Abstract
Cadmium telluride (CdTe) and iron oxide nanoparticles doped silica nanospheres were prepared by a multistep method. Iron oxide nanoparticles were first coated with silica and then modified with amino group. Thereafter, CdTe nanoparticles were assembled on the particle surfaces by their strong interaction with amino group. Finally, an outer silica shell was deposited. The final products were characterized by X-ray powder diffraction, transmission electron microscopy, vibration sample magnetometer, photoluminescence spectra, Fourier transform infrared spectra (FT-IR), and fluorescent microscopy. The characterization results showed that the final nanomaterial possessed a saturation magnetization of about 5.8 emu g−1and an emission peak at 588 nm when the excitation wavelength fixed at 380 nm.
Keywords: Iron oxide nanoparticles, CdTe, Fluorescent, Magnetic, Preparation
Introduction
Magnetic nanoparticles offer great potential in biomedical applications, including magnetic resonance imaging (MRI), targeted drug delivery, rapid biological separation, biosensors, and magnetic hyperthermia therapy. Among the magnetic nanomateials, iron oxide nanoparticles (including Fe3O4 and γ-Fe2O3) are by far the most commonly employed material because of their biocompatibility [1-4]. At the same time, cadmium telluride (CdTe) nanoparticles, as an important II–VI semiconductor material, have attracted considerable attention over the past decades. They have some unique physical and chemical properties, such as marvelous brightness, narrow and size-tunable emission, fairly high quantum yields, and good chemical and photo stability. These properties make CdTe nanocrystals suitable for biological applications, fabricating photoelectron devices and solar cells [5-11].
So the multifunctional nanomaterials which were composed of iron oxide nanoparticles and CdTe nanocrystals can enjoy both the advantages of iron oxide nanoparticles and CdTe QDs (quantum dots), they showed great potential in biological applications [12-14]. These multifunctional silica spheres are expected to serve as luminescent markers and are capable of being driven by an external magnetic field to a specific location [15]. For example, in a targeting drug-delivery system, the magnetic fluorescent nanoparticles labeled drugs could be easily administered and transported to the terminal under the guidance of an external magnetic field, resulting in a safer and more effective tissue-specific delivery of drugs.
Until now, various approaches have been employed to prepare the multifunctional nanoparticles composed of iron oxide nanoparticle and CdTe quantum dots (QDs). To the best of our knowledge, the major approach was layer-by-layer techniques [16-18]. And some other methods were also developed [19]. Although these approaches are very successful in preparing fluorescent magnetic nano- or micro-sized spheres, they are rather laborious and time-consuming or need more organic solvent. Better preparing techniques are still needed to develop. The previous study indicated QDs can be assembled on the silica surface by amino group, and Liu et al. [20] have modified the surface of silica coated iron oxide nanoparticles with CdSe by this principle. But until now, no one has combined iron oxide nanoparticles and CdTe by amino group. In this study, we prepare bi-functional silica nanospheres doped with iron oxide nanoparticles and CdTe QDs by this protocol for the first time. The preparation procedure was shown in Scheme 1, iron oxide nanoparticles were first coated with silica and then it was modified with amino group. After the CdTe nanocrystals assembled on the particle surfaces, a final silica shell was deposited. Herein, the synthesis and detailed structural, magnetic, and optical property of this multifunctional nanomaterial were presented.
Scheme 1.
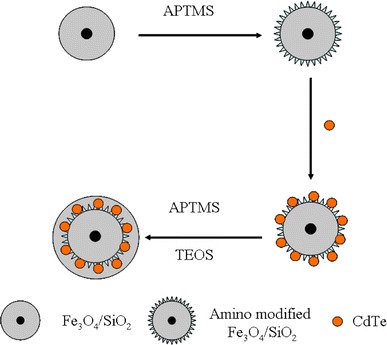
Preparation procedure of multifunctional nanoparticles composed of iron oxide nanoparticles and CdTe nanocrystals
Experimental Section
Chemical Reagents
Tellurium powder was purchased from Shanghai Chemical Reagents Factory, China. NaBH4was commercially available from Guangming Reagent Company, China. Ferric chloride (FeCl3 · 6H2O) and Ferrous chloride (FeCl2 · 4H2O) were purchased from Tianjin Shuangchuan Chemical Reagent Factory, China. TEOS was purchased from Tianjin Chemical Reagent Company, China. 3-aminopropyltrimethoxysilane (APTMS) was commercially available from Fluka. Other routine chemicals were purchased from Shanghai Reagents Factory of China. Doubly deionized water was used throughout the experiment. All chemical reagents were of analytical grade and used as received without further purification.
Preparation Procedure
Thioglycolic acid stabilized CdTe nanocrystals were prepared according to a reported procedure with some modification [21]. Typically, 6 mL of 0.02 M CdCl2 and 100 mL of water was mixed together, and then the pH of the mixture was adjusted to 11.3 in the presence of 26 μL of thioglycolic acid. After stirring and bubbling with nitrogen for 40 min, NaHSe prepared by 6.0 mg Te and 6.0 mg NaBH4 was added. After 1 h stirring, it was refluxed for 3 h.
Silica coated iron oxide nanoparticles were prepared as described by our previous work with some modification [22]. First, iron oxide nanoparticles were prepared from aqueous solution of Fe2+/Fe3+ by the addition of ammonia under magnetic stirring at room temperature. Then it was coated with silica. Typically, 2.5 mL of magnetic nanoparticles solution, 2.5 mL of distilled water, 30 mL of isopropyl alcohol, and 80 μL of TEOS were put together. The reaction was initiated by 0.8 mL of ammonia (25 wt%). After magnetic stirring for 10 h, the formed core–shell nanoparticles were collected by centrifugation and the silica coated iron oxide nanoparticles were denoted as FS nanoparticles.
The surfaces of FS nanoparticles were modified with amino group by treating with APTMS in refluxing toluene [23]. Typically, 260 mg of FS nanoparticles was added to 200 mL of toluene, and then refluxed for 6 h after 2.0 mL of APTMS was added.
In order to prepare CdTe assembled FS nanoparticles, 5.0 mg of the prepared CdTe and amino group modified FS nanoparticles were dissolved in 2 mL of water, respectively. Then the two dispersions were mixed together and stirred for 12 h. The final product was isolated by centrifugation and it was denoted as FSC nanoparticles.
The prepared FSC nanoparticles were further coated with an outer silica layer. After being dispersed in 1 mL of H2O and 4 mL of ethanol, 4 μL of APTMS and 10 μL of TEOS were added to the FSC dispersion under magnetic stirring, the procedure was preceded for 10 h [23]. Finally, the product (denoted as FSCS nanoparticles) was centrifuged and dispersed in water for further characterization.
Characterization
Photoluminescence (PL) spectra were made with a RF-5301 PC spectrofluorophotometer at room temperature. Fluorescence microscope was used to show the optical images of the product. X-ray diffraction (XRD) pattern of the product was performed on an X’Pertpro Philips X-ray diffractometer with CuKαradiation (λ = 0.154056). The scan range (2θ) was from 10° to 90°. Transmission electron microscopy (TEM) images were used to show the morphology and size of the as-prepared samples. Nicolet AVATAR 360 Fourier transform infrared spectra (FT-IR) spectrometer was used to demonstrate the chemical nature of the products in KBr pellets. Magnetic hysteresis loops of these core–shell nanoparticles were carried out with a vibration sample magnetometer (VSM, Lakeshore 730, America) at room temperature.
Results and Discussions
Figure 1a showed the XRD spectra of the FS nanoparticles. The peaks in the range between 10° and 90° demonstrated the iron oxide nanocrystals were Fe3O4 and/or γ-Fe2O3[24]. But it was not essential to distinguish them because they had similar magnetic property. The average particle size of the iron oxide nanoparticles was calculated to be about 10 nm by the <311> XRD peak [25]. Besides the peaks of iron oxide, there was a broad featureless XRD peak at low diffraction angle, which was corresponded to amorphous SiO2 shell.
Figure 1.
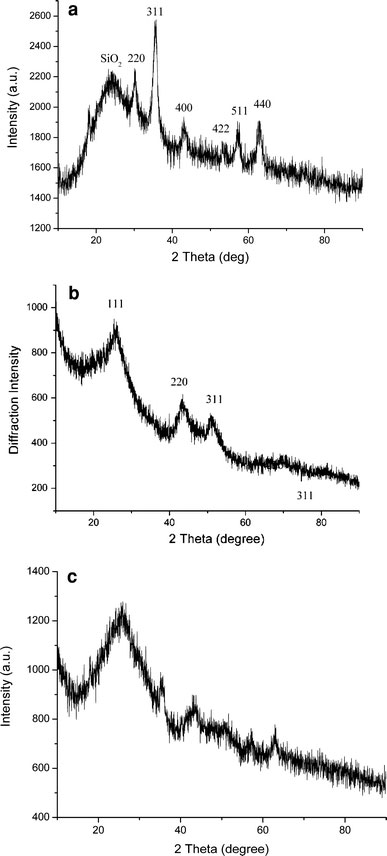
XRD patterns ofaFS;bCdTe;cFSCS nanoparticles
The XRD spectrum of CdTe nanoparticles was depicted in Fig. 1b. The diffraction peaks were similar with the previous reports [26,27]. It indicated the nanoparticles belonged to cubic structure. The average particle size of CdTe nanocrystallite was estimated to be about 3 nm by Scherrer formula.
Figure 1c showed the XRD pattern of FSCS nanoparticles. According to Fig. 1a, the diffraction peaks of iron oxide and silica were present in Fig. 1c. But the existence of CdTe in the particles could not be confirmed by the XRD spectrum because the peaks were ambiguous.
In order to investigate the morphology and size of the prepared nanoparticles in every step, TEM measurement was carried out (Fig. 2). As shown in Fig. 2a, CdTe nanoparticles were nearly monodisperse with an average particle size about 4 nm, which was a little bigger than the result obtained by XRD analysis. Figure 2b showed the TEM microphotographs of amino group modified FS nanoparticles. As observed, the black dots with a diameter of about 10 nm in the particles were iron oxide nanoparticles, which were surrounded by silica. The average diameter of the particles was 40 nm. Their size distribution was narrow, though aggregation appeared in some area. Amino group could not be observed by TEM. The TEM image of the CdTe nanocrystals assembled FS nanoparticles was shown in Fig. 2c. It can be seen that there were some dots on the amino group modified FS nanoparticles surface, which was corresponded to QDs. According to the previous study, the strong binding interaction between the amino groups and the QDs was the driven force for the modification of CdTe nanocrystals on the surface of FS nanoparticles [23,28,29]. Finally, the FSC nanoparticles were coated with an outer silica layer. As shown in Fig. 2d, the diameter of the FSCS particles was about 55 nm. But the particles were aggregated caused by the interactions between the magnetic nanoparticles. So the TEM results proved the existence of CdTe nanocrystal in the final multifunctional particles.
Figure 2.
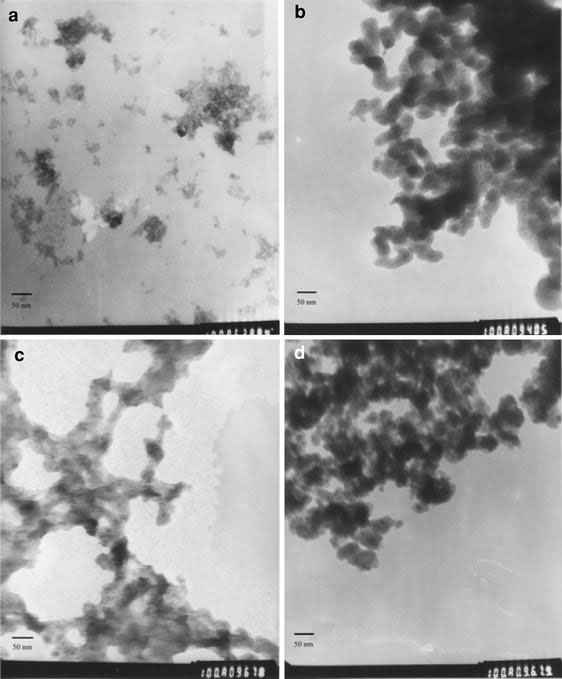
TEM images ofaCdTe;bamino modified silica coated iron oxide;cFS nanoparticles further assembled with CdTe QDs;dthe final multifunctional samples
The optical property of the prepared nanoparticles was investigated by PL spectra. Line a in Fig. 3 indicated the final multifunctional nanoparticles exhibited an emission peak at 588 nm when the excitation wavelength was fixed at 380 nm. At the same time, line b indicated the CdTe nanoparticles also showed an emission peak at 599 nm. This data suggested the PL signal of final multifunctional nanoparticles was coming from the CdTe nanocrystals, which also confirmed the existence of CdTe in the FSCS nanoparticles. But a blue-shift of the PL spectra was observed. As reported by the previous studies, the blue-shift may due to the CdTe nanocrystals may undergo corrosion during silica coating since thiol ligands must be completely removed from their surface, leaving the QDs entirely unprotected and resulting in this blue-shift in the maximum of the emission spectra [15].
Figure 3.
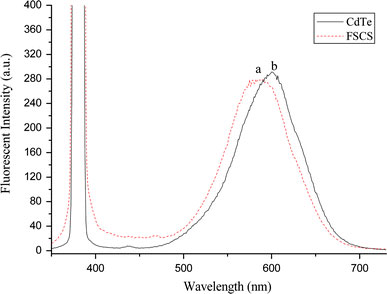
PL spectra ofaFSCS;bCdTe nanoparticles
The magnetic property of the final multifunctional nanoparticles was studied by VSM measurement. As shown by Fig. 4, the coercivity of the FSCS nanoparticles was zero. It indicated the nanoparticles were superparamagnetic. This was because the size of the iron oxide nanoparticles which was used to prepare the FSCS nanoparticles was about 10 nm [30]. The saturation magnetization (Ms) value of the final particles was 5.8 emu g−1. It was stronger than that of the particles prepared by another method [15].
Figure 4.

Magnetic hysteresis loop of the final bi-functional material measured at room temperature
Figure 5showed the fluorescence image of the CdTe nanoparticles and the final multifunctional material. As shown in Fig. 5a, the CdTe nanoparticles were presented as red dots. Figure 5b indicated the FSCS nanoparticles were also red dots, which indicated the hybrid particles can be detected by fluorescence microscope. Furthermore, the red color of the particles was consistent with the PL spectra analysis.
Figure 5.
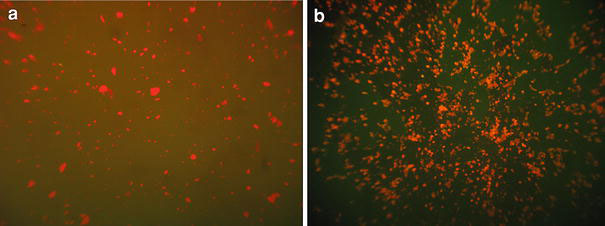
Fluorescent microscopy images ofaCdTe;bFSCS nanoparticles
As shown in Fig. 6a, the black FS nanoparticles can be driven by an external magnetic field. Figure 6b indicated the FSCS nanoparticles could also be collected by a magnet. The color of the FSCS nanoparticles in Fig. 6b was orange, which indicated the quantum dots had been entrapped in the silica matrix. In fact, Fig. 6further confirmed the existence of iron oxide and CdTe nanoparticles in the final samples.
Figure 6.
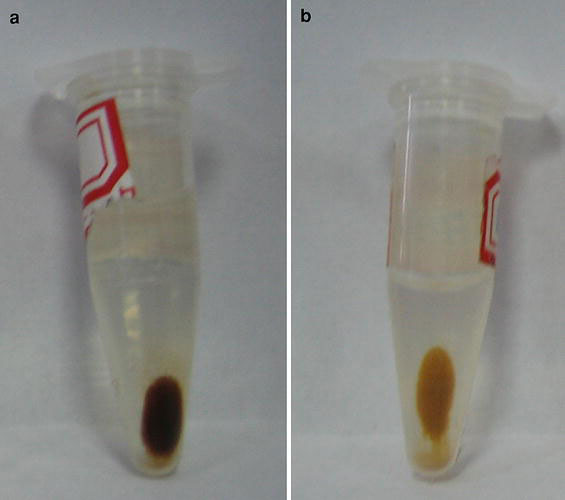
Photograph ofaFS nanoparticles;bFSCS nanoparticles driven by an external magnetic field
Conclusions
In summary, multifunctional silica spheres which were composed of CdTe and iron oxide nanoparticles was prepared by a convenient method. The TEM images proved the particle size was in nanometer range. But aggregation was occurred in some area. So improving their size distribution will be the further subject of our work. The PL spectra indicated the emission band of this material was located at 588 nm. The magnetization curves revealed the Ms value of the final particles was 5.8 emu g−1. The photograph indicated the color of the final particles was yellowish-brown in the daylight and it can be controlled by an external magnetic field. These results indicated the prepared multifunctional silica nanospheres, which were composed of iron oxide and quantum dots, possessed excellent magnetic and fluorescent properties.
Acknowledgments
This work was supported by the Open Subject Foundation of Key Laboratory for Magnetism and Magnetic Materials of MOE, Lanzhou University. We also kindly acknowledge the National Science Foundation of China (No. 20875040) for supporting this work.
References
- Lu AH, Salabas EL, Schüth F. Angew. 2007. p. 1222. COI number [1:CAS:528:DC%2BD2sXitF2lurs%3D] [DOI] [PubMed]
- Jeong U, Teng XW, Wang Y, Yang H, Xia YN. Adv. 2007. p. 33. COI number [1:CAS:528:DC%2BD2sXhslGrtLo%3D] [DOI]
- Wu W, He QG, Jiang CZ. Nanoscale Res. 2008. p. 397. COI number [1:CAS:528:DC%2BD1cXhsVyhtrvP] [DOI] [PMC free article] [PubMed]
- Zhang S, Chen XJ, Gu CR, Zhang Y, Xu JD, Bian ZP, Yang D, Gu N. Nanoscale Res. 2009. p. 70. COI number [1:CAS:528:DC%2BD1MXht1KgsL8%3D]; Bibcode number [2009NRL.....4...70Z] [DOI]
- Rogach AL, Franzl T, Klar TA, Feldmann J, Gaponik N, Lesnyak V, Shavel A, Eychmüller A, Rakovich YP, Donegan JF. J. Phys. Chem. C. 2007. p. 14628. COI number [1:CAS:528:DC%2BD2sXhtVChs7jM] [DOI]
- Komarala VK, Bradley AL, Rakovich YP, Byrne SJ, Gun’ko YK, Rogach AL. Appl. 2008. p. 123102. Bibcode number [2008ApPhL..93l3102K] [DOI]
- Wu XZ. Sol. Energy. 2004. p. 803. COI number [1:CAS:528:DC%2BD2cXhtVektrbM] [DOI]
- Yuan JP, Guo WW, Wang EK. Anal. 2008. p. 1141. COI number [1:CAS:528:DC%2BD1cXlvVSjug%3D%3D] [DOI] [PubMed]
- Wang X, Ozkan CS. Nano Lett. 2008. p. 398. COI number [1:CAS:528:DC%2BD1cXoslOkuw%3D%3D]; Bibcode number [2008NanoL...8..398W] [DOI] [PubMed]
- Ngo CY, Yoon SF, Loke WK, Cao Q, Lim DR, Wong V, Sim YK, Chua SJ. Nanoscale Res. 2008. p. 486. COI number [1:CAS:528:DC%2BD1cXhsVyhtrjJ]; Bibcode number [2008NRL.....3..486N] [DOI] [PMC free article] [PubMed]
- Jin L, Yu DD, Liu Y, Zhao XL, Zhou JG. Talanta. 2008. p. 1053. COI number [1:CAS:528:DC%2BD1cXhtVOmtb%2FJ] [DOI] [PubMed]
- You XG, He R, Gao F, Shao J, Pan BF, Cui DX. Nanotechnology. 2007. p. 035701. Bibcode number [2007Nanot..18c5701Y] [DOI] [PubMed]
- Li XZ, Wang L, Zhou C, Guan TT, Li J, Zhang YH. Clin. Chim. Acta. 2007. p. 168. COI number [1:CAS:528:DC%2BD2sXit1KisL4%3D] [DOI] [PubMed]
- He J, Chen JY, Wang P, Wang PN, Guo J, Yang WL, Wang CC, Peng Q. Nanotechnology. 2007. p. 415101. Bibcode number [2007Nanot..18U5101H] [DOI]
- Salgueiriño-Maceira V, Correa-Duarte MA, Spasova M, Liz-Marzán LM, Farle M. Adv. 2006. p. 509. [DOI]
- Hong X, Li J, Wang MJ, Xu JJ, Guo W, Li JH, Bai YB, Li TJ. Chem. 2004. p. 4022. COI number [1:CAS:528:DC%2BD2cXnsV2itL8%3D] [DOI]
- Gaponik N, Radtchenko IL, Sukhorukov GB, Rogach AL. Langmuir. 2004. p. 1449. COI number [1:CAS:528:DC%2BD2cXls1ajsw%3D%3D] [DOI] [PubMed]
- Zebli B, Susha AS, Sukhorukov GB, Rogach AL, Parak WJ. Langmuir. 2005. p. 4262. COI number [1:CAS:528:DC%2BD2MXjtl2gt78%3D] [DOI] [PubMed]
- Guo J, Yang WL, Wang CC, He J, Chen JY. Chem. 2006. p. 5554. COI number [1:CAS:528:DC%2BD28XhtFakur7L] [DOI]
- Liu B, Wang DP, Huang WH, Yu MJ, Yao AH. Mater. 2008. p. 2904. COI number [1:CAS:528:DC%2BD1cXhtFCju7rO] [DOI]
- Shavel A, Gaponik N, Eychmuller A. J. Phys. Chem. B. 2006. p. 19280. COI number [1:CAS:528:DC%2BD28Xptleksb8%3D] [DOI] [PubMed]
- Ren CL, Li JH, Chen XG, Hu ZD, Xue DS. Nanotechnology. 2007. p. 345604. Bibcode number [2007Nanot..18L5604R] [DOI]
- Wu CL, Zheng JS, Huang CB, Lai JP, Li SY, Chen C, Zhao YB. Angew. 2007. p. 5393. COI number [1:CAS:528:DC%2BD2sXotVSksbs%3D] [DOI] [PubMed]
- Casula MF, Jun YW, Zaziski DJ, Chan EM, Corrias A, Alivisatos AP. J. 2006. p. 1675. COI number [1:CAS:528:DC%2BD28XjvV2kuw%3D%3D] [DOI] [PubMed]
- Giri J, Guha Thakurta S, Bellare J, Kumar Nigam A, Bahadur D. J. 2005. p. 62. COI number [1:CAS:528:DC%2BD2MXktVagt7s%3D]; Bibcode number [2005JMMM..293...62G] [DOI]
- Lin YW, Hsieh MM, Liu CP, Chang HT. Langmuir. 2005. p. 728. COI number [1:CAS:528:DC%2BD2cXhtVOhsbzJ] [DOI] [PubMed]
- Deng DW, Yu JS, Pan Y. J. 2006. p. 225. COI number [1:CAS:528:DC%2BD28XkvVWqtrg%3D] [DOI] [PubMed]
- Kim J, Lee JE, Lee J, Jang Y, Kim SW, An K, Yu JH, Hyeon T. Angew. 2006. p. 4789. COI number [1:CAS:528:DC%2BD28Xns1KltLY%3D] [DOI] [PubMed]
- Liu N, Prall BS, Klimov VI. J. 2006. p. 15362. COI number [1:CAS:528:DC%2BD28XhtF2ntLbK] [DOI] [PubMed]
- Yamaura M, Camilo RL, Sampaio LC, Macêdo MA, Nakamura M, Toma HE. J. 2004. p. 210. COI number [1:CAS:528:DC%2BD2cXlslGhtrk%3D]; Bibcode number [2004JMMM..279..210Y] [DOI]


