Abstract
Hybrid magnetic nanostructures with high coercivity have immense application potential in various fields. Nickel (Ni) electrodeposited inside Cobalt (Co) nanotubes (a new system named Ni @ Co nanorods) were fabricated using a two-step potentiostatic electrodeposition method. Ni @ Co nanorods were crystalline, and they have an average diameter of 150 nm and length of ~15 μm. The X-ray diffraction studies revealed the existence of two separate phases corresponding to Ni and Co. Ni @ Co nanorods exhibited a very high longitudinal coercivity. The general mobility-assisted growth mechanism proposed for the growth of one-dimensional nanostructures inside nano porous alumina during potentiostatic electrodeposition is found to be valid in this case too.
Keywords: Magnetic nanowires, Nanorods, Hybrid nanostructures, Core–shell nanostructures, Mobility-assisted growth mechanism
Introduction
Nanostructured materials such as nanowires, nanotubes and nanorods are drawing considerable attention of the scientific community because of their tremendous application potential in various fields such as solar cells, field sensors, bioseparation and medical therapy [1]. Designing and controlling the morphology and growth of these nanowire and nanotubes will surely impact the development of nanotechnology [2,3]. The landmark paper on carbon nanotubes by Iijima [4] led to a surge in research activities in the area of organic and inorganic one-dimensional nanostructures [2]. Inorganic one-dimensional nanostructures like nanotubes and nanowires assume significance because of their diverse utilities in sensor technology, high density magnetic storage, delivery vehicles, catalysis and selective separation [5,6]. Various methods are in vogue for the synthesis of metal nanotubes and nanowires. These include various wet-chemical routes [5,7-9] and physical techniques such as electrochemical deposition, pulse laser deposition and molecular beam epitaxy [10-12].
Metallic magnetic nanotubes/wires of Ni, Co and Fe and also their alloys such as FePt, CoPt, NiFe, NiZn, CoCu and FeB were investigated in great detail due to their application potential in diverse fields such as perpendicular recording, cell separation, diagnosis, therapeutics and magnetic resonance imaging [2,13-18]. Most of these structures are based on pore wall modification or wet-chemical methods [12]. Magnetic nanostructures synthesized via the earlier-mentioned routes are often impure and rendered useless for applications [2]. Template-assisted technique is an elegant technique for fabricating one-dimensional structures, and most of the reported template-assisted methods are based on the chemical modification of porous templates such as etched polymer membrane or anodized alumina (AAO). Template-assisted electrodeposition is a simple, low-cost and unique method for the preparation of one-dimensional structures with very high purity and control [2].
Controlled synthesis of smart nanostructures based on magnetic materials assumes important due to their potential applications in various fields and the possibility for manipulating these structures using an external magnetic field [19,20]. Earlier, the authors reported the synthesis of Nickel nanowires (Ni NWs), Cobalt nanowires (Co NWs) [13] and Cobalt nanotubes (Co NTs) [2] employing different precursors by a single step potentiostatic electrodeposition technique. A general mobility-assisted growth mechanism has been proposed for the growth of one-dimensional nanostructures during electrodeposition for the first time, and the veracity of the mobility-assisted mechanism inside porous alumina has been tested using different precursors. Recently, the authors also tested the veracity of mobility-assisted growth mechanism inside MWCNTs and could fabricate co-axial multifunctional nanostructures of MWCNTs and Co NTs [20].
Core–Shell nanostructures represent a novel class of hybrid materials, where composition and microstructure varies through the radial direction [1]. The Co–Ni system is special due to the fact that the magnetic properties, especially, its coercivity can be tuned by varying the Co content [15]. Cobalt is known for its contribution in modifying the magnetic properties because of its high uniaxial anisotropy. However, this is more true in the bulk and the magnetic interactions taking place at the interface at Ni @ Co could be entirely different, where they are in the nano regime. Several groups attempted to synthesize various magnetic alloys using template-assisted electrodeposition [14-17], and they achieved this by mixing the electrolyte precursors in different compositional ratios. The lacuna of such techniques is the unpredictability in the magnetic properties such as coercivity of the resultant one-dimensional structures after electrodeposition. Co-axial hybrid magnetic structures synthesized via a two-step electrodeposition technique can possibly surpass this problem by controlling the deposition of one of the components. It was shown earlier that a single-step template-assisted electrodeposition method could be employed for the fabrication of one-dimensional magnetic nanostructures [2,13]. The authors successfully fabricated various multifunctional nanostructures and concluded that a mobility-assisted mechanism is responsible for the growth of such nanostructures [20]. Co nanotubes could be fabricated using template-assisted growth and if these structures can be employed as further template for electrodeposition, systems such as Ni @ Co could be fabricated. Such a method of preparation for hybrid magnetic nanostructures was not found to be attempted earlier. Moreover, the growth parameters can be easily optimized. This paper reports the fabrication of such a one-dimensional system namely Ni @ Co nanorods, which is essentially a core–shell architecture (Ni as core and Co as shell) and studies on their structural and magnetic properties.
Experimental
Alumina membranes (AAO template, Whatman) of high purity and uniform pore density, with average pore diameter ~150 nm and thickness ~60 μm, were employed for electrodeposition. Initially, a layer of Ag (about 200 nm thickness) was thermally evaporated onto one side of the AAO template which acted as the working electrode for the electrochemical deposition. The electrodeposition was carried out on the nanopores, using a standard three electrode potentiostat system (Princeton E.G & G 273 A). Ag/AgCl was the reference electrode, and platinum was used as the counter electrode. 0.2 M Cobalt acetate was used as the precursor for electrodeposition for making cobalt nanotubes, and the deposition was carried out for a time period of 1 h. Ni NWs have been electrodeposited in to these Co NTs using 0.2 M nickel sulfate hexahyrate (NiSO4·6H2O) in 0.1 M Boric acid (H3BO3) as electrolyte for 1 h. Schematic diagram showing the synthesis of Ni @ Co nanorods is depicted in Fig. 1. The X-ray diffraction (XRD) pattern of the Ni @ Co nanorods embedded in alumina template was recorded using Cu Kα radiation, λ = 1.5418 Å (Rigaku Dmax-C). Field Emission Scanning Electron Microscope (JSM-6335 FESEM) was employed to study the morphology of Ni @ Co nanorods. Individual nanorods were separated by etching out the alumina using 3 M sodium hydroxide (NaOH) solution and decanting the dissolved alumina using magnetic separation. Magnetization measurements were carried out using a SQUID magnetometer (MPMS-5S XL Quantum Design) by keeping the nanorods inside the alumina pores in order to retain the alignment intact. Transmission electron microscopy (TEM) experiments were performed using JEM 2010 transmission electron microscope.
Figure 1.
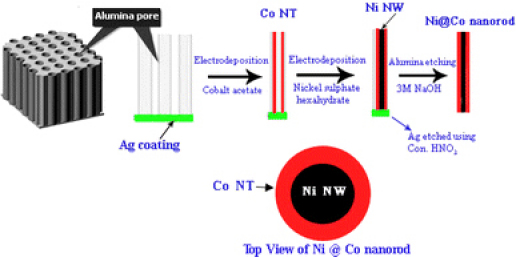
Schematic diagram showing the synthesis of Ni @ Co nanorods
Results and Discussion
The formation of Co NT and the subsequent formation of Ni NW inside Co NT (Fig. 1) are consistent with the mobility-assisted growth mechanism proposed earlier by the authors on nanoporous alumina [2,13]. There we could synthesize Co NTs having very high coercivity and high aspect ratio using cobalt acetate as precursor for electrodeposition and Ni NWs of high crystallinity and aspect ratio using nickel sulfate hexahydrate as precursor. It is to be note worthy that testing the veracity of this mobility-assisted growth mechanism for other porous membranes such as metallic membrane is being attempted for the first time. In generalizing this mobility-assisted growth mechanism, it is to be concluded that mobility of the cation and hydration layer are important parameters determining the morphology of one-dimensional structure after electrodeposition. Figure 2a shows the TEM of Co NT synthesized using Cobalt acetate. TEM of Ni NW is shown in Fig. 2b, and the inset depicts the electron diffraction (ED) pattern of Ni NWs.
Figure 2.
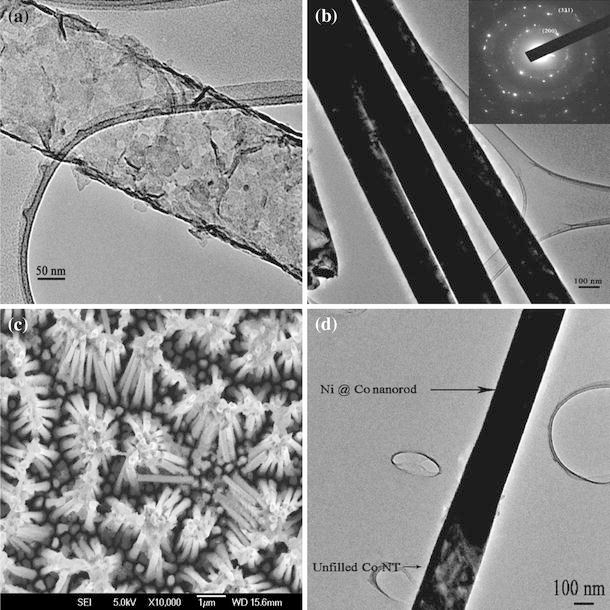
a TEM image of Co NT, b TEM image of Ni NW (inset: ED of Ni NW), c FESEM image of Ni @ Co nanorods, and d TEM image of Ni @ Co nanorod, after removing the alumina membrane
High crystallinity of Ni NWs is evident from the ED pattern, and the formation of face-centered cubic (fcc) Ni is also verified. Figure 2c depicts the FESEM images of Ni @ Co nanorods. Co NTs have been electrodeposited inside AAO membrane using Cobalt acetate as described earlier for 1 h, and then Ni is electrodeposited using NiSO4·6H2O also for 1 h. This has resulted into Ni-filled Co nanotubes (Ni @ Co nanorods) of length 15 μm and of diameter ~150 nm. The formation of a core–shell nanostructure with Co NT as shell and Ni NW as core is abundantly clear from the TEM image (Fig. 2d). It is to be noted that from the TEM image, some portion of the Co NTs remain unfilled. It can also be seen from the top portion of the FESEM image (Fig. 2c) that Ni is not completely filled inside Co NTs. The growth of nanowires/nanotubes initiates from the bottom portion of the alumina template. The incomplete filling of Ni may be due to the difference in the growth rate between Ni and Co, as their precursors are being different. Moreover, the extra hydration layer in Ni ions also may reduce the mobility and in turn the growth rate. This has supporting evidence from the energy dispersive spectroscopy (EDS).
The compositional analysis of these nanorods has been carried out using EDS and is shown in Fig. 3.
Figure 3.
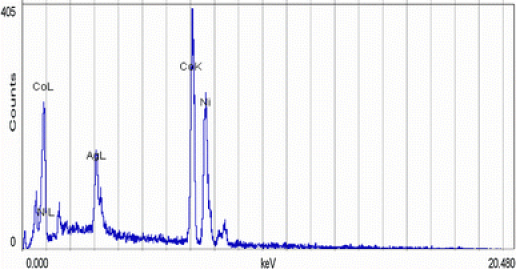
EDS of Ni @ Co nanorods
The presence of Co and Ni is evident from the EDS. Small amount of silver (Ag) is detected here which are perhaps from the back coating, which served as the working electrode during electrodeposition. It is also clear from the EDS that atomic percentage of Ni is less than that of Co. XRD (Fig. 4) indicates that Ni @ Co core–shell structure is crystalline in nature and constitutes two separate phases, a fcc belonging to Ni and a hexagonally closed packed (hcp) Co.
Figure 4.
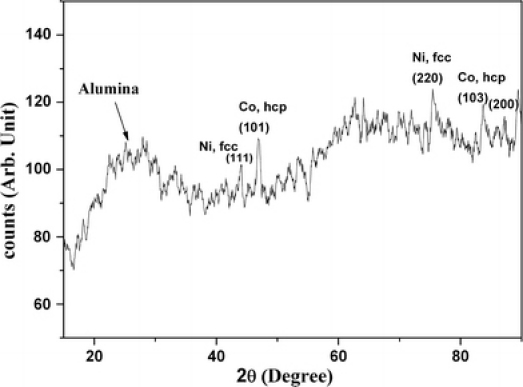
XRD pattern of Ni @ Co nanorods
Broad features appearing in the 15–35° 2θ range arise from the amorphous alumina. This is in agreement with the earlier reports [21]. The phase formation is consistent with our earlier reports on Co NTs and Ni NWs [2,13]. In order to investigate the magnetic properties of crystalline Ni @ Co nanorods, room temperature and low temperature (6 K) magnetic properties of the Ni @ Co nanorods were conducted using a SQUID magnetometer. Figure 5a and 5b depict the room temperature and low temperature M(H) curves of Ni @ Co nanorods measured parallel to the nanorods.
Figure 5.
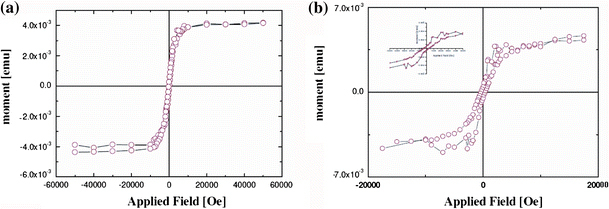
M(H) curves of Ni @ Co nanorods; a at room temperature b at 6 K
The Ni @ Co nanorods display a room temperature coercivity of 200 Oe. This coercivity is much higher than the bulk coercivity values of both the Ni (Hc = 0.7 Oe) and Co (Hc = 10 Oe) [22]. The enhanced coercivity in Ni @ Co nanorods emanate from the enhanced shape anisotropy. Li et al. reported [14] a similar coercivity value for Co nanotubes synthesized via template-assisted synthesis; however, the values were smaller than our earlier reports on Co NTs of very high aspect ratio [2]. This is due to the fact that the shape anisotropy of the samples mentioned in the earlier report is much higher (aspect ratio of Co NTs is ~330) than that of the present (aspect ratio of Ni @ Co nanorods is ~100). The coercivity value for Ni @ Co nanorods is higher than that reported for Ni NWs [12] possessing a higher aspect ratio, and this is due to the presence of cobalt. This indicates that one can tailor the coercivity of these heterostructures by controlling the aspect ratio as well as cobalt content. M(H) curve at 6 K exhibit an enhanced coercivity of ~380 Oe. This is much higher than the other reported values of Co-based alloy nanowires [15]. The enhancement in coercivity at low temperatures is consistent with the monotonic increase of uniaxial anisotropy constant with decreasing temperature, with the basic assumption that the shape anisotropy is independent of temperature for high aspect ratio tubes [23].
Similar to Co NTs [2], Co NWs and Ni NWs [13], squareness ratio (Mr/Ms) of the Ni @ Co nanorods is small. This may be due to the very high magnetic dipolar interrod interaction. This type of hybrid magnetic system with higher aspect ratio can render very high coercivity with the higher contribution of shape anisotropy and higher coercivity hybrid nanorods can find applications in fields such as data storage where a high coercivity is required. This can be achieved by extending the electrodeposition for longer deposition times and aspect ratio up to three times (~330) that of the present value (~100), using the AAO template of 60 μm thickness.
Conclusions
A novel magnetic nanostructure called Ni @ Co nanorods with Ni NW as core and Co NT as shell was synthesized using a two-step electrodeposition method. Structural studies indicate the formation of Ni and Co in two phases. Magnetic studies showed that Ni @ Co nanorods exhibited high longitudinal coercivity, and they can find applications in various fields where high coercivity is required. Understanding the growth mechanism also opens possibility for tuning the magnetic properties by extending the electrodeposition for longer times to obtain very high coercivity hybrid nanowires.
Acknowledgments
TNN acknowledges the financial support received from Interconnect Focus Center at Rensselaer Polytechnic Institute, Troy, New York, USA. TNN thanks Council of Scientific and Industrial Research, India for financial support in the form of CSIR-SRF.
References
- Liu Z, Elbert D, Chien CL, Searson PC. Nano. 2008. p. 2166. COI number [1:CAS:528:DC%2BD1cXosF2gsLw%3D]; Bibcode number [2008NanoL...8.2166L] [DOI] [PubMed]
- Narayanan TN, Shaijumon MM, Ajayan PM, Anantharaman MR. J. Phys. Chem. C. 2008. p. 14281. COI number [1:CAS:528:DC%2BD1cXhtValurnF] [DOI]
- Meng GW, Jung YJ, Cao A, Vajtaj R, Ajayan PM. PNAS. 2005. p. 7074. COI number [1:CAS:528:DC%2BD2MXks12gu74%3D]; Bibcode number [2005PNAS..102.7074M] [DOI] [PMC free article] [PubMed]
- Iijima S. Nature. 1991. p. 56. COI number [1:CAS:528:DyaK38Xmt1Ojtg%3D%3D]; Bibcode number [1991Natur.354...56I] [DOI]
- Bao J, Tie C, Xu Z, Zhou Q, Shen D, Ma Q. Adv. 2001. p. 21. [DOI]
- Steinhart M, Wehrsphon RB, Gosele U, Wendroff JH. Angew. 2004. p. 1334. COI number [1:CAS:528:DC%2BD2cXivFSksb4%3D] [DOI] [PubMed]
- Yanagishita T, Nishio K, Masuda H. Adv. 2005. p. 2241. COI number [1:CAS:528:DC%2BD2MXhtVKqsbvK] [DOI]
- Nielsch K, Castano FJ, Matthias S, Lee W, Ross CA. Adv. 2005. p. 4.
- Lee W, Scholz R, Nielsch K, Gosele U. Angew. 2005. p. 6050. COI number [1:CAS:528:DC%2BD2MXhtVKqs7nE] [DOI] [PubMed]
- Martin CR. Science. 1991. p. 1961. Bibcode number [1994Sci...266.1961M] [DOI] [PubMed]
- Heydon GP, Hoon SR, Farley AN, Tomlinson SL, Valera MS, Attenborough K, Schwarzacher W. J. 1997. p. 1083. COI number [1:CAS:528:DyaK2sXisFGltbk%3D]; Bibcode number [1997JPhD...30.1083H] [DOI]
- Sharif R, Shamaila S, Ma M, Yao LD, Yu RC, Han XF, Khaleeq-ur-Rahman M. Appl. 2008. p. 032505. Bibcode number [2008ApPhL..92c2505S] [DOI]
- Narayanan TN, Shaijumon MM, Ci L, Ajayan PM, Anantharaman MR. Nano. 2008. p. 465. COI number [1:CAS:528:DC%2BD1MXhtFSjtr3N] [DOI]
- Li D, Thompson RS, Bergmann G, Lu JG. Adv. 2008. p. 1. [DOI]
- Talapatra S, Tang X, Padi M, Kim T, Vajtai R, Sastry GVC, Shima M, Deevi SC, Ajayan PM. J. 2008. p. 2271. Bibcode number [2008JMatS..44.2271T] [DOI]
- Li XZ, Wei XW, Ye Y. Mater. 2009. p. 578. COI number [1:CAS:528:DC%2BD1MXhtVKgtA%3D%3D] [DOI]
- Wu CU, Lin HL, Shau NL. J. 2006. p. 198. COI number [1:CAS:528:DC%2BD28XhtFSnsrk%3D] [DOI]
- Fu L, Yang J, Bi Q, Liu W. Nanoscale Res. 2009. p. 11. COI number [1:CAS:528:DC%2BD1MXht1Kgs70%3D]; Bibcode number [2009NRL.....4...11F] [DOI] [PMC free article] [PubMed]
- Ou FS, Shaijumon MM, Ajayan PM. Nano. 2008. p. 1853. COI number [1:CAS:528:DC%2BD1cXmsVKhur0%3D]; Bibcode number [2008NanoL...8.1853O] [DOI] [PubMed]
- Narayanan TN, Suchand Sandeep CS, Shaijumon MM, Ajayan PM, Philip R, Anantharaman MR. Nanotechnology. 2009. p. 285702. COI number [1:STN:280:DC%2BD1MvjvFGksw%3D%3D] [DOI] [PubMed]
- Cao H, Wang L, Qiu Y, Wu Q, Wang G, Zhang L, Liu X. Chem. 2006. p. 1500. COI number [1:CAS:528:DC%2BD28Xnt1Olu7s%3D] [DOI] [PubMed]
- Chikazumi S. Physics of magnetism. Wiley, New York; 1964. [Google Scholar]
- Henry Y, Ounadjela K, Piraux L, Dubois S, George JM. Duvail Eur. Phys. J. B. 2001. p. 35. COI number [1:CAS:528:DC%2BD3MXktlOrsbw%3D]; Bibcode number [2001EPJB...20...35H] [DOI]


