34, CD45, CD14, and CD11b have been postulated as criteria of determination.34 Endothelial progenitor cell (EPC) home to sites of endothelial injury and ischemia, where they proliferate, differentiate, and integrate into the endothelial layer or exert a paracrine function by producing vascular growth factors. In response to ischemic injury, EPC are mobilized from the bone marrow. Unfortunately, there is no specific marker to identify an EPC, but it was suggested to use CD34, CD133, and KDR as markers for circulating EPC in human subjects.35 This suggests that chondrocyte-like cells in our animal model of atherosclerosis rather seem to be descended from myeloid precursors or EPC as of mesenchymal stem cell origin. Taken together, a dynamic interaction of initial crystallization of calcium and phosphate within apoptotic leukocytes with a local milieu of hypoxia and mechanical stress as well as an immune response of last resort might explain the occurrence of chondrocyte-like cells within atherosclerotic vasculature.
To uncover the origin of chondrocyte metaplasia is important to develop adequate prevention or therapeutic strategies for atherosclerotic calcification. Morony et al13 recently demonstrated that atherosclerotic calcification could significantly be reduced in the same mouse model by treatment of LDLr−/− mice with recombinant osteoprotegerin (Fc-OPG). The authors hypothesized that the attenuation of vascular calcification was achieved by systemic long-term RANKL inhibition via OPG treatment. RANKL is expressed predominantly in bone and myelomonocytic cells but rarely detected in unaffected vasculature.13 RANKL involvement was described to be needed in survival of leukocytes from myeloid origin36 and differentiation of leukocytes. Here we demonstrated a RANKL positive staining in clusters of hypertrophic chondrocyte-like cells and close proximity to MOMA-2-positive macrophages, presenting evidence that RANKL indeed plays a significant role in the transdifferentiation of myeloid precursors. Our results would render an explanation for the Fc-OPG treatment to that effect that bone marrow-derived precursor cells infiltrate the plaque actively and differentiate into chondrocytes as induced by RANKL. OPG treatment might inhibit the RANKL effect on myeloid transdifferentiation. Interestingly, vascular intimal calcification as induced by chondrocyte-like cells could be decreased suggesting that the process of atherosclerosis and vascular calcification can be uncoupled thereby revealing thrilling new prospects.
In summary, our findings suggest a crucial role for myeloid CD34+/CD13+ precursor cells derived from bone marrow in the development of chondrocyte-like cells in atherosclerotic intimal calcification of LDLr−/− mice. Moreover, the results may render an explanation for the therapeutic effect of Fc-OPG treatment in the same mouse model displaying features of the metabolic syndrome.
Acknowledgments
We thank Dr. Rüdiger Noel for advice in matters of animal maintenance and welfare. We are indebted to Prof. Dr. Jürgen Westermann, Dr. Peter Koenig, and Gurdrun Knebel (Institute of Anatomy) for supporting this work, to Dr. Jan Marxsen (Institute of Hematology) for valuable discussions, and to Dr. Roger Nadrowitz for conducting the irradiation experiments.
Footnotes
Address reprint requests to Lars Doehring, M.D., Medizinische Klinik II, Universitaetsklinikum Schleswig-Holstein, Campus Luebeck, Ratzeburger Allee 160, 23538 Luebeck, Germany. E-mail: Lars.Doehring@uk-sh.de.
Supported by Atherogenomics 01GS0831.
L.C.D. and C.H. contributed equally to this work.
References
- Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GR, Partridge J. Coronary calcification score: the coronary-risk impact factor. Lancet. 2004;363:557–559. doi: 10.1016/s0140-6736(04)15544-x. [DOI] [PubMed] [Google Scholar]
- Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann NY Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, Maekawa K, Yamakawa T, Imanishi Y, Inaba M, Nishizawa Y. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417–425. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med. 2006;260:192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- Mathew S, Davies M, Lund R, Saab G, Hruska KA. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur J Clin Invest. 2006;36(Suppl 2):43–50. doi: 10.1111/j.1365-2362.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- Duer MJ, Friscic T, Proudfoot D, Reid DG, Schoppet M, Shanahan CM, Skepper JN, Wise ER. Mineral surface in calcified plaque is like that of bone: further evidence for regulated mineralization. Arterioscler Thromb Vasc Biol. 2008;28:2030–2034. doi: 10.1161/ATVBAHA.108.172387. [DOI] [PubMed] [Google Scholar]
- Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr−/− mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- Hikita A, Yana I, Wakeyama H, Nakamura M, Kadono Y, Oshima Y, Nakamura K, Seiki M, Tanaka S. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-κB ligand. J Biol Chem. 2006;281:36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Fandrich F, Varoga D, Wruck CJ, Mentlein R, Helfenstein A, Hoseas D, Dressel S, Tillmann B, Ruhnke M. Programmable cells of monocytic origin (PCMO): a source of peripheral blood stem cells that generate collagen type II-producing chondrocytes. J Orthop Res. 2008;26:304–313. doi: 10.1002/jor.20516. [DOI] [PubMed] [Google Scholar]
- Shafer J, Davis AR, Gannon FH, Fouletier-Dilling CM, Lazard Z, Moran K, Gugala Z, Ozen M, Ittmann M, Heggeness MH, Olmsted-Davis E. Oxygen tension directs chondrogenic differentiation of myelo-monocytic progenitors during endochondral bone formation. Tissue Eng. 2007;13:2011–2019. doi: 10.1089/ten.2006.0063. [DOI] [PubMed] [Google Scholar]
- Boisvert WA, Rose DM, Boullier A, Quehenberger O, Sydlaske A, Johnson KA, Curtiss LK, Terkeltaub R. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler Thromb Vasc Biol. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- Bu H, Ma Y, Cheng J, Li S, He Q, Li Y. Immune reconstitution in immunosuppressed pigs with human immune competent cells. Hua Xi Yi Ke Da Xue Xue Bao. 2000;31:165–168. [PubMed] [Google Scholar]
- Van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska KA. Vascular smooth muscle cells in the pathogenesis of vascular calcification. Circ Res. 2009;104:710–711. doi: 10.1161/CIRCRESAHA.109.195487. [DOI] [PubMed] [Google Scholar]
- Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer LL, Sage AP, Tintut Y. Nanoscale architecture in atherosclerotic calcification. Arterioscler Thromb Vasc Biol. 2008;28:1882–1884. doi: 10.1161/ATVBAHA.108.175711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- Wahlgren CM, Zheng W, Shaalan W, Tang J, Bassiouny HS. Human carotid plaque calcification and vulnerability: relationship between degree of plaque calcification, fibrous cap inflammatory gene expression and symptomatology. Cerebrovasc Dis. 2009;27:193–200. doi: 10.1159/000189204. [DOI] [PubMed] [Google Scholar]
- Schulz RM, Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 2007;36:539–568. doi: 10.1007/s00249-007-0139-1. [DOI] [PubMed] [Google Scholar]
- Concaro S, Gustavson F, Gatenholm P. Bioreactors for tissue engineering of cartilage. Adv Biochem Eng Biotechnol. 2009;112:125–143. doi: 10.1007/978-3-540-69357-4_6. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop Relat Res. 1997:254–269. [PubMed] [Google Scholar]
- Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43:32–39. doi: 10.1016/j.bone.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7(Suppl 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann NY Acad Sci. 2008;1143:123–150. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]