Abstract
Lymphangiogenesis plays an important role in tumor metastasis, wound healing, and immune reactions, such as after organ transplantation. Furthermore, novel antilymphangiogenic drugs are moving into clinical medicine, but so far nothing is known about a potential genetic heterogeneity influencing lymphangiogenesis. Using the mouse cornea micropocket assay (VEGF-C) and the suture-induced corneal neovascularization model in different inbred and wild–derived mouse strains (Balb/cAnNCrl, C57BL/6NCrl, 129S1/SvImJ, SJL/JCrl, Cast/EiJ, FVB/NCrl), significant differences in the lymphangiogenic response were detected: the lymphvascularized area varied up to 1.9-fold in the micropocket assay and up to 1.7-fold in the suture-induced neovascularization model between the “low-responder” strain BALB/c and the “high-responder” strain FVB in response to the same stimulus. Furthermore, the number of physiological lymphatic vascular extensions into the marginal zone of the normally alymphatic cornea in untreated eyes again showed a difference of 1.6-fold between low- and high-responders. An anti-inflammatory (prednisolone acetate) and a specific anti(lymph)angiogenic therapy (blocking anti–VEGFR-3 antibody) had different effects on the lymphvascularized area in BALB/c mice and FVB mice, suggesting a different responsiveness to antilymphangiogenic treatments. These data for the first time demonstrate significant differences in the lymphangiogenic response of several mouse strains and suggest underlying genetic factors influencing the lymphangiogenic response. These considerations need to be taken into account when using different mouse strains to study lymphangiogenesis and may also explain different success of antilymphangiogenic treatments in tumor patients.
Lymphangiogenesis is the formation of novel lymphatic vessels from pre-existing ones. The lymphatic vasculature is spread throughout the whole body with some exceptions like cartilage, central nervous system, and the cornea. It is found in all higher vertebrates as a second vascular system beside the blood vascular system and maintains the fluid balance and blood pressure1 in the body. In addition, the lymphatic system contributes to the immune surveillance of the body. Via the lymphatic vessels antigen-presenting cells can be transported to the regional lymph nodes and initiate an immune response. This is of special interest in the clinical setting of graft rejection.2 Lymphangiogenesis also plays an important role in cancer metastasis3 and is suggested to be involved in the pathogenesis of disorders such as inflammatory arthritis4 and chronic airway inflammation.5
Contrary to angiogenesis, where substantial progress in understanding the molecular mechanisms and regulation-pathways was gained in the last decades, lymphangiogenesis research was long hampered by the absence of specific molecular markers. This changed with the discovery of specific molecular markers, such as LYVE-1,6 Podoplanin,7 Prox1,8 and VEGFR-39 and various in vitro and in vivo models in the last few years.
However, available information concerning the field of lymphangiogenesis research still lags behind hemangiogenesis (e.g., in the field of genetic diversity). Genetic heterogeneity of angiogenesis in mice was first reported in 2000 by Rohan et al,10 who showed that—dependent on the genetic background—the response to growth factor–induced angiogenesis varies significantly between different inbred mouse strains. Strain-dependent differences were also published for the density and surface area of the resting limbal vessels after bFGF-induced neovascularization in the cornea.11 Genetic diversity influencing angiogenesis-regulating genes12 is implicated in altering the susceptibility to angiogenesis-dependent diseases like cancer, diabetic retinopathy,13 psoriasis,14 and others. In contrast to the evidence for genetic heterogeneity on angiogenesis, to date little is known in this context about lymphangiogenesis.
In this study we analyzed the presence of strain-dependent differences in lymphatic vessel growth in a standard corneal neovascularization model, the micropocket assay. We further determined the differences in an inflammatory context, because inflammation is the main trigger for pathological lymphangiogenesis in clinical settings.15,16 Therefore, in addition we used the murine model of suture-induced inflammatory corneal neovascularization. The normal cornea is devoid of blood and lymph vessels.2,17 However, due to an inflammatory stimulus the angiogenic privilege can be overcome resulting in a parallel ingrowth of blood and lymphatic vessels.15 Therefore, the cornea serves as an ideal model to study neovascularization under pathological conditions in vivo.18 Interindividual differences in lymphangiogenesis may affect the risk for graft/tissue rejection after transplantation and furthermore determine the response to an antilymphangiogenic treatment (e.g., in oncology).
Materials and Methods
Mice and Anesthesia
For the suture-induced inflammatory corneal neovascularization assay female mice between 6 and 9 weeks of age were analyzed as described previously.13 Animal care and experiments were performed according to the local animal care committee and were in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Before surgery, mice were deeply anesthetized with an intraperitoneal injection using a combination of Ketamine (Ketanest-S®, 8 mg/kg) and Xylazine (Rompun®, 0.1 ml/kg). The following mice strains were used for analyzing differences in the lymphangiogenic response: C57BL/6NCrl, 129S1/SvImJ, Balb/cAnNCrl, FVB/NCrl, SJL/JCrl, and Cast/EiJ (Charles River, Sulzfeld, Germany). To determine the sensitivity to antilymphangiogenic drugs, Balb/cAnNCrl and FVB/NCrl mice were used (Charles River, Sulzfeld, Germany).
Suture-Induced Inflammatory Corneal Neovascularization Assay
The mouse model of suture-induced inflammatory corneal neovascularization was used as described previously.19 Before corneal surgery, each animal was deeply anesthetized. Briefly, three 11-0 nylon sutures (Serag Wiessner, Naila, Germany) were placed intrastromally with two stromal incursions extending over 120° of corneal circumference each. The outer point of suture placement was chosen near the limbus, and the inner suture point was chosen near the corneal center equidistant from the limbus to obtain standardized vascularization responses. Sutures were left in place for the duration of the particular experiment.
To compare the lymphangiogenic responses of the different inbred mouse strains induced by the corneal neovascularization assay the sutures were left in place for 14 days.20 The mice were killed after 14 days, and the cornea with limbus was excised.
Cornea Micropocket Assay
The mouse corneal micropocket assay was performed as described previously with modifications.21 Briefly, after animals were deeply anesthetized a corneal micropocket was dissected toward the nasal limbus. Sucralfate pellets coated with hydron (Sigma-Aldrich Chemie GmbH, Munich, Germany) containing 200 ng recombinant human VEGF-C (RELIATech GmbH, Wolfenbuettel, Germany) were implanted 0.5 to 0.9 mm from the limbus. The pellets were created by dissolving 20 μg recombinant human VEGF-C in PBS (Dulbecco’s phosphate buffered saline, PromoCell GmbH, Heidelberg, Germany). To the suspension 4.5 mg sucralfate and 4.5 μl of 12% hydron in ethanol were added and then the suspension was pipetted onto a nylonmesh (10 × 10 squares) with a pore size of 400 μm (Fisher Scientific GmbH, Schwerte, Germany). Such pellets contained 200 ng VEGF-C and 45 μg sucralfate. Antibiotic ointment (ofloxacin) was applied to each operated eye postoperatively. On postoperative day 7 the mice were killed, the cornea with limbus was excised, and the lymphangiogenic response was measured.
Sensitivity to an Anti-Inflammatory and Antilymphangiogenic Treatment
To determine the sensitivity to an anti-inflammatory and antilymphangiogenic therapy again the suture-induced inflammatory corneal neovascularization assay was used as described above and the sutures remained in the corneal stroma for 10 days. Before the corneal neovascularization assay the central cornea was marked with a 1.5-mm-diameter trephine and deepithelialized to ensure a similarly good penetration of the eye drops. The treatment group received prednisolone acetate 1% eye drops beginning on the day of surgery for 10 days (Inflanefran® Forte 10 mg/ml, three eye drops daily, 5 μl per drop; Pharm-Allergan GmbH, Ettlingen, Germany). The control group received an equal volume of saline solution (NaCl 0.9%). After 10 days the mice were killed and the cornea with limbus was excised.
For analyzing the sensitivity to a anti–VEGFR-3 antibody (mF4-31C1, ImClone Systems Inc., New York, NY,22 generous gift by Bronislaw Pytowski) the sutures were left in the corneal stroma for 7 days.23 The treatment group received the anti–VEGFR-3 antibody mF4-31C1 on the day of surgery and on day 3 and 7 intraperitoneally (25 μg/g).23 Control mice received an equal amount of control IgG solution (rat IgG-Isotype Control, Abcam, Cambridge, UK). After 7 days the mice were killed and the cornea with limbus was excised.
Preparation of Corneal Wholemounts and Immunohistochemistry
Preparation of corneal wholemounts from the corneal neovascularization assay was done as described previously.19 Briefly, corneal wholemounts were rinsed in PBS and fixed in acetone for 30 minutes at room temperature. After additional three washing steps in PBS and blocking with 2% bovine serum albumin (BSA) in PBS for 2 hours, corneas were stained overnight at 4°C with rabbit anti-mouse LYVE-1 antibody (1:200 dilution; AngioBio Co., Del Mar, California). On day two after a washing step, LYVE-1 was detected using a Cy3-conjugated secondary goat anti-rabbit antibody (1:100 dilution; Dianova, Hamburg, Germany). After a final washing step the wholemounts were transferred to Superfrost®-slides (SuperFrost; Menzel-Glaser, Braunschweig, Germany), covered with DAKO® fluorescent mounting medium (Glostrup, Denmark), and stored at 4°C in the dark.
Morphometrical Analysis of Lymphangiogenesis
The stained wholemounts were analyzed with a fluorescence microscope (BX51, Olympus Optical Co., Hamburg, Germany), and digital pictures were taken with a 12-bit monochrome CCD camera (F-View II, Soft Imaging System, Münster, Germany). Each wholemount picture was assembled out of nine or twelve pictures (depending on the size of the cornea) taken at ×100 magnification.
Neovascularization Area
Concerning the outgrowth of lymphatic vessels in the inflammation-induced corneal neovascularization model and in the corneal micropocket assay, the area covered with lymphatic vessels was detected with an algorithm established in the image analyzing program cell^ (Olympus Deutschland GmbH, Hamburg, Germany): before analysis gray value images of the wholemount pictures were modified by several filters.24 Lymphatic vessels were detected by threshold setting including the bright vessels and excluding the dark background as described previously.24
Invasion Area
The measurement of the invasion area was done as described previously.25 Briefly, the sprouts of the lymphatic vessels were connected, and the whole area enclosed between the limbus and the line connecting the sprouts was measured and related to the whole cornea.
Centrally Directed Vascular Extensions and Resting Limbal Vasculature
The number of centrally directed vascular extensions (sprouts) from the main limbal lymphatic vessel and the surface area of the resting limbal lymphatic vasculature were determined from wholemounts of the untreated eye of the different mouse strains.26 Sprouts refers to physiological extensions from the physiologically lymphvascularized conjunctiva into the physiologically alymphatic cornea with the “limbus” being the border.
Statistical Analysis
To analyze the VEGF-C–induced and the suture-induced lymphvascularized area of different mouse strains, the mean lymphvascularized area of the Balb/cAnNCrl wholemounts (reference strain) was defined as being 100%; vascularized areas of the other strains were then related to this value. The same was done for analyzing the invasion area and the area of the resting limbal vasculature in naïve corneas. Concerning the sensitivity to an antilymphangiogenic and anti-inflammatory therapy, the mean vascularized area of the Balb/cAnNCrl control wholemounts was defined as being 100%; vascularized areas of the other strains were then related to this value.
Subsequent statistical analysis was done by InStat 3 Version 3.06 (GraphPad Software Inc, San Diego, CA). Graphs were drawn using Prism4, Version 4.03 (GraphPad Software, San Diego, CA). Figures 1 to 3 were reported as “Box-and-whiskers graph” with a box representing 50% of all values (box extends from the 25th to the 75th percentile) and a line at the median (the 50th percentile). The whiskers of the boxes extend to the lowest and highest value. In case of Figure 4 the bar graph shows the mean of the values with standard deviation (SD). Figures 5 to 7 were reported as “Box-and-whiskers graph”. Additionally, all means and SD for the box plots are given in the results part.
Results
Corneal Inflammation-Induced Lymphangiogenesis Varies between Different Mouse Strains
To investigate whether there are differences in inflammation-induced pathological lymphangiogenesis in different mouse strains we compared the outgrowth of pathological lymphatic vessels into the corneas of Balb/cAnNCrl (n = 21), C57BL/6NCrl (n = 20), SJL/JCrl (n = 19), 129S1/SvImJ (n = 19), Cast/EiJ (n = 17), and FVB/NCrl mice (n = 19) using two different image analysis methods to quantify lymphangiogenesis.
Quantification of Lymphangiogenesis by Measuring the Neovascularization Area
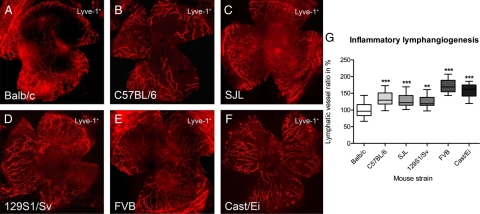
Fourteen days after the inflammatory insult by suture-placement, the total surface area of the vessels ingrown in the previously avascular cornea was assessed. The lymphvascularized area of all tested murine strains was significantly different from the reference strain Balb/cAnNCrl (Figure 1, A–F).
Figure 1.
Strain-dependency of inflammatory lymphangiogenesis in different mouse strains. A–F: Representative wholemounts of murine corneas (original magnification, ×100) with Lyve-1+ lymphatic vessels. A: Balb/cAnNCrl. B: C57BL/6NCrl. C: SJL/JCrl. D: 129S1/SvImJ. E: FVB/NCrl. F: Cast/EiJ. G: Inflammatory lymphangiogenesis varied up to 1.7-fold between different strains. The mean lymphvascularized area of Balb/cAnNCrl was defined as 100%. The mean lymphvascularized area for Balb/cAnNCrl was 100 ± 22% (n = 21), for C57BL/6NCrl 132 ± 22% (n = 20), for SJL/JCrl 128 ± 18% (n = 19), for 129S1/SvImJ 126 ± 18% (n = 19), for FVB/NCrl 172 ± 20% (n = 19), and for Cast/EiJ 159 ± 19% (n = 17), respectively. G: Boxplot where the box represents 50% of all values and the whiskers show the lowest and highest value. Statistical evaluation was done by analysis of variance with Bonferroni multiple comparison post test. **P < 0.01, ***P < 0.001.
The mean lymphvascularized area for Balb/cAnNCrl was 100 ± 22% (n = 21), for C57BL/6NCrl 132 ± 22% (n = 20), for SJL/JCrl 128 ± 18% (n = 19), for 129S1/SvImJ 126 ± 18% (n = 19), for FVB/NCrl 172 ± 20% (n = 19), and for Cast/EiJ 159 ± 19% (n = 17), respectively. Compared with Balb/cAnNCrl the lymphvascularized area in the C57BL/6NCrl strain was increased 1.3-fold (P < 0.001), 1.3-fold in the SJL/JCrl (P < 0.001), 1.3-fold in the 129S1/SvImJ (P < 0.01), 1.6-fold in the Cast/EiJ (P < 0.001), and 1.7-fold in the FVB/NCrl strain (P < 0.001), analysis of variance with Bonferroni multiple comparison post test (Figure 1G). In summary, the resulting strain-dependent rank order of inflammation-induced lymphangiogenesis was: FVB/NCrl > Cast/EiJ > C57BL/6NCrl > SJL/JCrl > 129S1/SvImJ > Balb/cAnNCrl.
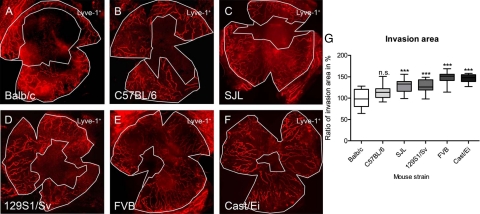
Quantification of Lymphangiogenesis by Measuring the Invasion Area
To evaluate strain-dependent differences we used a second method to quantify the fraction of the cornea into which vessels were grown.25 Whereas the neovascularized area describes the area directly covered by pathological lymphatic vessels in the cornea, the invasion area allows quantifying the part of the total corneal area where vessels are present. Similar to the neovascularization area the results of the invasion area showed a statistically significant difference between Balb/cAnNCrl and other strains, except for the C57BL/6NCrl (Figure 2, A–F). Corresponding to the minor neovascularization area, the Balb/cAnNCrl had the lowest invasion area with an increase of 1.5-fold in the FVB/NCrl strain. The mean invasion area was 100 ± 20% for Balb/cAnNCrl, 115 ± 16% for C57BL/6NCrl (P > 0.05), 129 ± 16% for SJL/JCrl (P < 0.001), 128 ± 14% for 129S1/SvImJ (P < 0.001), 148 ± 14% for FVB/NCrl (P < 0.001), and 146 ± 10% for Cast/EiJ (P < 0.001), respectively (Figure 2G, analysis of variance with Bonferroni multiple comparison post test).
Figure 2.
Strain-dependency of the invasion area of lymphatic vessels in different mouse strains. A–F: Representative wholemounts of murine corneas (original magnification, ×100) with Lyve-1+ lymphatic vessels (as seen in FIgure 1). Invasion area is defined as the area that is enclosed between the two white lines. A: Balb/cAnNCrl. B: C57BL/6NCrl. C: SJL/JCrl. D: 129S1/SvImJ. E: FVB/NCrl. F: Cast/EiJ. G: Invasion area of lymphatic vessels varied up to 1.5-fold between the different strains. The mean vascularized area of Balb/cAnNCrl was defined as 100%. The mean invasion area was 100 ± 20% for Balb/cAnNCrl, 115 ± 16% for C57BL/6NCrl (P > 0.05), 129 ± 16% for SJL/JCrl (P < 0.001), 128 ± 14% for 129S1/SvImJ (P < 0.001), 148 ± 14% for FVB/NCrl (P < 0.001), and 146 ± 10% for Cast/EiJ (P < 0.001), respectively. G: Boxplot where the box represents 50% of all values and the whiskers show the lowest and highest value. Statistical evaluation was done by analysis of variance with Bonferroni multiple comparison post test. ***P < 0.001.
Corneal VEGF-C-Induced Lymphangiogenesis Varied Between Different Mouse Strains
We further compared the lymphvascularized area between different mouse strains with the standard angiogenic neovascularization model, the cornea micropocket assay. The mean lymphvascularized area induced by 200 ng VEGF-C for Balb/cAnNCrl was 100 ± 28% (n = 15), for C57BL/6NCrl 143 ± 35% (n = 16), for SJL/JCrl 132 ± 48% (n = 11), for 129S1/SvImJ 132 ± 35% (n = 14), for FVB/NCrl 194 ± 55% (n = 21), and for Cast/EiJ 177 ± 63% (n = 13), respectively. The mean lymphvascularized area significantly differed between the Balb/cAnNCrl and FVB/NCrl with an increase of 1.9-fold (P < 0.001) and between Balb/cAnNCrl and Cast/EiJ with an increase of 1.8-fold (P < 0.01). The C57BL/6NCrl, SJL/JCrl, and 129S1/SvImJ strain showed an increase of 1.4-fold, 1.3-fold, and 1.3-fold, respectively, (P > 0.05; Kruskal–Wallis analysis with Dunn’s multiple comparison test; Figure 3). Similar to the suture-induced lymphangiogenesis the resulting strain-dependent rank order of VEGF-C–induced lymphangiogenesis was: FVB/NCrl > Cast/EiJ > C57BL/6NCrl > SJL/JCrl, 129S1/SvImJ > Balb/cAnNCrl.
Figure 3.
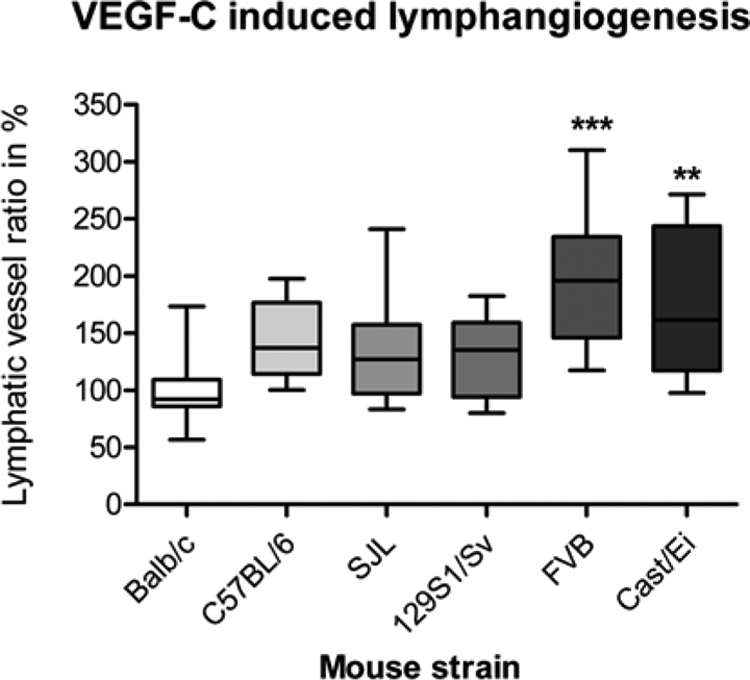
Strain-dependency of lymphangiogenesis induced by VEGF-C pellets in the murine corneal micropocket assay. Lymphangiogenesis induced by the lymphangiogenic growth factor (VEGF-C) differed significantly between Balb/cAnNCrl and FVB/NCrl (P < 0.001) and Balb/cAnNCrl and Cast/EiJ (P < 0.01). The mean lymphvascularized area induced by 200 ng VEGF-C for Balb/cAnNCrl was 100 ± 28% (n = 15), for C57BL/6NCrl 143 ± 35% (n = 16), for SJL/JCrl 132 ± 48% (n = 11), for 129S1/SvImJ 132 ± 35% (n = 14), FVB/NCrl 194 ± 55% (n = 21), and for Cast/EiJ 177 ± 63% (n = 13), respectively. VEGF-C-induced lymphangiogenesis varied up to 1.9-fold between different murine strains. The figure shows a boxplot where the box represents 50% of all values and the whiskers show the lowest and highest value. Statistical evaluation was done by Kruskal-Wallis analysis with Dunn’s multiple comparison post test. **P < 0.01, ***P < 0.001.
Resting Limbal Vasculature in Naïve Corneas Varied between Different Mouse Strains
To investigate whether there are also differences in the resting physiological lymphatic vasculature in diverse mouse strains we compared the number of centrally directed vascular extensions (sprouts) from the conjunctiva into the normally avascular cornea (at the so called “limbus”) and the area of the resting limbal vasculature in naïve corneas of Balb/cAnNCrl (n = 13), C57BL/6NCrl (n = 16), SJL/JCrl (n = 19), 129S1/SvImJ (n = 19), FVB/NCrl (n = 19), and Cast/EiJ mice (n = 13).
Number of Physiological Limbal Lymphatic Vascular Extensions
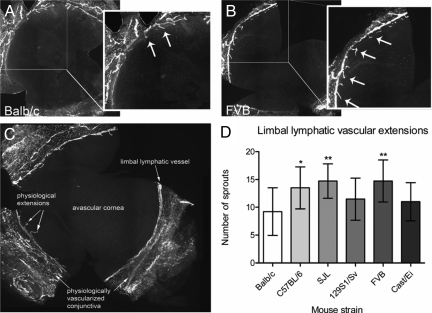
To evaluate whether differences between the mouse strains with respect to lymphatic vessels could also be found in a physiological situation we counted the number of the centrally directed vascular extensions (sprouts) from the main limbal lymphatic vessel into the cornea from flatmounts of the untreated eye (Figure 4, A and B). Limbal sprouts refer to physiological small extensions of conjunctival lymphatic vessels into the physiological alymphatic corneal stroma (Figure 4C). Statistical analysis showed that the number of sprouts significantly differed between Balb/cAnNCrl and C57BL/6NCrl (P < 0.05), Balb/cAnNCrl, and SJL/JCrl (P < 0.01) as well as between Balb/cAnNCrl and FVB/NCrl (P < 0.01; Figure 4D). The lowest number of vascular extensions was found for the Balb/cAnNCrl with a mean of 9 ± 4 extensions (n = 13). The mean number for C57BL/6NCrl was 14 ± 4 (n = 16), for SJL/JCrl 15 ± 3 (n = 19), for 129S1/SvImJ 11 ± 4 (n = 19), FVB/NCrl 15 ± 4 (n = 19), and Cast/EiJ 11 ± 3 (n = 13). No statistical difference was observed between Balb/cAnNCrl and 129S1/SvImJ, and Balb/cAnNCrl and Cast/EiJ (analysis of variance with Bonferroni multiple comparison post test). The rank order found for the physiological extensions was: FVB/NCrl, SJL/JCrl > C57BL/6NCrl > 129S1/SvImJ, Cast/EiJ > Balb/cAnNCrl.
Figure 4.
Number of physiological limbal lymphatic vascular extensions in untreated corneas showed significant differences between murine strains. A and B: Representative wholemounts of murine corneas (original magnification, ×100), with Lyve-1+ lymphatic vessels. Arrows show small centrally directed vascular extensions (sprouts) of Lyve-1+ lymphatic vessels from the physiologically vascularized conjunctiva into the physiologically avascular cornea. A: Balb/cAnNCrl. B: FVB/NCrl. C: The main limbal lymphatic vessel going circumferentially around the cornea separates the physiologically vascularized conjunctiva from the physiological avascular cornea. The physiological small vascular extensions (sprouts) grow out from conjunctival vessels and are centrally directed from the main limbal vessel toward the avascular cornea. D: The number of sprouts significantly differed between Balb/cAnNCrl and C57BL/6NCrl (1.5-fold), BALB/c and SJL/JCrl (1.6-fold), as well as between Balb/cAnNCrl and FVB/NCrl (1.6-fold). *P < 0.05, **P < 0.01. Statistical evaluation was done by analysis of variance with Bonferroni multiple comparison post test.
Resting Limbal Vasculature
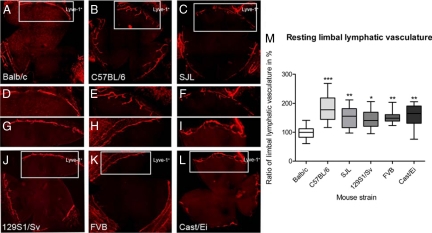
Due to the fact that after an inflammatory stimulus new lymphatic vessels grow out from the limbus, we also analyzed the strain dependency of the total surface area of the resting limbal vasculature in the untreated eye (Figure 5, A–L). Total surface area means the corneal area covered by the circular limbal lymphatic vessel and the physiological extension from the main limbal lymphatic vessel into the cornea. Similar to the results of the neovascularization area the lowest resting limbal vessel area was determined for Balb/cAnNCrl. The mean of the resting limbal vessel area for the different strains was as follows: Balb/cAnNCrl 100 ± 23%, C57BL/6NCrl 182 ± 43%, SJL/JCrl 155 ± 38%, 129S1/SvImJ 145 ± 33%, FVB/NCrl 151 ± 19%, and Cast/EiJ 159 ± 39%. Significant differences could be determined between Balb/cAnNCrl and C57BL/6NCrl (P < 0.001), Balb/cAnNCrl and SJL/JCrl (P < 0.01), Balb/cAnNCrl and 129S1/SvImJ (P < 0.05), Balb/cAnNCrl and Cast/EiJ (P < 0.01), as well as between Balb/cAnNCrl and FVB/NCrl (P < 0.01) Statistical evaluation was done with the Kruskal–Wallis analysis with Dunn’s multiple comparison post test. In contrast to the other measurements the highest total surface area in the naïve cornea was found for the C57BL/6NCrl (Figure 5M). Compared with the strain-dependent rank order of the neovascularization area, the rank order changed to C57BL/6NCrl > Cast/EiJ > SJL/JCrl > FVB/NCrl > 129S1/SvImJ > Balb/cAnNCrl.
Figure 5.
Area of resting limbal lymphatic vasculature of untreated corneas. A–L: Representative wholemounts of murine corneas with resting limbal vasculature (original magnification, ×100 of Lyve-1+ lymphatic vessels). A: Balb/cAnNCrl. B: C57BL/6NCrl. C: SJL/JCrl. J: 129S1/SvImJ. K: FVB/NCrl. L: Cast/EiJ. D–I show details of the limbal vasculature of the strains. M: The area of the resting limbal vasculature (i.e., physiological extensions of conjunctival lymphatic vessels into avascular cornea) varied 1.8-fold between Balb/cAnNCrl and C57BL/6NCrl, 1.5-fold between Balb/cAnNCrl and SJL/JCrl, 1.4-fold between Balb/cAnNCrl and 129S1/SvImJ, 1.5-fold between Balb/cAnNCrl and FVB/NCrl, and 1.6-fold between Balb/cAnNCrl and Cast/EiJ. Statistical evaluation was done by Kruskal-Wallis analysis with Dunn’s multiple comparison post test. *P < 0.05, **P < 0.01, ***P < 0.001.
To further evaluate whether the differences in the resting limbal vasculature might influence the outcome of inflammation-induced lymphangiogenesis we compared the resting limbal vessel area with the inflammation-induced lymphangiogenesis of the corresponding strain. The ratio of the induced lymphvascularization to resting limbal vessel area was 3.8 for Balb/cAnNCrl, 2.6 for C57BL/6NCrl, 3.3 for SJL/JCrl and 129S1/SvImJ, 4.0 for Cast/EiJ, and 4.8 for FVB/NCrl. Therefore the rank order changed to FVB/NCrl > Cast/EiJ > Balb/cAnNCrl > 129S1/SvImJ, SJL/JCrl > C57BL/6NCrl.
Sensitivity of Different Strains to Inhibitors of Lymphangiogenesis
To determine whether the observed strain-dependent differences in lymphangiogenesis also influence the sensitivity to antilymphangiogenic treatment options, we analyzed the effect of i) the unspecific anti-inflammatory steroid prednisolone acetate 1% and ii) the specific lymphangiogenesis-blocking anti–VEGFR-3 antibody mF4-31C122,23 on inflammation-induced lymphangiogenesis in the low-responder strain Balb/cAnNCrl and the high-responder strain FVB/NCrl.
Sensitivity to Prednisolone Acetate 1%
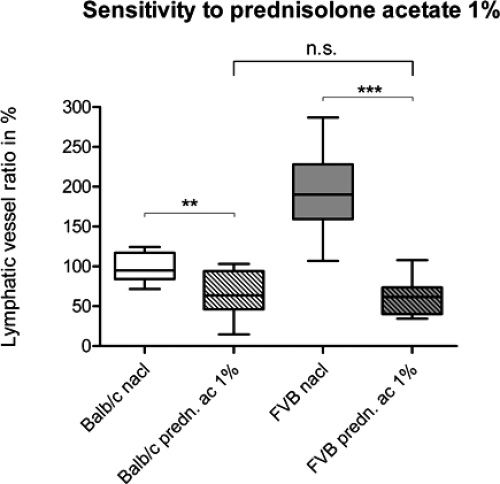
Treatment of Balb/cAnNCrl and FVB/NCrl with the anti-inflammatory steroid prednisolone acetate 1% after suture placement revealed a twofold higher reduction in the lymphvascularization area for FVB/NCrl versus Balb/cAnNCrl. For both strains the reduction of the vascularized area was found to be statistically significant: for Balb/cAnNCrl from 100 ± 18% in the control group (n = 9) to 66 ± 27% in the treatment group (n = 10, P = 0.0064); for FVB/NCrl, from 194 ± 52% in the control group (n = 9) to 62 ± 24% in the treatment group (n = 8, P < 0.0001; unpaired t test; Figure 6).
Figure 6.
Effect of an anti-inflammatory therapy on inflammation-induced lymphangiogenesis in low- and high-responder murine strains. Lymphatic vascularization after suture placement and topical application of saline solution (0.9%) and prednisolone acetate 1% for ten days, three eye drops daily, 5 μl per drop. The reduction of the neovascularization area was 34% in the Balb/cAnNCrl strain (P = 0.0064) and 68% in the FVB/NCrl strain (P < 0.0001). After treating the mice with prednisolone acetate 1% the initially significantly different lymphvascularized areas (P = 0.0007) between Balb/cAnNCrl and FVB/NCrl mice reached a similar level in both strains (P = 0.7064). **P < 0.01, ***P < 0.001. Statistical evaluation was done with an unpaired t test with Welch correction.
In line with the results from the lymphvascularized area after suture placement the mean of the Balb/cAnNCrl (100 ± 18%) and the FVB/NCrl (194 ± 52%) control group, treated with saline solution (0.9%) differed significantly (P = 0.0007, unpaired t test with Welch correction). No significant difference could be observed after the treatment with prednisolone acetate 1% (P = 0.7064, unpaired t test). This showed a strong antilymphangiogenic effect of prednisolone acetate 1% in both strains with different levels of lymphangiogenesis inhibition (decrease of 34% for Balb/cAnNCrl versus 68% for FVB/NCrl) but resulting in similar levels of final lymphvascularized areas.
Sensitivity to Anti-VEGFR-3 Antibody mF4-31C1
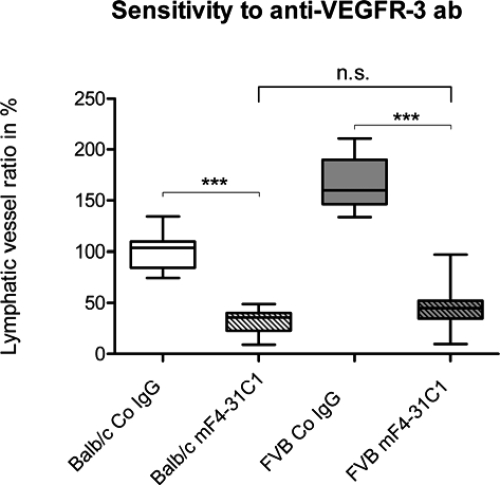
To determine whether there is a difference in the sensitivity between low and high responders to a specific antilymphangiogenic drug, the anti–VEGFR-3 antibody mF4-31C1, we treated Balb/cAnNCrl (n = 11) and FVB/NCrl (n = 11) mice with mF4-31C1. Again the levels of lymphvascularization between the control groups, treated with equal amounts of isotype control, varied significantly between FVB/NCrl and Balb/cAnNCrl. FVB/NCrl mice (n = 11) showed a 1.7-fold higher lymphvascularized area compared with Balb/cAnNCrl (n = 10; P < 0.0001, unpaired t test). Similar to the previous experiment, the neovascularization area of lymphatic vessels under mF4-31C1 treatment decreased significantly in both strains: for Balb/cAnNCrl from 100 ± 17% in the control group (n = 10) to 33 ± 12% in the treatment group (n = 11, P < 0.0001); for FVB/NCrl from 166 ± 25% in the control group (n = 11) to 45 ± 22% in the treatment group (n = 11, P < 0.0001; unpaired t test). The final level of lymphvascularization after mF4-31C1-treatment showed no significant difference between Balb/cAnNCrl and FVB/NCrl (P = 0.1359; unpaired t test; Figure 7).
Figure 7.
Effect of a specific antilymphangiogenic therapy (blocking anti–VEGFR-3 antibody mF4-31C1) on inflammatory lymphangiogenesis in low- and high-responder murine strains. Neovascularization of lymphatic vasculature after suture placement and intraperitoneal application of either a blocking anti-VEGFR-3 antibody (mF4-31C1, 25 μg/g) or an equal amount of control IgG on day of surgery and on day three and seven post surgery. The decrease in neovascularization was 67% (P < 0.0001) in the Balb/cAnNCrl strain and 73% (P < 0.0001) in the FVB/NCrl strain. After treating the mice with mF4-31C1 the significant differing vascularization areas (P < 0.0001) between Balb/cAnNCrl and FVB/NCrl reached a similar level in both strains (P = 0.1359). ***P < 0.001. Statistical evaluation was done with an unpaired t test.
Discussion
The experiments shown in this study for the first time demonstrate that the genetic background is an important predictor for the extent of growth factor–induced (VEGF-C) and inflammation-induced lymphangiogenesis. Depending on the mouse strain, the lymphvascularized area induced by the same stimulus varied up to 1.9-fold in the VEGF-C corneal micropocket assay and up to 1.7-fold in the suture-induced inflammatory corneal neovascularization model. In the corneal micropocket assay the lymphangiogenic response of the Balb/cAnNCrl mice was significantly lower compared with the FVB/NCrl and Cast/EiJ mice. In the inflammatory model the lymphangiogenic response found in the Balb/cAnNCrl mice was significantly and consistently lower compared with all other mouse strains, especially to the FVB/NCrl and Cast/EiJ mice, which were found to be high responders. These results were in line with findings on the invasion area of the cornea that was significantly greater in the FVB/NCrl and Cast/EiJ compared with the Balb/cAnNCrl strain. Thus not only the area that is covered by vessels but also the fraction of the cornea where vessels are present varied between the low- and the high-responder mouse strains after an inflammatory stimulus. This suggests that strain-related differences not only influence the total lymphvascular surface area but furthermore the vessel length and also the distance of the vessels to the center of the cornea.
The strain-related rank order in the lymphangiogenic response reported herein differs from the strain-related rank order found for the genetic heterogeneity of angiogenesis in several mouse strains.10 Several reasons could account for the observed differences: i) Inherent differences may exist between the genetic background determining the hem- versus the lymphangiogenic response (e.g., expression levels of endogenous inhibitors could differ between strains); ii) Different growth factors and different stimuli were used to generate the neovascular response: the (hem)angiogenic neovascularization response was induced by implanting a pellet containing the growth factor bFGF and thereby directly stimulating blood vessel growth with only minimal inflammatory activity.21 In our study, however, VEGF-C pellets were implanted, specifically stimulating lymphatic vessel growth. In the second model in this study the applied stimulus (suture placement) elicits corneal neovascularization via an immune response after induction of inflammation and therefore includes many compounds of the inflammatory cascade, but predominantly VEGFs. Therefore in this model inflammation per se plays a greater role compared with the low-inflammatory pellet model.
Although we primarily focused on classical laboratory inbred mouse strains we further included the Cast/EiJ strain. The Cast/EiJ strain belongs to the wild derived mouse strains that are known to be largely genetically pure in contrast to the classical mouse strains, which derive from a mixture of different subspecies of the genus Mus.27,28 The results suggest that our observation also pertains to wild–derived strains, although further studies have to analyze this possibility in detail.
We further determined the sensitivity of different murine strains to specific and unspecific inhibitors of lymphangiogenesis. The response to a treatment with an indirect antilymphangiogenic drug (prednisolone acetate) was compared with a specific inhibitor of lymphangiogenesis (blocking anti–VEGFR-3 antibody) in a low- and high-responder mouse strain. It was previously shown that inhibition of VEGFR-3 signaling in the inflamed cornea specifically inhibits corneal lymphangiogenesis.23 Treating the low-responder Balb/cAnNCrl and the high-responder strain FVB/NCrl with prednisolone acetate or anti–VEGFR-3 antibody resulted in a different amount of inhibition of the lymphvascularized area. This suggests a different sensitivity among the strains to an anti-inflammatory and also to a specific anti(lymph)angiogenic therapy, both affecting the lymphangiogenic response. Taken together these findings may implicate also interindividual variety in inflammatory lymphangiogenesis in humans that could affect, for example, the responsiveness to an antilymphangiogenic therapy not only in tumor patients. This finding should be specifically relevant when suboptimal tissue drug levels are reached in contrast to suprathreshold values delivered here.
Interestingly although the FVB/NCrl mice showed an up to 1.7-fold increase in the lymphvascularized area after inflammation compared with the Balb/cAnNCrl and although the levels of pharmacological inhibition of lymphangiogenesis varied between the strains, the final lymphvascularized area after treatment reached a similar level in both strains for the anti-inflammatory and the specific anti(lymph)angiogenic therapy as well. That may relate to the fact that both treatments were applied in a very high concentration, but the underlying mechanisms for the observed strain-dependent differences of lymphangiogenesis remain to be studied in more detail. It may well be that underlying genetic factors influencing the immune amplification cascade are responsible for the observed differences in lymphangiogenesis between Balb/cAnNCrl and FVB/NCrl mice.
This is in line with the observation that endotoxin injection induced a stronger inflammatory response in FVB mice (resulting in a severe uveitis) compared with the BALB/c (developing only a mild uveitis).29 In addition, there are several known examples of strain dependency of inflammatory reactions (e.g., in allergen-induced airway disease and atherosclerosis resistance).30,31,32,33,34 However, because VEGF-C–induced lymphangiogenesis and also the resting physiological limbal lymphatic vasculature in the naïve untreated corneas varied significantly between the strains, it is likely that there are also genetic differences affecting developmental lymphangiogenesis: significantly more pre-existing vasculature was found in FVB/NCrl than in Balb/cAnNCrl and also significant more vascular extensions from the main limbal lymphatic vessels in FVB/NCrl mice, both attributing a relevance to a different lymphangiogenic potential between the strains. A strain dependency in the resting limbal vessel surface area was also described for growth factor–induced hemangiogenesis.11
Taken together, we for the first time provide evidence that the genetic background significantly influences lymphangiogenesis and furthermore the sensitivity to an antilymphangiogenic therapy in mice. The information on the genetic heterogeneity of lymphangiogenesis reported in this study has to be taken into account when using mouse models for studying lymphangiogenesis. Furthermore, a detailed knowledge of genetic factors influencing lymphangiogenesis could help to understand different susceptibility to lymphangiogenesis-dependent diseases and different responsiveness to antilymphangiogenic therapies in patients. Last but not least these results pave the way to the identification of novel endogenous activators and inhibitors of lymphangiogenesis using low- and high-responding murine strains.
Acknowledgments
We thank Prof. Dr. André Reis for his helpful suggestions and Dr. Bronislaw Pytowski for the kind gift of the anti-VEGFR-3 antibody mF4-31C1.
Footnotes
Address reprint requests to Claus Cursiefen, M.D., Department of Ophthalmology, Friedrich-Alexander University Erlangen-Nuremberg, Schwabachanlage 6, 91054 Erlangen, Germany. E-mail: Claus.Cursiefen@uk-erlangen.de.
Supported by the Interdisciplinary Center for Clinical Research (IZKF) Erlangen (A9) and the German Research Foundation (DFG: Sonderforschungsbereich SFB 643: B10).
References
- Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis–impact on cancer metastasis. J Leukoc Biol. 2006;80:691–696. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM, Boyce BF, Xing L. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9:R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Horvat R, Amann G, Kowalski H, Kerjaschki D. [Podoplanin–a specific marker for lymphatic endothelium expressed in angiosarcoma]. Verh Dtsch Ges Pathol. 1999;83:270–275. [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan RM, Fernandez A, Udagawa T, Yuan J, D'Amato RJ. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- Chan CK, Pham LN, Chinn C, Spee C, Ryan SJ, Akhurst RJ, Hinton DR. Mouse strain-dependent heterogeneity of resting limbal vasculature. Invest Ophthalmol Vis Sci. 2004;45:441–447. doi: 10.1167/iovs.03-0869. [DOI] [PubMed] [Google Scholar]
- Rogers MS, D'Amato RJ. The effect of genetic diversity on angiogenesis. Exp Cell Res. 2006;312:561–574. doi: 10.1016/j.yexcr.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Crawford TN, Alfaro DV, 3rd, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009;5:8–13. doi: 10.2174/157339909787314149. [DOI] [PubMed] [Google Scholar]
- Heidenreich R, Rocken M, Ghoreschi K. Angiogenesis: the new potential target for the therapy of psoriasis? Drug News Perspect. 2008;21:97–105. doi: 10.1358/dnp.2008.21.2.1188196. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Schlotzer-Schrehardt U, Kuchle M, Sorokin L, Breiteneder-Geleff S, Alitalo K, Jackson D. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Invest Ophthalmol Vis Sci. 2002;43:2127–2135. [PubMed] [Google Scholar]
- Regenfuss B, Bock F, Parthasarathy A, Cursiefen C. Corneal (lymph)angiogenesis–from bedside to bench and back: a tribute to Judah Folkman. Lymphat Res Biol. 2008;6:191–201. doi: 10.1089/lrb.2008.6348. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Dietrich T, Bachmann B, Pytowski B, Cursiefen C. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol. 2008;246:115–119. doi: 10.1007/s00417-007-0683-5. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Hos D, Horn F, Martus P, Cursiefen C. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye Res. 2008;87:462–470. doi: 10.1016/j.exer.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Dastjerdi MH, Al-Arfaj KM, Nallasamy N, Hamrah P, Jurkunas UV, Pineda R, 2nd, Pavan-Langston D, Dana R. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127:381–389. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hos D, Bachmann B, Bock F, Onderka J, Cursiefen C. Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp Eye Res. 2008;87:427–432. doi: 10.1016/j.exer.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- Wade CM, Kulbokas EJ, 3rd, Kirby AW, Zody MC, Mullikin JC, Lander ES, Lindblad-Toh K, Daly MJ. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- Li Q, Peng B, Whitcup SM, Jang SU, Chan CC. Endotoxin induced uveitis in the mouse: susceptibility and genetic control. Exp Eye Res. 1995;61:629–632. doi: 10.1016/s0014-4835(05)80056-9. [DOI] [PubMed] [Google Scholar]
- Stein O, Dabach Y, Ben-Naim M, Halperin G, Stein Y. Lower macrophage recruitment and atherosclerosis resistance in FVB mice. Atherosclerosis. 2006;189:336–341. doi: 10.1016/j.atherosclerosis.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Thurston G, Murphy TJ, Baluk P, Lindsey JR, McDonald DM. Angiogenesis in mice with chronic airway inflammation: strain-dependent differences. Am J Pathol. 1998;153:1099–1112. doi: 10.1016/S0002-9440(10)65654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol. 2003;285:L32–L42. doi: 10.1152/ajplung.00390.2002. [DOI] [PubMed] [Google Scholar]
- Zhu W, Gilmour MI. Comparison of allergic lung disease in three mouse strains after systemic or mucosal sensitization with ovalbumin antigen. Immunogenetics. 2009;61:199–207. doi: 10.1007/s00251-008-0353-8. [DOI] [PubMed] [Google Scholar]