Abstract
Nanomaterials have widely been used in the field of biological and biomedicine, such as tissue imaging, diagnosis and cancer therapy. In this study, we explored the cytotoxicity and photodynamic effect of different-sized ZnO nanoparticles to target cells. Our observations demonstrated that ZnO nanoparticles exerted dose-dependent and time-dependent cytotoxicity for cancer cells like hepatocellular carcinoma SMMC-7721 cells in vitro. Meanwhile, it was observed that UV irradiation could enhance the suppression ability of ZnO nanoparticles on cancer cells proliferation, and these effects were in the size-dependent manner. Furthermore, when ZnO nanoparticles combined with daunorubicin, the related cytotoxicity of anticancer agents on cancer cells was evidently enhanced, suggesting that ZnO nanoparticles could play an important role in drug delivery. This may offer the possibility of the great potential and promising applications of the ZnO nanoparticles in clinical and biomedical areas like photodynamic cancer therapy and others.
Keywords: ZnO nanoparticle, SMMC-7721, Photodynamic cancer therapy (PDT), Size effect, Drug delivery
Introduction
With the development of nanotechnology, nanomaterials are receiving increasing interest in the relative research and industrial applications for their unique characteristics. As one of the most important application, nanomaterials are now widely studied and applied in biological and biomedical field [1-6]. Nanoscale materials, such as nanoparticles [7-9], nanorods [10], nanowires [11,12], nanotubes [13] and nanofiber [14,15], have been explored in many biomedical applications because of their novel properties, such as the high volume/surface ratio, surface tailorability and multifunctionality [7-10,16-18]. Moreover, the intrinsic optical [19,20], magnetic [21,22] and biological properties of nanomaterials offer remarkable opportunities to study and regulate complex biological processes for biomedical applications in an unprecedented manner [23-25]. Fundamentally, life itself is a collective of processes at nanoscale within cells [15,26].
Nanoparticles (NPs) and nanosized objects are being incorporated rapidly into clinical medicine and particularly into the field of medical oncology [27]. As the energy donor, quantum dots have the possibility for energy transfer between quantum dot particles and cell molecules such as active oxygen and give them a potential to induce generation of reactive oxygen species and/or free radicals and to provoke apoptosis of the cells [28,29]. The dual nature of UV-mediated cytotoxicity of quantum dots and their energy donor capacity could open a new area of quantum dot application in biology and medicine, as novel photosensitizers or at least as potentiators of the conventional photosensitizing drugs in photodynamic therapy (PDT) of cancer. Some reports have shown that some kinds of quantum dots (QDs) can act as photosensitizers and potentiators of classical photosensitizers to overcome the limitations of organic dye-based PDT [30-32].
In this study, we initially investigated the effect of different-sized ZnO nanoparticles on cytotoxicity of hepatocellular carcinoma cells (SMMC-7721). Combined with the MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide) assay, real-time cell electronic sensing (RT-CES) study and fluorescence microscope image, we explored the nanoparticles’ cytotoxicity for human cancer cells in vitro and compared the distinction of different size ZnO nanoparticles affecting the cell proliferation. As the good photocatalysis material, the photodynamic effect of these different-sized ZnO nanoparticles has also been investigated by combination with UV irradiation, which could be further utilized as a good photosensitizer with the potential application in PDT. Meanwhile, the synergetic cytotoxicity of the anticancer agent daunorubicin (DNR) with ZnO nanoparticles was explored to inhibit the cancer cell proliferation in vitro, and the real-time cell electronic sensing (RT-CES) assay also provided the dynamic process and binding behavior between cells and related agents.
Experimental
Reagents and Materials
Three different-sized ZnO nanoparticles capped with aminopolysiloxane, i.e., ZP5, ZP6 and ZP7, were purchased from Jiangsu Changtai Nanometer Material Co., Ltd. The average diameters of ZP5, ZP6 and ZP7 were about 20, 60 and 100 nm, respectively, observed by transmission electron microscopy (TEM) using a JEOL JEM-2100 transmission electron microscope. Daunorubicin was purchased from Farmitalis Co. Italian. MTT was purchased from Sigma (USA), and all other agents were analytical pure.
Cell Culture
SMMC-7721 cancer cells (purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) were maintained in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Sigma, USA), 100 U/ml penicillin (Sigma, USA) and 100 μg/ml streptomycin (Sigma, USA) and grown at 37°C in a 5% CO2 humidified environment.
MTT Assay for the Proliferation of SMCC 7721
The effect of different size ZnO nanoparticles’ concentrations on SMMC-7721 cancer cells was observed by MTT assay. The final concentrations of ZnO nanoparticles were 1.56, 3.12, 6.25, 12.5, 25 and 50 μg/mL, respectively. Initially, 1 × 104 cells were seeded into each well containing 200 μL cell culture mediums in 96-well plate and incubated for 24 h and then added the relevant materials and incubated at 37°C with 5% CO2 for 72 h. Then, 20 μL, 5 mg/mL MTT solution was added into the well and continue cultured for about 4 h. The culture medium was discarded, and 200 μL DMSO was added following the shaking about 10 min. The UV absorption was measured at 490 nm. Controls were cultivated under the same conditions without addition of ZnO nanoparticles. The relevant experiments were repeated thrice independently. The inhibition efficiency (%) was expressed as follows: (1−[A]test/[A]control) × 100, where [A]test and [A]control represent the optical density at 490 nm for the test and control experiments, respectively.
The Effect of UV Irradiation for the Proliferation of SMCC 7721 in the Presence of ZnO Nanoparticles
The procedure of cell culture and treatment of nanoparticles was similar with the above phase of MTT assay. Upon application of UV irradiation, the UVC (λ = 254 nm) was provided by a germicidal lamp in the clean bench, and its average intensity is 0.1 mW/cm2 at the working plane. The effect of different-sized ZnO nanoparticles for SMMC 7721 cell proliferation in the presence of UV irradiation for 180 s has been explored by MTT assay. Every experiment was repeated at least three times independently.
In Vitro RT-CES Cytotoxicity Assay for SMMC-7721 Proliferation
The cell culture condition, the starting cell number, and cell culture medium volume used for the 16× sensor device were similar to that of MTT assay. Different size ZnO nanoparticles were seeded in the plate with the concentration of 2.5, 5.0, and 10.0 μg/mL, respectively. Then, the effect of UV irradiation was studied by using MTT assay. In order to study the synergistic cytotoxicity of ZnO nanoparticles and DNR, DNR was introduced into the system as the negative control with concentration about 1.0 × 10−7 mol/L. The correlative controls were also seeded in the same plate simultaneously. Once the cells were added to the sensor wells, the sensor devices were placed into the incubator, and the real-time cell index (CI) data acquisition was initiated by the RT-CES analyzer (ACEA Bioscience. Inc. USA).
Olympus IX71 Inverted Fluorescence Microscopy
The experiment was performed as described in the literature [33]. The SMMC-7721 cells were seeded on the coverslips in six-well plates (1 × 105 cells/well) and cultured for 24 h at 37°C with 5% CO2, then daunorubicin with 1.0 × 10−5 mol/L and different size ZnO nanoparticles with 2.5 μg/mL were injected to the cells system. Meanwhile, the cells treated with the same concentration of solvent were taken as control experiments. All specimens were subsequently incubated for 1 h at 37°C with 5% CO2, and quickly washed with PBS, followed by fixation with 4% formaldehyde for 5 min. Finally, specimens were observed by inverted fluorescence microscopy (Olympus IX71, Japan).
Statistics
Data were expressed as the mean ± SD (standard deviation) from at least three independent experiments. One-tailed unpaired Student’s t-test was used for significance testing, and P < 0.05 is considered significant.
Results and Discussion
Cytotoxicity of Different-Sized ZnO Nanoparticles for SMMC7721 Cancer Cells
It is already known that the unique properties and size effect of semiconductor nanomaterials may play an important role in the possible biomedical application, especially in the photodynamic therapy (PDT). In this contribution, we have explored the cytotoxic effect of ZnO nanoparticles, named ZP5, ZP6 and ZP7 with the average diameters of about 20, 60 and 100 nm, as shown in the supporting information, on SMMC-7721 cancer cells. According to the MTT results, as shown in Fig. 1, it was observed that the cytotoxicity was in a dose-dependent manner, which was similar with the literature report [34]. The relevant IC50 values of ZP5, ZP6 and ZP7 nanoparticles’ cytotoxicity on SMMC7721 cell lines were about 27.01, 36.60 and 21.70 μg/mL, respectively (shown in Fig. 1). From the viability and the values of IC50, we observe that there was no apparent difference in the cytotoxicity between the different size ZnO nanoparticles. Besides, our observations demonstrate that although these ZnO nanoparticles can greatly inhibit cancer cell proliferation in vitro at higher concentrations, they have little effect on target cancer cells at lower concentrations.
Figure 1.
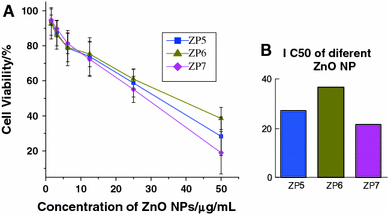
a Cell viability of SMMC-7721 cells at various concentrations of different-sized ZnO nanoparticles.Error bars indicate standard deviation. b The comparison of IC 50 values for different-sized ZnO NPs
In general, there are two mainly different actions for nanomaterials to act toxic effects on target cells: first, a chemical toxicity based on the chemical composition, such as release of (toxic) ions, particle surface catalyzed reactions or formation of reactive oxygen species; second, the stress or stimuli caused by the surface, size and/or shape of the particles [35]. In this study, three different-sized ZnO nanoparticles could exert cytotoxic effect on SMMC-7721 cells at different concentrations. While in scale from 20 to 100 nm, there was little difference in cytotoxicity at the identical concentration of ZnO NPs. This is different from some other reports, such as the cytotoxic effect of carbon-based nanomaterials is size-dependent [36].
The Effect of Nano ZnO and UV Irradiation on SMMC 7721 Cell Proliferation
As the energy donors [28], the semiconductor nanomaterials could have a very important application in the photodynamic therapy (PDT) for energy transfer between quantum dot particles and cell molecules (such as triplet oxygen, reducing equivalents and pigments). Derfus and colleagues provoked the hypothesis that while the cytotoxicity of quantum dots, mediated by UV irradiation, is harmful for normal cell viability, it may be very useful in killing cancer cells [31,32]. After the semiconductor, nanomaterial is excited by the UV irradiation, the semiconductor nanomaterial can release the oxygen free radical/oxyradical, and the oxyradical was considered as the main mediator of photocytotoxicity in photodynamic therapy, causing biomembrane oxidation and degradation.
In view of the above consideration, in this study, we have explored the effect of UV irradiation combined with different-sized ZnO nanoparticles to inhibit the cancer cell proliferation. As shown in Fig. 2, after the UV irradiation for about 180 s, the cell viability of SMMC 7721 was apparently decreased. According to the curves, we can find that this decrease was also enhanced when the nano ZnO concentration was increased, following the dose-dependent manner. After UV irradiation, the related IC 50 values were 16.74, 13.17 and 8.58 μg/mL, respectively (as shown in Fig. 3). So, these three different-sized ZnO nanoparticles could exert remarkable inhibition effect on SMMC 7721 cells proliferation under UV irradiation compared to that without UV irradiation, demonstrating that ZnO nanoparticles could greatly exert cancer cell-killing effect under UV irradiation, and this effect was in the dose-dependent manner.
Figure 2.
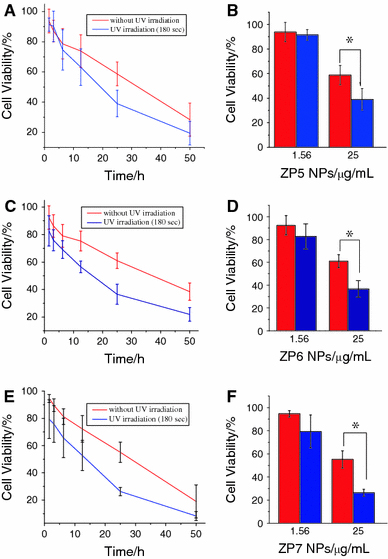
Cell viability of SMMC-7721 cancer cells treated with different concentrations of different-sized ZnO NPs in the presence and absence of UV irradiation. a Target cancer cells treated with ZP5 NPs and b the comparison of cell viability in different ZP5 NPs concentration with/without UV irradiation; c Target cancer cells treated with ZP5 NPs and d the comparison of cell viability in different ZP6 NPs concentration with/without UV irradiation; e Target cancer cells treated with ZP5 NPs and f the comparison of cell viability in different ZP7 NPs concentration with/without UV irradiation
Figure 3.
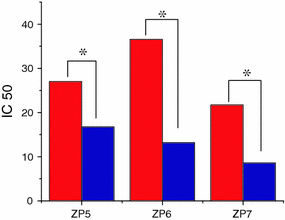
The comparison of IC 50 values of three different-sized ZnO NPs for SMMC 7721 cytotoxicity in the presence and absence UV irradiation
Based on the effect of different-sized ZnO nanoparticles in the absence/presence of UV irradiation on SMMC 7721 cell lines, it was evident that all these ZnO nanoparticles have the similar inhibition capacity on target cancer cells, and UV irradiation could greatly enhance this inhibition effect on SMMC-7721 cells in vitro when treated with ZnO nanoparticles. These observations demonstrated that in this nano-scale level, ZnO nanoparticles could play an important role in inhibiting cancer cell proliferation, and UV irradiation can further enhance this effect. It was considered that the light excites the photosensitizing agent, resulting in formation of ROS, believed to be responsible for the cascade of cellular and molecular events in which the following result is selective tumor destruction [37]. After UV irradiation, nano-sized ZnO particles could efficiently induce the formation of ROS and further attack the cell membrane (mainly by lipid peroxidation), proteins (such as enzyme deactivation) or even nucleic acids. And the attack of the photogenerated ROS on the cell membrane can lead to the membrane destruction, then results in the changes in the permeability on the target cell membrane, which causes the efflux of cytoplasm and the apoptosis or death of the target caner cells [28,29,32].
The RT-CES Dynamic Study for the Inhibition of ZnO Nanoparticles on SMMC-7721 Cancer Cells in the Presence of UV Irradiation
The real-time cell electronic sensing (RT-CES) assay could provide dynamic information to identify the interaction between target cells and reagents. The basic principle of the RT-CES system is to monitor the changes in electrode impedance induced by the interaction between testing cells and electrodes, where the presence of the cells will lead to an increase in the electrode impedance. The more cells attached to the sensor, the higher the impedance that could be monitored with RT-CES. The RT-CES array has been proven to be a valuable and reliable way for real-time monitoring of dynamic changes induced by cell-chemical interaction [38-41]. Since the relevant test is labeling free, the RT-CES assay allows real-time, automatically and continually monitoring cellular status changes during the whole process of the cell-chemical interaction. Thus, in this work we introduced the RT-CES assay to study the dynamic response of target cancer cells exposure to ZnO nanoparticles.
As shown in Figs. 4, 5 and 6, our observations demonstrate that when the ZnO nanoparticles were injected into the cell system, the electrode impendence would be lower compared with that of negative system in the absence of nanoparticles, in which the electrode impedance became higher following the culture time. And this decrease affected by nanoparticles was also dose dependent and time dependent. Compared with the results of different size nanoparticles’ effects as shown in Figs. 3a, 4a and 5a, we can find that there was no any notable difference. These results were coherent with that of our MTT assay.
Figure 4.
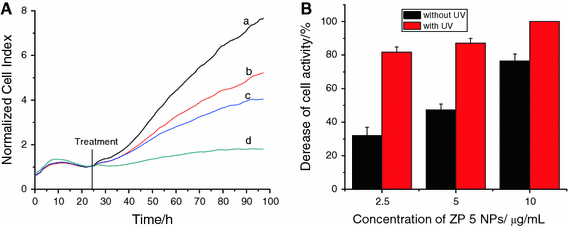
Dynamic response of SMMC-7721 cells exposure to ZP5 ZnO nanoparticles. a in the absence of UV irradiation:aZnO nanoparticle-free (control),b2.5 μg/mL of ZnO nanoparticles,c5.0 μg/mL of ZnO nanoparticles and d10.0 μg/mL of ZnO nanoparticles; b The comparison of decrease for cell activity in the absence/presence of UV (irradiated for 180 s) after ZP 5 NPs was incubated in the cell system for about 72 h
Figure 5.
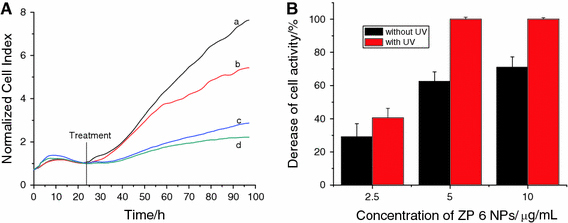
Dynamic response of SMMC-7721 cells exposure to ZP6 ZnO nanoparticles. a in the absence of UV irradiation:aZnO nanoparticle-free (control),b2.5 μg/mL of ZnO nanoparticles,c5.0 μg/mL of ZnO nanoparticles and d10.0 μg/mL of ZnO nanoparticles; b The comparison of decrease for cell activity in the absence/presence of UV (irradiated for 180 s) after ZP 6 NPs was incubated in the cell system for about 72 h
Figure 6.
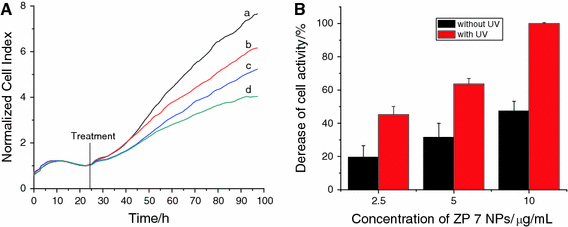
Dynamic response of SMMC-7721 cells exposure to ZP7 ZnO nanoparticles. a in the absence of UV irradiation:aZnO nanoparticle-free (control),b2.5 μg/mL of ZnO nanoparticles,c5.0 μg/mL of ZnO nanoparticles and d10.0 μg/mL of ZnO nanoparticles; b The comparison of decrease for cell activity in the absence/presence of UV (irradiated for 180 s) after ZP 7 NPs was incubated in the cell system for about 72 h
Furthermore, when ZnO nanoparticles were stimulated by the UV irradiation for about 180 s, we can notice that the electrode impendence was extremely decreased, caused by the caner cells massive death in a short culture time (shown in Supporting Information). Compared with Figs. 3b, 4b and 5b, we can find that when ZP 5 ZnO nanoparticles sized about 20 nm were only about 2.5 μg/mL, the electrode impedance evidently decreased about more than 80% comparing with the negative control because of the massive death of SMMC-7721. And there was no evidently size-dependent manner for 20 nm ZnO nanoparticle in the cytotoxicity of cell proliferation. For ZP 6 ZnO nanoparticles sized about 60 nm, there was a decrease of about 40% after 72-h incubation with the nanoparticle concentration about 2.5 μg/mL, and the decrease of the electrode impedance would be more than 95% when the concentration was about 5.0 and 10.0 μg/mL, respectively. As the ZP 7 ZnO nanoparticles sized about 100 nm, there was a decrease about 40% in about 2.5 μg/mL and about 63% decrease in about 5.0 μg/mL. And there was an extremely decrease when ZP 7 was about 10.0 μg/mL. As shown in Fig. 7, the comparison of cell inhibition for different-sized and different concentration ZnO NPs in the absence/presence of UV irradiation indicates that UV irradiation could enhance the suppression ability of ZnO nanoparticles on cancer cells proliferation. More importantly, this cytotoxicity for SMMC-7721 proliferation caused by ZnO nanoparticles after UV irradiation was in the size-dependent manner, i.e., the smaller the related nanoparticle size, the higher the cytotoxicity of cancer cell proliferation.
Figure 7.
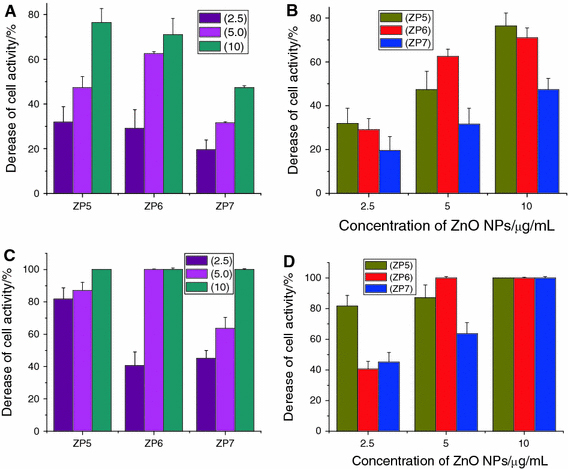
a Decrease of cell index after different concentration ZnO NPs was incubated in the cell system for about 72 h without UV irradiation. b Comparison of cell inhibition for different-sized ZnO NPs in the absence of UV irradiation. c Decrease in cell index after different concentration ZnO NPs was incubated in the cell system for about 72 h with UV irradiation for 180 s. d Comparison of cell inhibition for different-sized ZnO NPs in the presence of UV irradiation for 180 s
The Synergistic Effect of ZnO Nanoparticles for Daunorubicin Cytotoxicity
To further explore the bio-application of ZnO nanoparticles in the PDT, we have combined the nano ZnO with daunorubicin (DNR), a widely used clinical chemotherapeutic agent, to study the possible synergistic effect. As shown in Fig. 8 as well as in the supporting information, our studies indicate that when there was only daunorubicin (1.0 × 10−7 mol/L) in the cell system, the dynamic response of SMMC-7721 cancer cells gradually grew with the increase in the culture time, and the electrode impendence increased correspondingly. While ZnO nanoparticles with different concentrations were injected into the DNR drug system, the electrode impendence is observed to apparently decrease. It is noted that when the nanoparticles concentration was only 2.5 μg/mL, the electrode impedance decreased apparently. Especially, after the related treatment by combining DNR with nano ZnO for about 10 h, the electrode impendence would have the apparent decrease and the effect of nanoparticle size was not very notable.
Figure 8.
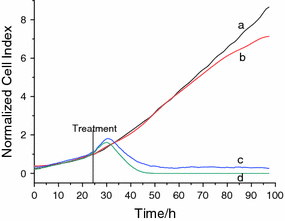
Dynamic response of SMMC-7721 cells exposure to daunorubicin (1.0 × 10−7 mol/L) with ZP5 NPs.a negative control (ZnO nanoparticle-free);b daunorubicin (1.0 × 10−7 mol/L);c2.5 μg/mL of ZnO NPs and daunorubicin (1.0 × 10−7 mol/L) and d5.0 μg/mL of ZnO NPs and daunorubicin (1.0 × 10−7 mol/L). The information of ZP6 and ZP7 was shown in the supporting information
If the ZnO nanoparticles were irradiated by UV light, the generated ROS made the membrane destruction which may cause the efflux of cytoplasm and/or make more daunorubicin molecules enter into cancer cells and induce the target cell killing. So, ZnO nanoparticles could play an important role to enhance the synergistic cytotoxicity of daunorubicn in the target cancer therapy, or the ZnO nanoparticles may act as the role of drug delivery carries.
Moreover, as a widely used clinical chemotherapeutics agent in cancer therapy, daunorubicin has the good characteristic of fluorescence, suggesting that it can be used in the fluorescence microscope image of the target cancer cells. As shown in Fig. 9, if there was only daunorubicin in the cell system, the excited fluorescence was not strong. However, when the combination of daunorubicin and ZnO nanoparticles was injected into the cell system, the excited fluorescence in cancer cells was extremely enhanced, and there was no any difference between the different size ZnO nanoparticles, which was coherent with the above studies of MTT and RT-CES. So, it is obvious that ZnO nanoparticles could play an important role in the drug delivery to carry daunorubicin into the target caner cells thus enhance the drug accumulation and strengthen the drug cytotoxicity to restrain SMMC-7721 cells proliferation. This raises the possibility to utilize ZnO nanoparticles as one of the efficient photosensitizers in cancer PDT, which may also provide other promising application in biological and biomedical engineering.
Figure 9.
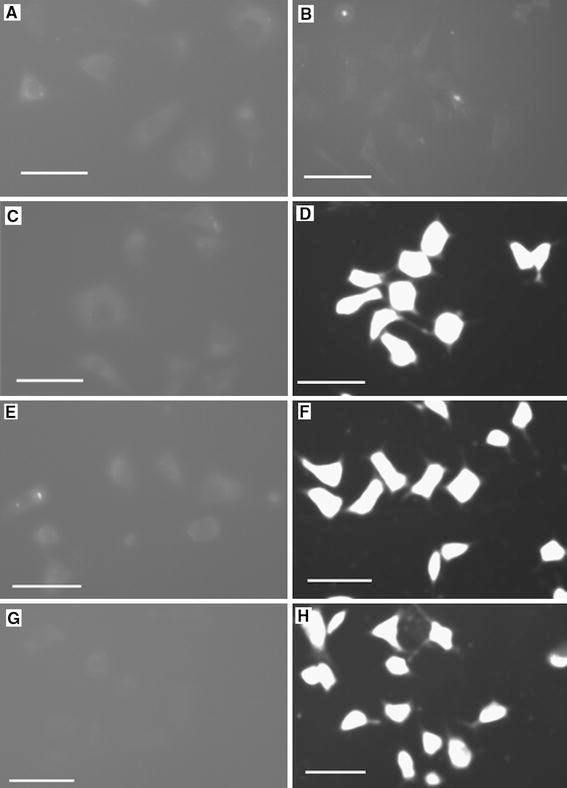
Inverted fluorescence micrographs of SMMC-7721 cells after incubation with a cells only; b daunorubicin; c ZP5 ZnO nanoparticles; d ZP5 ZnO nanoparticles and daunorubicin; e ZP6 ZnO nanoparticles; f ZP6 ZnO nanoparticles and daunorubicin; g ZP7 ZnO nanoparticles; h ZP7 ZnO nanoparticles and daunorubicin. Here, the concentration of daunorubicin is 1.0 × 10−5 mol/L,bar20 μm
In summary, our studies demonstrate that the relevant ZnO nanoparticles play an important role as the nano-sized drug carries. Semiconductor fluorescent NPs could be developed for simultaneous detection and localization of multiple solid cancer biomarkers, enabling the personalization of therapeutic regimens for each patient [29,42]. Additionally, inorganic NPs can be readily conjugated with tumor-specific ligands and used for tumor-selective delivery of chemotherapeutic or hormonal agents [20,22]. Meanwhile, it was observed that all these three different size ZnO nanoparticles could have effective proliferation inhibition capacity on target cancer cells, and UV irradiation could greatly enhance the inhibition effect on target cancer cells in vitro. The possible process for cellular cytotoxicity of ZnO nanoparticles and daunorubicin in the PDT could be illustrated in Scheme 1, suggesting that ZnO particles could play an important role in the drug delivery to enhance the accumulation and the synergistic cytotoxicity of daunorubicin in the target SMMC-7721 cells.
Scheme 1.
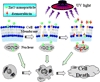
The schematic image of ZnO nanoparticle cytotoxicity and the PDT process cooperated with daunorubicin in vitro
Conclusion
In this contribution, our observations demonstrate that different-sized ZnO nanoparticles exposed to SMMC-7721 cancer cells could exert dose-dependent cytotoxicity suppression in vitro, and this cytotoxicity of nanoparticles was time depended and dose depended, while the size-depended effect was not clear in the scope from 20 to 100 nm. UV irradiation could readily enhance the proliferation suppression ability of ZnO nanoparticles on cancer cells. More importantly, these effects were size dependent, while the smaller the nanoparticle size, the higher the cytotoxicity of cancer cell proliferation caused by ZnO nanoparticle. Meanwhile, if ZnO nanoparticles combined with daunorubicin, cytotoxicity of DNR for SMMC-7721 cancer cells was especially enhanced. These observations suggest that ZnO nanoparticles could play an important role in the PDT and have the great potential and promising applications in clinical and biomedical engineering.
Supplementary Material
Figure S1 The TEM images of ZnO nanoparticles. (A) TEM image of the ZnO nanoparticle named ZP5 and (a) the corresponding HR-TEM image of the marked part A. (B) TEM image of the ZnO nanoparticle named ZP6 and (b) the corresponding HR-TEM image of the marked part B. (C) TEM image of the ZnO nanoparticle named ZP7 and (c) the corresponding HR-TEM image of the marked part C.
Figure S2 Dynamic response of SMMC-7721 cells exposure to ZP5 ZnO nanoparticles in the presence of UV irradiation for 180s: (a) ZnO nanoparticle-free (control); (b) 2.5 μg/mL of ZnO nanoparticles,(c) 5.0 μg/mL of ZnO nanoparticles, and (d) 10.0 μg/mL of ZnO nanoparticles.
Figure S3 Dynamic response of SMMC-7721 cells exposure to ZP6 ZnO nanoparticles in the presence of UV irradiation for 180s: (a) ZnO nanoparticle-free (control); (b) 2.5 μg/mL of ZnO nanoparticles,(c) 5.0 μg/mL of ZnO nanoparticles, and (d) 10.0 μg/mL of ZnO nanoparticles.
Figure S4 Dynamic response of SMMC-7721 cells exposure to ZP7 ZnO nanoparticles in the presence of UV irradiation for 180s: (a) ZnO nanoparticle-free (control); (b) 2.5 μg/mL of ZnO nanoparticles,(c) 5.0 μg/mL of ZnO nanoparticles, and (d) 10.0 μg/mL of ZnO nanoparticles.
Figure S5 Dynamic response of SMMC-7721 cells exposure to daunorubicin (1.0 ×10-7 mol/L) with ZP5 NPs. A: ZP 6 NPs and B: ZP 7 NPs. For A and B, a: negative control (ZnO nanoparticle-free); b: daunorubicin (1.0 ×10-7M); c: 2.5 μg/mL of ZnO NPs and daunorubicin (1.0 x 10-7M); and d: 5.0 μg/mL of ZnO NPs and daunorubicin (1.0 ×10-7M).
Acknowledgments
This work is supported by National Natural Science Foundation of China (90713023, 20675014 and 20535010), National Basic Research Program of China (No. 2010CB732404), the Chinese Ministry of Science and Technology (2007AA022007 and 2008DFA51180) and the Natural Science Foundation of Jiangsu Province (BK2008149).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Gao JH, Xu B. Nano Today. 2009. pp. 37–51. COI number [1:CAS:528:DC%2BC3cXkvFGit7s%3D] [DOI]
- Lewis LN. Chem. 1993. pp. 2693–2730. COI number [1:CAS:528:DyaK3sXmsFOhs7Y%3D] [DOI]
- Yezhelyev M, Yacoub R, O’Regan R. Nanomedicine. 2009. pp. 83–103. COI number [1:CAS:528:DC%2BD1cXhsFajsbrI] [DOI] [PubMed]
- Xiao Liu C. Nano Biomed. Engine. 2009. pp. 1–18.
- Ji JJ, Ruan J, Cui DX. Nano Biomed. Engine. 2010. pp. 100–128. COI number [1:CAS:528:DC%2BC3cXksFens7s%3D]
- Wang Z, Ruan J, Cui DX. Nanoscale Res. 2009. pp. 593–605. COI number [1:CAS:528:DC%2BD1MXnt1Sktrw%3D]; Bibcode number [2009NRL.....4..593W] [DOI] [PMC free article] [PubMed]
- Tian FR, Prina-Mello A, Estrada G, Beyerle A, Möller W, Schulz H, Kreyling W, Stoeger T. Nano Biomed Eng. 2009. pp. 19–38. COI number [1:CAS:528:DC%2BC3cXksFegu7Y%3D] [DOI]
- Cui DX, Han YD, Li ZM, Song H, Wang K, He R, Liu B, Liu HL, Bao C, Huang P, Ruan J, Gao F, Yang H, Cho HS, Ren QS, Shi DL. Nano Biomed. 2009. pp. 94–112. COI number [1:CAS:528:DC%2BC3cXksFensro%3D]
- Wang T, Hu Y, Zhang L, Jiang L, Chen Z, He NY. Nano Biomed. 2010. pp. 46–61. COI number [1:CAS:528:DC%2BC3cXksFens74%3D]
- Chen SH, Ji YX, Lian Q, Wen YL, Shen HB, Jia NQ. Nano Biomed. 2010. pp. 19–31. COI number [1:CAS:528:DC%2BC3cXksFensrY%3D]
- Bechara SL, Judson A, Popat KC. Biomaterials. 2010. pp. 3492–3501. COI number [1:CAS:528:DC%2BC3cXisFSlsLk%3D] [DOI] [PubMed]
- Bellamkonda R, John T, Mathew B, DeCoster M, Hegab H, Davis DJ. Micromech. 2010. pp. 1–6. COI number [1:CAS:528:DC%2BC3cXit1Wlsr0%3D] [DOI]
- Chen DF, Wu XB, Wang JX, Han BS, Zhu P, Peng CH. Nano Biomed. 2010. pp. 119–129.
- He CL, Zhang L, Wang HS, Zhang F, Mo XM. Nano Biomed. 2009. pp. 91–99.
- Li YQ, Li ZY, Zhou XP, Yang P. Nano Biomed. 2010. pp. 32–45. COI number [1:CAS:528:DC%2BC3cXksFensrc%3D] [DOI]
- Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT, Orr BG, Baker JR, Holl MB. Bioconjugate Chem. 2006. pp. 728–734. COI number [1:CAS:528:DC%2BD28XktVSksr0%3D] [DOI] [PubMed]
- Mecke A, Lee DK, Ramamoorthy A, Orr BG, Holl MB. Biophys. 2005. pp. 4043–4050. COI number [1:CAS:528:DC%2BD2MXhtlWms77K] [DOI] [PMC free article] [PubMed]
- Park H, Lee S, Chen LX, Lee EK, Shin SY, Lee YH, Son SW, Oh CH, Song JM, Kang SH, Choo J. Phys. 2009. pp. 7444–7449. COI number [1:CAS:528:DC%2BD1MXhtVSmtrrJ] [DOI] [PubMed]
- Fisher BR, Eisler HJ, Stott NE, Bawendi MG. J. 2004. pp. 143–148. COI number [1:CAS:528:DC%2BD3sXps1SmsbY%3D] [DOI]
- Everts M, Saini V, Leddon JL, Kok RJ, Stoff-Khalili M, Preuss MA, Millican CL, Perkins G, Brown JM, Bagaria H, Nikles DE, Johnson DT, Zharov VP, Curiel DT. Nano. 2006. pp. 587–591. COI number [1:CAS:528:DC%2BD28Xit1OrsLs%3D]; Bibcode number [2006NanoL...6..587E] [DOI] [PubMed]
- Lin XM, Sorensen CM, Klabunde KJ, Hadjipanayis GC. Langmuir. 1998. pp. 7140–7146. COI number [1:CAS:528:DyaK1cXntlansr8%3D] [DOI]
- Sonvico F, Mornet S, Vasseur S, Dubernet C, Jaillard D, Degrouard J, Hoebeke J, Duguet E, Colombo P, Couvreur P. Bioconjugate Chem. 2005. pp. 1181–1188. COI number [1:CAS:528:DC%2BD2MXpsleqsrs%3D] [DOI] [PubMed]
- Allen TM, Cullis PR. Science. 2004. pp. 1818–1822. COI number [1:CAS:528:DC%2BD2cXitFehu70%3D]; Bibcode number [2004Sci...303.1818A] [DOI] [PubMed]
- Wang G, Huang T, Murray RW, Menard L, Nuzzo RG. J. 2005. pp. 812–813. COI number [1:CAS:528:DC%2BD2MXkvVOmsQ%3D%3D] [DOI] [PubMed]
- Tiefenauer LX, Kuhne G, Andres RY. Bioconjugate Chem. 1993. pp. 347–352. COI number [1:CAS:528:DyaK3sXlslKktrY%3D] [DOI] [PubMed]
- Mann S. Angew. 2008. pp. 5306–5320. COI number [1:CAS:528:DC%2BD1cXovF2lu7o%3D] [DOI] [PubMed]
- Bao CC, Yang H, Sheng P, Song H, Ding XH, Liu B, Lu YC, Hu GH, Cui DX. Nano Biomed. 2010. pp. 74–87.
- Claap AR, Medintz IL, Mauro JM, Fisher BR, Bawendi MG, Mattoussi H. J. 2004. pp. 301–310. COI number [1:CAS:528:DC%2BD3sXpslWntr8%3D] [DOI] [PubMed]
- Bakalova R, Ohba H, Zhelev Z, Nagase T, Jose R, Ishikawa M, Baba Y. Nano Lett. 2004. pp. 1567–1573. COI number [1:CAS:528:DC%2BD2cXmtVKlu7c%3D]; Bibcode number [2004NanoL...4.1567B] [DOI]
- Delehanty JB, Boeneman K, Bradburne CE, Robertson K, Medintz IL. Expert. 2009. pp. 1091–1112. COI number [1:CAS:528:DC%2BD1MXhtFGhur3P] [DOI] [PubMed]
- Derfus AM, Chan WC, Bhatia SN. Nano Lett. 2004. pp. 11–18. COI number [1:CAS:528:DC%2BD3sXps1SmtLo%3D]; Bibcode number [2004NanoL...4...11D] [DOI] [PMC free article] [PubMed]
- Macdonald IJ, Dougherty TJ. J. Porphyr. Phthalocyanines. 2001. pp. 105–129. COI number [1:CAS:528:DC%2BD3MXht1SgtLY%3D] [DOI]
- Nifli AP, Theodoropoulos PA, Munier S, Castagnino C, Roussakis E, Katerinopoulos HE, Vercauteren J, Castanas EJ. J. 2007. pp. 2873–2878. COI number [1:CAS:528:DC%2BD2sXjsVOrsLo%3D] [DOI] [PubMed]
- Guo DD, Wu CH, Li JY, Guo AR, Li QN, Jiang H, Chen BA, Wang XM. Nanoscale Res. 2009. pp. 1395–1402. COI number [1:CAS:528:DC%2BD1MXhsFWqsbnK]; Bibcode number [2009NRL.....4.1395G] [DOI] [PMC free article] [PubMed]
- Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. Environ. 2006. pp. 4374–4381. COI number [1:CAS:528:DC%2BD28Xit1Wms7g%3D] [DOI] [PubMed]
- Magrez S, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B, Forro l. Nano Lett. 2006. pp. 1121–1125. COI number [1:CAS:528:DC%2BD28Xksl2ktrw%3D]; Bibcode number [2006NanoL...6.1121M] [DOI] [PubMed]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J Natl. 1998. pp. 889–905. COI number [1:CAS:528:DyaK1cXktFOqsLY%3D] [DOI] [PMC free article] [PubMed]
- Xing JZ, Zhu LJ, Gabos S, Xie L. Toxicol. In Vitro. 2006. pp. 995–1004. COI number [1:CAS:528:DC%2BD28Xmt1Oru70%3D] [DOI] [PubMed]
- Xing JZ, Zhu L, Jackson JA, Gabos S, Sun XJ, Wang XB, Xu X. Chem. 2005. pp. 154–161. COI number [1:CAS:528:DC%2BD2MXps1Ogtw%3D%3D] [DOI] [PubMed]
- Zhu J, Wang XB, Xu X, Abassi YA. J. Immunol. Methods. 2006. pp. 25–33. COI number [1:CAS:528:DC%2BD28XhtlGmsLY%3D] [DOI] [PubMed]
- Mossman T. Immunol. Methods. 1983. pp. 55–63. [DOI]
- Matsumura Y, Gotoh M, Muro K, Yamada Y, Shirao K, Shimada Y, Okuwa M, Matsumoto S, Miyata Y, Ohkura H, Chin K, Baba S, Yamao T, Kannami A, Takamatsu Y, It K, Takahashi K. Ann. 2004. pp. 517–525. COI number [1:STN:280:DC%2BD2c7gvVersQ%3D%3D] [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The TEM images of ZnO nanoparticles. (A) TEM image of the ZnO nanoparticle named ZP5 and (a) the corresponding HR-TEM image of the marked part A. (B) TEM image of the ZnO nanoparticle named ZP6 and (b) the corresponding HR-TEM image of the marked part B. (C) TEM image of the ZnO nanoparticle named ZP7 and (c) the corresponding HR-TEM image of the marked part C.
Figure S2 Dynamic response of SMMC-7721 cells exposure to ZP5 ZnO nanoparticles in the presence of UV irradiation for 180s: (a) ZnO nanoparticle-free (control); (b) 2.5 μg/mL of ZnO nanoparticles,(c) 5.0 μg/mL of ZnO nanoparticles, and (d) 10.0 μg/mL of ZnO nanoparticles.
Figure S3 Dynamic response of SMMC-7721 cells exposure to ZP6 ZnO nanoparticles in the presence of UV irradiation for 180s: (a) ZnO nanoparticle-free (control); (b) 2.5 μg/mL of ZnO nanoparticles,(c) 5.0 μg/mL of ZnO nanoparticles, and (d) 10.0 μg/mL of ZnO nanoparticles.
Figure S4 Dynamic response of SMMC-7721 cells exposure to ZP7 ZnO nanoparticles in the presence of UV irradiation for 180s: (a) ZnO nanoparticle-free (control); (b) 2.5 μg/mL of ZnO nanoparticles,(c) 5.0 μg/mL of ZnO nanoparticles, and (d) 10.0 μg/mL of ZnO nanoparticles.
Figure S5 Dynamic response of SMMC-7721 cells exposure to daunorubicin (1.0 ×10-7 mol/L) with ZP5 NPs. A: ZP 6 NPs and B: ZP 7 NPs. For A and B, a: negative control (ZnO nanoparticle-free); b: daunorubicin (1.0 ×10-7M); c: 2.5 μg/mL of ZnO NPs and daunorubicin (1.0 x 10-7M); and d: 5.0 μg/mL of ZnO NPs and daunorubicin (1.0 ×10-7M).


