Abstract
pH-sensitive micelles with hydrophilic core and hydrophilic corona were fabricated by self-assembling of triblock copolymer of poly(methylacrylic acid)-poly(ethylene glycol)-poly(methylacrylic acid) at lower solution pH. Transmission electron microscopy and laser light scattering studies showed micelles were in nano-scale with narrow size distribution. Solution pH value and the micelles concentration strongly influenced the hydrodynamic radius of the spherical micelles (48–310 nm). A possible mechanism for the formation of micelles was proposed. The obtained polymeric micelle should be useful for biomedical materials such as carrier of hydrophilic drug.
Keywords: Self-assembly, Micelles, Double-hydrophilic, Triblock copolymers
The self-assembly behavior of double-hydrophilic block copolymers under the influence of a given external stimulus, such as a change in pH, temperature, or ionic strength, has attracted considerable interests due to their potential application in areas such as biomimetic chemistry, molecular switch, and controlled drug delivery [1-4]. Many systems, such as poly(ethylene glycol)-block-poly(N-isopropylacrylamide) [5], poly(styrenesulfonic acid)-block-poly(methacrylic acid) [6], and poly(ethylene glycol)-block-poly(methacrylic acid) [7], have been investigated.
PMAA is one of the most commonly used hydrophilic polymers that possess several specific properties such as bioactive, pH, and ionic strength responsive properties. A number of polymeric micelles formed from PMAA-based amphiphilic diblock copolymers have been investigated for various applications [8,9]. PEG is a noncharged, hydrophilic, and nonimmunogenic polymer that has found wide chemical, biomedical, and industrial applications [10,11]. Complexation of PMAA with PEG has been extensively studied [12]. Also, the micellization of PEG-b-PMAA diblock copolymers with linear structure, as well as with branched or graft architecture has recently been studied [7]. However, there is no report on the self-assembly behavior of double-hydrophilic triblock copolymer of PEG and PMAA (PMAA-b-PEG-b-PMAA). Actually, triblock copolymer display very rich phase behavior and morphologies in solution, because of their special compositions and properties [13,14]. Therefore, it is very interesting to have insight into the self-assembly behavior of this novel double-hydrophilic triblock copolymer PMAA-b-PEG-b-PMAA in aqueous solutions.
Amphiphilic block copolymers of poly(tert-butyl methacrylate)-block-poly(ethylene glycol)-block-poly(tert-butyl methacrylate) were first prepared by using bromo-terminated difunctional PEG as macroinitiators by the atom transfer radical polymerization. After the removal of thetert-butyl group by hydrolysis, triblock copolymers of PMAA-b-PEG-b-PMAA were obtained (Scheme 1). The resulted triblock copolymer was characterized by size exclusion chromatography (SEC) using polystyrene standard and had anMnof 11 K and a polydispersity of 1.3. The number of structural units of PMMA and PEG block is 100 and 46, respectively. The typical procedure of the preparation of triblock copolymer micelles in selective solvents is shown as follows: triblock copolymer PMAA-b-PEG-b-PMAA (0.05 g) were dissolved in 100 mL dimethylformamide (DMF), followed by the filtration of the solution through a 0.45 μm Teflon membrane filter (Chromatographic Specialties Inc.). The micelles were formed by the addition of the resulting DMF solution into water with lower pH value at a rate of 1 drop per 60 s with continuous stirring. The micelles solution was stirred for 24 h, and then dialyzed in water using a cellulose dialyzer tube. The hydrodynamic radius,Rh, of the micelles was measured by laser light scattering (ALV500E, ALV Co., Germany) and nanosphere morphologies were directly observed using a transmission electron microscope (TEM; Hitachi H-7010A).
Scheme 1.

The chemical structure of double-hydrophilic triblock copolymer PMAA-b-PEG-b-PMAA and mechanism for the formation of micelles
Typical TEM image of micelles is shown in Fig. 1. As shown in the figure, spherical micelles with a narrow size distribution were obtained from double-hydrophilic triblock copolymer PMAA-b-PEG-b-PMAA. According to the calculation results from TEM, the size of the micelles is ca. 92 nm. The average size and size distribution of micelles (PDI) could also be determined from DLS measurement. The hydrodynamic diameter was 96 nm from DLS method, which was well consistent with the TEM results (Fig. 2). And PDI value by DLS was 0.053, also showing narrow size distribution. Figure 2shows the dependence ofRhand polydispersity of micelles on the solution pH value. MinimumRhvalue and the narrowest PDI occurred at pH 2.6. When pH value decreased to 1.7, theRhvalue increased abruptly to 310 nm, whereas an increase beyond 2.6 also led to the increase inRhvalue along with a slight increase in the polydispersity.
Figure 1.
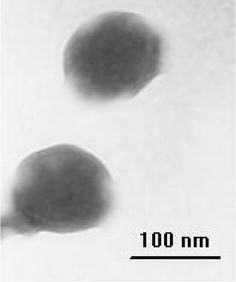
TEM images of PMAA-b-PEG-b-PMAA polymeric micelles at pH 2.6
Figure 2.

The effects of pH value on theRhand size distribution of PMAA-b-PEG-b-PMAA micelles The concentration of micelles is 5 × 10−4
Based on the fact that the fabrication as well as the average size and size distribution of PMAA-b-PEG-b-PMAA micelles were strongly dependent on the pH value, a possible mechanism for the formation of PMAA-b-PEG-b-PMAA micelles at lower pH value was proposed and shown in Scheme 1. It is well-known that nonionized PMAA is water-soluble and has an hypercoiled conformation as result of the intramolecular hydrogen bonding of the MAA units when the solution pH was less than 6 [15]. Therefore, above pH 6, PMAA-b-PEG-b-PMAA chains were in extended chain conformation due to the electrostatic repulsion between the PMAA segments [8]. Decreasing the solution pH down to 6 caused the conformation change of PMAA segments from extended chain to hypercoil. This change of conformation resulted in the aggregation of PMAA chain segments into micellar core. Simultaneously, the middle block PEO self-assembled into micellar corona due to the back-folding and looping of PEO chain [13,16]; therefore, the generation of micelles with water-soluble PMAA core and hydrophilic PEO loop chain corona was realized. The reason why minimum Rh and the narrowest size distribution occurred at pH 2.6 was not clear at this moment. Possibly, 2.6 was a critical pH value, and PMAA chains would more tightly aggregate at this value, resulting in firmly fixed micelles, whereas a further decrease below 2.6 caused much more aggregation of PMAA segments, leading to unstable micelles and even precipitation of the polymers.
The application of these pH-sensitive micelles with hydrophilic core and hydrophilic corona as a carrier of hydrophilic drug will be subsequently reported [17,18].
The National Natural Science Foundation of China (NSFC50673038) is acknowledged for supporting this research.
References
- Zhang LF, Eisenberg A. Science. 1995. p. 1728. COI number [1:CAS:528:DyaK2MXmsFCisbk%3D]; Bibcode number [1995Sci...268.1728Z] [DOI] [PubMed]
- Chécot F, Lecommandoux S, Gnanou Y, Klok H. Angew. 2002. p. 1339. [DOI] [PubMed]
- Jiang K, Chi F, Li B, Jiang B. Chem. 2008. p. 492. COI number [1:CAS:528:DC%2BD1cXmvVWqtbc%3D] [DOI]
- Tao Y, Liu R, Liu X, Chen M, Yang C, Ni Z. Chem. 2008. p. 1162. COI number [1:CAS:528:DC%2BD1cXhsVCltrjL] [DOI]
- Zhang W, Shi L, Wu K, An Y. Macromolecules. 2005. p. 5743. COI number [1:CAS:528:DC%2BD2MXks1KgsLw%3D] [DOI]
- Tauer K, Khrenov V, Shirshova N, Nassif N. Macromol. 2005. p. 187. COI number [1:CAS:528:DC%2BD2MXmvVeht7k%3D] [DOI]
- Holappa S, Karesoja M, Shan J, Tenhu H. Macromolecules. 2002. p. 4733. COI number [1:CAS:528:DC%2BD38Xjt12ru7Y%3D] [DOI]
- Arimura H, Ohya Y, Ouchi T. Biomacromolecules. 2005. p. 720. COI number [1:CAS:528:DC%2BD2MXjs1Kqsg%3D%3D] [DOI] [PubMed]
- Burkhardt M, Ruppel M, Tea S, Drechsler M, Schweins R, Pergushov DV, Gradzielski M, Zezin AB, Muller AHE. Langmuir. 2008. p. 1769. COI number [1:CAS:528:DC%2BD1cXhtFantLk%3D] [DOI] [PubMed]
- Hu X, Liu S, Chen X, Mo G, Xie Z, Jing X. Biomacromolecules. 2008. p. 553. COI number [1:CAS:528:DC%2BD1cXptl2gtg%3D%3D] [DOI] [PubMed]
- Fujioka M, Sato H, Tsuchiya K, Ogino K. Chem. 2008. p. 350. COI number [1:CAS:528:DC%2BD1cXjsFChsLY%3D] [DOI]
- Oyama HT, Tang WT, Frank CW. Macromolecules. 1987. p. 474. COI number [1:CAS:528:DyaL2sXhtVaqtrk%3D] [DOI]
- Chang C, Wei H, Quan C, Li Y, Liu J, Wang Z, Cheng S, Zhang X, Zhuo R. J. 2008. p. 3048. COI number [1:CAS:528:DC%2BD1cXltlWls7g%3D] [DOI]
- Li J, Li X, Ni XP, Leong KW. Macromolecules. 2003. p. 2661. COI number [1:CAS:528:DC%2BD3sXitlGku7w%3D] [DOI]
- Gohy JF, Varshney SK, Jerome R. Macromolecules. 2001. p. 3361. COI number [1:CAS:528:DC%2BD3MXisV2qtbg%3D] [DOI]
- Yuan J, Xu Z, Cheng S, Feng L. Eur. 2002. p. 1537. COI number [1:CAS:528:DC%2BD38Xjsl2jsb0%3D] [DOI]
- Tikhonov VE, Stepnova EA, Babak VG, Krayukhina MA, Berezin BB, Yamskov IA. React. 2008. p. 436. COI number [1:CAS:528:DC%2BD1cXhsFWgt7o%3D] [DOI]
- Wang L-P, Wang Y-P, Pei X-W, Peng B. React. 2008. p. 649. COI number [1:CAS:528:DC%2BD1cXhsFWgu7g%3D] [DOI]


