SUMMARY
The activity of keratinocytes in the hair follicle is regulated by signals from a specialized mesenchymal niche, the dermal papilla. Here, mice expressing cre recombinase in the dermal papilla were developed to probe the interaction between follicular keratinocyte populations and the dermal papilla in vivo. Inactivation of the β-catenin gene within the dermal papilla of fully developed hair follicles results in dramatically reduced proliferation of the progenitors and their immediate progeny that generate the hair shaft and, subsequently, premature induction of the destructive phase of the hair cycle (catagen). It also prevents regeneration of the cycling follicle from stem cells resident in the permanent portion of the follicle. Gene expression analysis reveals that β-catenin activity in the dermal papilla regulates at least two signaling pathways, FGF and IGF, that can mediate the inductive effects of the DP on keratinocytes. This study reveals a reciprocal signaling loop that employs Wnt/β-catenin signaling in both epithelial progenitor cells and their mesenchymal niche to govern and coordinate the interactions that are essential for the function of these two compartments.
Keywords: niche, β-catenin, hair follicle, dermal papilla
Introduction
Hair follicles undergo cycles of growth (anagen), regression (catagen), quiescence (telogen) and regeneration. During the active growth phase (anagen), the mature hair follicle is composed primarily of keratinocytes arranged in concentric layers of differentiated cell types that comprise the hair shaft (HS), inner root sheath (IRS) and outer root sheath (ORS). Keratinocytes in direct contact with the dermal papilla (DP), a specialized mesenchymal component embedded in the hair bulb at the base of the follicle, undergo asymmetric divisions to renew this “matrix stem cell” compartment and to generate progeny that are displaced away from the DP and undergo a few divisions before differentiating to form the constituents of the HS and IRS (Legue and Nicolas, 2005). Growth of the hair occurs as these constituents are added to the base of the inner layers, the IRS and HS, which are extruded through the ORS towards the surface of the skin.
At the end of the anagen phase, proliferation in the hair matrix ceases. Some matrix cells differentiate to form the terminal structure of the hair (club), while the rest apoptose as the catagen phase begins (Ito et al., 2004). Thus while “matrix stem cells” exhibit asymmetric division, self-renewal and oligopotency, they are a transient population analogous to those more commonly referred to as committed progenitors in other systems. This population will be referred to as Matrix Progenitor cells Abutting the DP (MPADs) to avoid confusion with nominal stem cell populations in the follicle or the suprabasal cells of the matrix sometimes referred to as matrix progenitors. During the catagen phase, the majority of the ORS of the lower follicle undergo apoptosis. The DP is drawn up with the regressing epithelial strand to lie adjacent to the base of the permanent portion of the follicular epithelium, the secondary germ. A quiescent phase of variable length precedes regeneration of the lower follicle. At the onset of a new anagen phase, keratinocytes in the secondary germ adjacent to the DP proliferate and form a new pool of MPADs. The Cd34+ “follicular bulge stem cells” and possibly other putative stem cell populations repopulate to the secondary germ to sustain follicular regeneration (Ito et al., 2004; Jaks et al., 2008).
DP cells generate signals that regulate the behavior of keratinocytes in the follicle during the hair cycle. Extirpation and grafting studies broadly support a role for reciprocal inductive and instructive signaling between DP and keratinocytes (Ibrahim and Wright, 1977; Jahoda et al., 1984; Jahoda et al., 1993; McElwee et al., 2003), but the physical disruption of follicles involved in such studies complicates their interpretation. The fact that many of the genetic pathways active in the DP are either required in mesenchymal cells for the formation of the follicle during embryogenesis or are active in keratinocytes of the follicle as well has hampered the analysis of gene function in the DP. In the absence of an experimental approach to manipulate gene activity in the DP of follicles that have already undergone normal development, it has not been possible to distinguish whether the phenotypic consequences of genetic perturbation result from the indirect consequences of earlier abnormalities in hair follicle development or from ongoing roles of the DP in regulating keratinocyte behavior.
Wnt/β-catenin signaling plays important roles in hair follicle morphogenesis and regeneration. Ablation of β-catenin in embryonic epidermis prevents hair follicle formation while forced expression of constitutively activated β-catenin in the epidermis results in expansion of hair follicle fate during development (Huelsken et al., 2001; Zhang et al., 2008) and de-novo formation of hair follicles in adults (Gat et al., 1998; Lo Celso et al., 2004). Wnt/β-catenin signaling also plays critical roles in the activation of keratinocytes in the permanent follicle to initiate hair follicle regeneration (Lowry et al., 2005; Van Mater et al., 2003). In addition, β-catenin signaling is active in the keratinocytes of the hair bulb and specifies the differentiation of a subset of their descendents into cell types of the hair shaft (Merrill et al., 2001).
While the direct role of β-catenin signaling in the keratinocyte compartment is established, the function of this pathway in the DP is less clear. Activation of β-catenin in the mesenchyme during dermal development is observed before the initiation of follicle formation and precedes the activation of this pathway in the epidermis (Noramly et al., 1999; Zhang et al., 2009). Pathway activity is then detected in the dermal condensate, a transient mesenchymal compartment that differentiates into the DP (DasGupta and Fuchs, 1999; Zhang et al., 2009). The failure to detect activity of the pathway by reporter assays at later stages and tissue recombination studies with Lef-1 deficient mesenchyme have been taken as evidence suggesting the pathway is inactive in mature DP (DasGupta and Fuchs, 1999; Kratochwil et al., 1996). However, the question of whether activation of the pathway in the DP is sustained during the anagen phase has remained controversial (Maretto et al., 2003).
To directly test the function of specific gene pathways in the DP, mouse strains that allow genetic manipulation in these cells within the context of fully-formed hair follicles in vivo were developed. These were used to analyze the role of β-catenin signaling in the DP of the anagen follicle. Here we report that sustained β-catenin function in the dermal papilla is required throughout the growth phase to maintain proliferation of adjacent MPADs and their progeny and to direct hair shaft morphogenesis, in part by regulating the expression of secreted growth factors that can influence keratinocyte behavior. While there is no apparent requirement for β-catenin in the DP during the catagen and telogen stages, regeneration of the follicle from stem cells in the permanent portion of the follicle is also defective in these mice.
Results
DP specific expression of cre recombinase
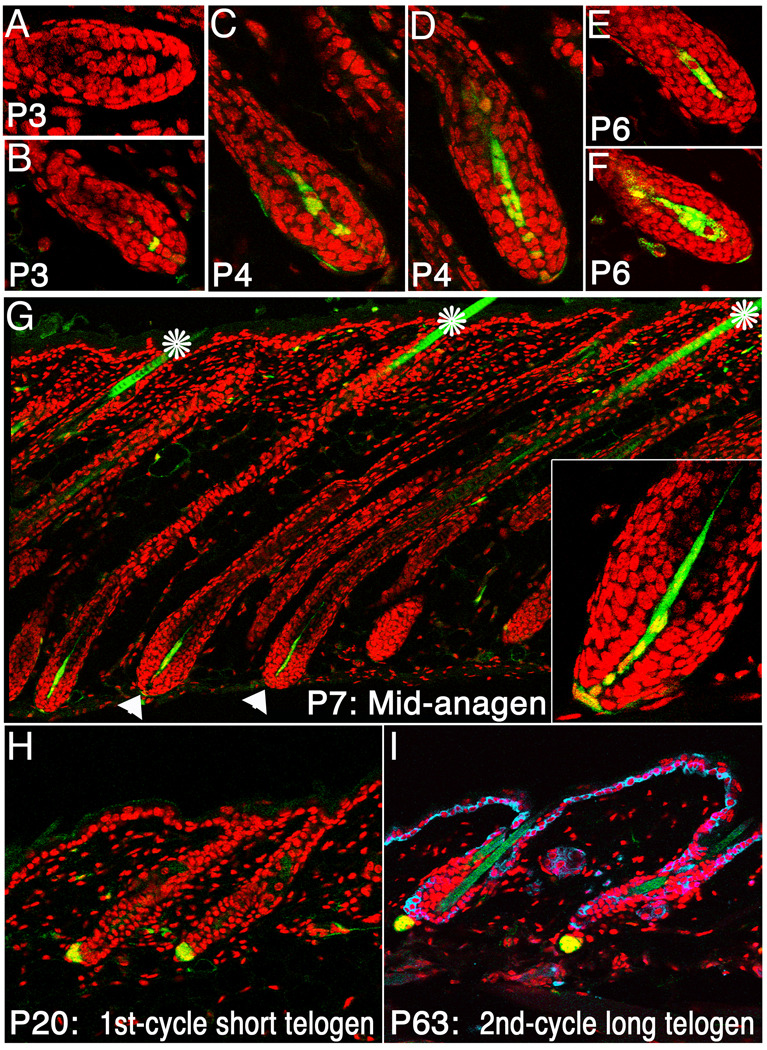
Sequences encoding cre recombinase were inserted into the Corin gene locus to generate Cor-cre mice. Within the skin, Corin is specifically expressed in the DP (Enshell-Seijffers et al., 2008). Disruption of the Corin gene does not alter hair follicle development or cycling, although mice homozygous for a Corin mutation have increased pheomelanin production on an Agouti background (Enshell-Seijffers et al., 2008). Although Corin transcripts were detected in the DP of wild type mice from the earliest stages of hair follicle morphogenesis when the DP first forms from the dermal condensate (Enshell-Seijffers et al., 2008), cre activity is not detected until after birth. Cre-mediated activation of the r26YFP reporter (Srinivas et al., 2001) in Cor-cre/+; r26YFP/+ mice was first observed at P3 (Fig. 1A,B). At this stage most hair follicles lack cells expressing YFP (Fig. 1A), but the number of YFP-positive cells in the DP increases rapidly (Fig. 1C–F) and by P7 YFP is detected in virtually all DP cells of all hair types as well as some cells in the proximal dermal sheath (DS) (Fig. 1G). Within the skin, Cre activity is largely restricted to the DP/DS, but some YFP-positive cells are found at low frequency in the dermis. This pattern of DP-specific expression of YFP remains unchanged throughout the hair cycle and persists as the mice age (Fig. 1H,I). In contrast to the highly efficient targeting of DP cells, the rare labeled cells outside the DP are variable in position and number, represent a negligible fraction of the dermis and are unlikely to impact follicle behavior. The fact that cre activity is first observed postnatally renders this Cor-cre line a powerful tool to manipulate gene expression in the DP after the formation of hair follicles.
Figure 1. Cre activity is largely confined to the dermal papilla.
Cre-mediated activation of the r26YFP locus reveals the timing and distribution of cre recombinase activity in Cor-cre/+; r26YFP/+ mice. YFP (Green) was first detected at P3 in some but not all follicles (A,B) and increasing numbers of DP cells that express YFP were observed through the mid-anagen phase (C–F). By P7, virtually all DP cells express YFP (G, arrowheads). Inset in G shows higher magnification of a hair bulb. The hair shaft is autofluorescent and its green color is not an indication of cre activity (asterisk). Cre activity remains restricted to the DP as the mice age, as shown during the first (H) and second (I) telogen phases. In I, K14 staining (blue) labels the basal layer of the inter-follicular epidermis and the ORS of the hair follicle. Nuclei-red.
β-catenin is required in the DP for normal hair morphogenesis
The Cor-cre line was used to delete a conditional allele of β-catenin (Brault et al., 2001) to evaluate the role for β-catenin signaling in regulating follicular keratinocytes indirectly by its action in the dermal papilla of the anagen hair follicle. Steps of hair follicle development thought to depend on β-catenin activity, including formation of dense dermis, hair placodes and DP (Andl et al., 2002), occur in the presence of an intact β-catenin gene in mice of the genotype Cor-cre/+; Ctnnb1Del/Flox; r26YFP/+. Deletion of the floxed β-catenin allele (Ctnnb1Flox) (Brault et al., 2001) to a null allele (Ctnnb1Del) occurs in fully-functional DP cells during the early to mid-anagen phase of the hair cycle. Mice of the genotype Cor-cre/+; Ctnnb1+/Flox; r26YFP/+ or +/+; Ctnnb1Del/Flox; r26YFP/+ were phenotypically indistinguishable from wild type. Cor-cre/+; Ctnnb1+/Flox; r26YFP/+ were used as controls throughout this study.
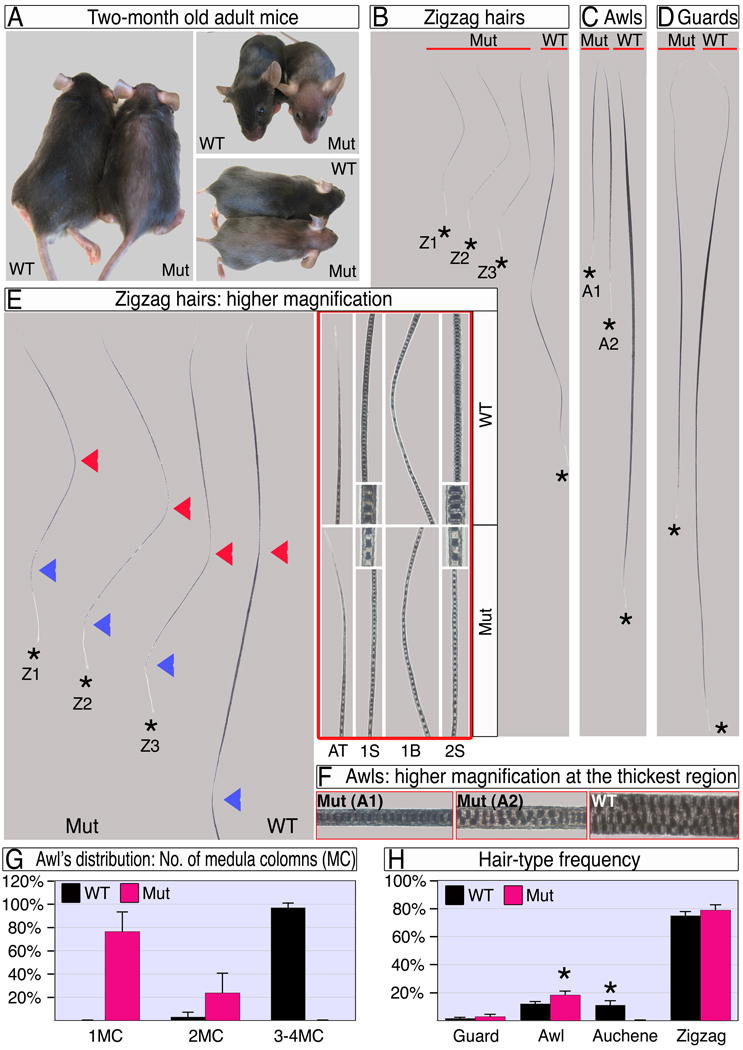
Mouse pelage consists of four hair types with distinctive morphologies. Of the two straight hair types, the long thin guard hairs form first (e14), while the shorter, thicker awl hairs are thought to form in the second wave of folliculogenesis (e16). Auchenes and zigzag hairs are characterized by single and multiple oblique angle bends in the hair shaft respectively and are thought to form last (e18). At P5, when YFP expression reveals that cre activity in the DP is becoming prevalent, all follicles are generating hair shafts but guard and awl hair follicles have been doing so for longer. When scored at the end of the first hair cycle, all hairs in the mutant pelage are dramatically shorter and thinner than wild type (Fig. 2A–G). Mutant awl hairs are less than half the length of wild type and reduced in thickness to a single column of medulla cells from the normal 3 to 4 (Fig 2C, F, G). Guard hairs are also reduced in length, but less dramatically so as might be expected from the longer period of growth prior to deletion (Fig 2D).
Figure 2. Ablation of β-catenin in the DP results in dramatic hair shortening and thinning.
(A) Adult wild type and mutant mice. Note the epidermis is visible through the thin mutant hair coat. (B–D) Zigzag, awl and guard hair-types after the first hair cycle. The apical tips of all hairs are aligned next to the red line and hair-clubs are marked by stars. Mutant hairs within a hair-type population are classified into sub-types based on their different length and/or thickness. Z1–Z3 in B represent a first segment 50% or less, between 50–100%, and similar in length to wild type respectively. A1–A2 in C represent 1 or 2 columns of medulla cells respectively. (E) Mutant zigzag hairs and the apical portion of a wild type zigzag are shown at left. The first and second bends from the apical tip are marked by red and blue arrowheads, respectively. At right, framed with red line, higher magnifications of the apical tip (AT), mid-domain of the first segment (1S), first bend (1B) and mid-region of the second segment (2S) reveal the reduced thickness in mutant hair along the entire hair shaft. Insets show the thickness and organization of the medulla column. (F) Higher magnifications of the thickest region of awl hairs show the number, structure and organization of the medulla columns. (G) Distribution of awl hairs according to the number of medulla columns (MC) at the thickest region. (H) Frequency of hair types in wild type and mutant. Two-tailed unpaired students T-test was employed (*, p<0.0001). See also Figure S1.
Zigzag hairs are the most abundant hair type (Fig. 2H). In wild type, these hairs consist of four segments separated by oblique angle bends (Fig 2B). Although the length of the first segment in mutant hairs varies substantially from similar to wild type to 40% reduction, the second segment is consistently between 50–60% of wild type length (Fig 2B,E; Z1–Z3). The third and fourth segments are absent in the mutant. Both remaining segments are thinner than the corresponding wild type.
Auchene hairs, characterized by a single oblique angle bend (Fig. S1), are apparently absent in mutant mice, and the frequency of hairs scored as awls increases (Fig. 2H). While this may reflect a role for β-catenin in hair type determination, an alternative and more likely explanation is that changes in the structure of mutant hairs affect the characters used to distinguish these hair types (Fig. S1, Fig. 2C,F). The distal segment of an auchene hair is morphologically indistinguishable from a reduced awl, so that premature termination of auchene growth before formation of the single bend would phenocopy awl structure.
Changes in either the rate of proliferation in the hair bulb, the duration of the proliferative phase, or both could cause the reduced length of the hair coat. Growth and segmentation of zigzag hairs are not directly coupled, so that the length of individual segments is a good indicator of growth rate, while the number of segments correlates with the length of the anagen phase of the hair cycle (Hebert et al., 1994; Millar et al., 1999). The structure of the zigzag hairs suggests both proliferation rates and duration of the hair cycle are affected when β-catenin is deleted in the DP. The reduced length and thickness of each segment implies proliferation rates are reduced in the mutant, starting shortly after hair shaft formation begins. The variable length of the first segment is consistent with the variable deletion efficiency among follicles predicted by YFP expression at the stage this segment is forming, while the more consistent length of the second segment correlates with more uniform deletion predicted by mid-anagen. The lack of proximal segments suggests that the anagen phase is terminated prematurely.
Compromising β-catenin in the DP reduces proliferation rates of MPADs and their progeny
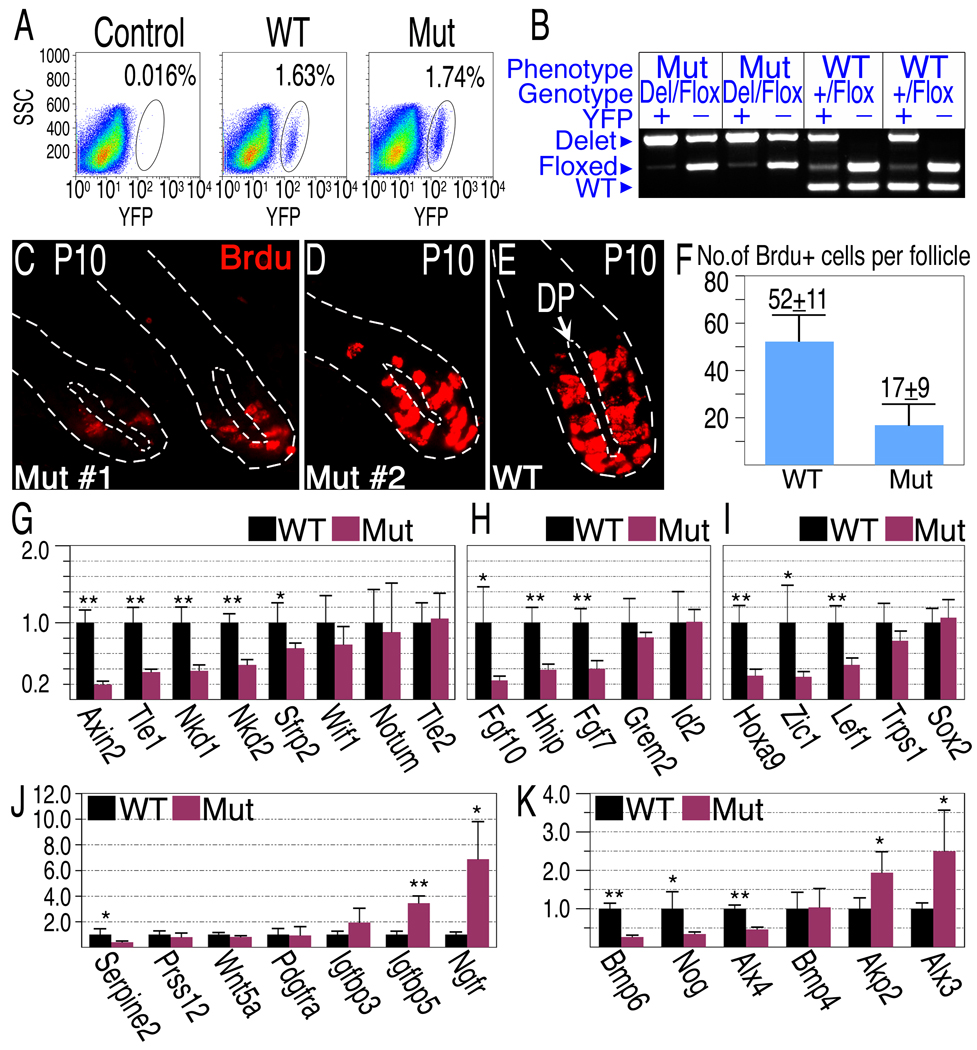
At P9, both wild type and mutant follicles are in the anagen phase and morphologically indistinguishable in skin sections (Fig S2A–B). There is no apparent change in DP structure or DP-cell numbers, and virtually all DP cells in the mutant express YFP as in littermate controls (Fig S2C). The lack of obvious effect on hair bulb morphology in the mutant may belie underlying molecular alterations that affect hair morphogenesis or simply reflect inefficient deletion of the β-catenin gene at this stage. Deletion efficiency was estimated by purifying DP cells from individual mutant and control P9 animals by FACS sorting cells expressing the activated r26YFP allele (Fig. 3A–B). The proportion of YFP positive cells is similar in wild type and mutant mice (Fig. 3A), confirming the lack of observable alteration in DP-cell number or labeling at this stage. Genotyping the sorted YFP+ cells revealed efficient deletion of the β-catenin gene. About 90% of YFP+ cells lack a functional β-catenin gene (Fig. 3B and data not shown).
Figure 3. Deletion of β-catenin in the DP results in reduced proliferation rates of matrix cells and alterations of gene expression in the DP.
(A) FACS analysis of dissociated single cells derived from a back skin of “wild type” (Cor-cre/+; Ctnnb1+/Flox ;r26YFP/+), mutant (Cor-cre/+; Ctnnb1Del/Flox; r26YFP/+) and YFP-negative control (+/+; Ctnnb1+/+;r26YFP/+) P9 mice. (B) Deletion efficiency of the β-catenin gene is 90% at P9. YFP positive (DP) and negative (control) cells were FACS sorted from individual mice and used to genotype the β-catenin gene. Representative examples at P9 are shown. Note that “wild type” mice carry one allele of floxed β-catenin. (C–F) BrdU incorporation in P10 mice. Representative examples from two mutants (C,D) and one wild-type (E) are shown to demonstrate BrdU incorporation (red) in the bulb region. The inner and outer dashed lines demarcate the DP and hair follicle respectively. (F) The average number of BrdU-positive cells in the hair matrix per follicle per section is shown. (G–K) Gene expression analysis of Wnt/β-catenin target and DP signature genes. DP cells purified from individual P9 mice were analyzed for gene expression by real-time PCR (mean±s.d.; n=8 for each genotype). The Y axis represents fold change in expression with wild type levels set to 1. Two-tailed unpaired students T-test was employed (*, p<0.005; **p<0.0001). (G) β-catenin target genes known to be involved in negative feedback loop of Wnt/β-catenin pathway. (H) Other confirmed and inferred β-catenin target genes. (I–J) Transcriptional regulators (I), secreted factors and transmembrane receptors (J) known to be expressed in the DP. (K) Components of the Bmp signaling pathway expressed in the DP and Bmp signaling target genes shown to be modulated in the DP in-vitro. See also Figure S2.
While wild type and mutant follicles at P10 reveal similar and apparently normal midanagen morphologies (data not shown), analysis of BrdU incorporation in the hair bulb at P10 confirmed that deletion of β-catenin in the dermal papilla results in decreased proliferation of adjacent MPADs and their immediate progeny (Fig. 3C–F). The number of BrdU-positive cells in the hair bulb is reduced by 67% in mutant follicles.
Alterations of gene expression in DP lacking β-catenin
To identify signals that are regulated by β-catenin activity in the DP compartment, DP cells were purified from mutant and controls at P9 and real-time PCR was employed to evaluate changes in gene expression (Fig. 3G–K). Axin2 is a direct transcriptional target of the β-catenin pathway and its expression is used as an indicator of pathway activity (Jho et al., 2002). Axin2 expression in wild type DP and its 5-fold reduction in mutant DP (Fig. 3G) confirm the activity of the β-catenin signaling pathway in wild type DP cells and its effective reduction in the mutant. β-catenin signaling is often accompanied by the induction of inhibitory factors that negatively modulate the β-catenin pathway. Among these, Tle1, Nkd1, Nkd2 and Sfrp2 transcripts are also reduced in the mutant DP (Fig. 3G), confirming not only that the β-catenin pathway is active in the DP but that its activity is held in check by negative feedback. Several transcriptional regulators (Zic1, HoxA9, Alx3 and 4, Tle1) also show altered expression in mutant DP (Fig. 3I). This raises the possibility that indirect regulation by β-catenin within DP cells may also contribute to cell autonomous alteration in the expression of signaling genes.
The dermal papilla expresses components of several families of inter-cellular signaling factors known to act on follicular keratinocytes (Rendl et al., 2005). These include BMPs, FGFs, Wnts and Igfs as well as secreted proteins that modulate the activities of these signaling molecules. Fgf7, Fgf10 and Igf-1 promote keratinocyte proliferation (Barreca et al., 1992; Greco et al., 2009). Consistent with the observed reduction in matrix cell proliferation, levels of Fgf7 and Fgf10 transcripts are dramatically reduced in the mutant DP (Fig. 3H) while Igfbp5 transcripts, which encode a secreted inhibitor of IGF signaling, are increased fourfold in the absence of β-catenin in DP (Fig. 3J).
The BMP and Wnt signaling pathways are also active in matrix keratinocytes. Wnt5a is prominently expressed in the DP, but its level is not significantly different when β-catenin is deleted in these cells. In DP lacking β-catenin, Bmp4 expression is unchanged, but the level of Bmp6 transcripts, which are slightly less abundant than that of Bmp4 transcripts in wild type DP (data not shown), drops 4 fold in the mutant (Fig. 3K). Expression of the BMP inhibitor Gremlin 2 is unchanged, while Noggin is reduced 4 fold (Fig. 3H,K).
Ablation of β-catenin in the DP results in premature induction of catagen
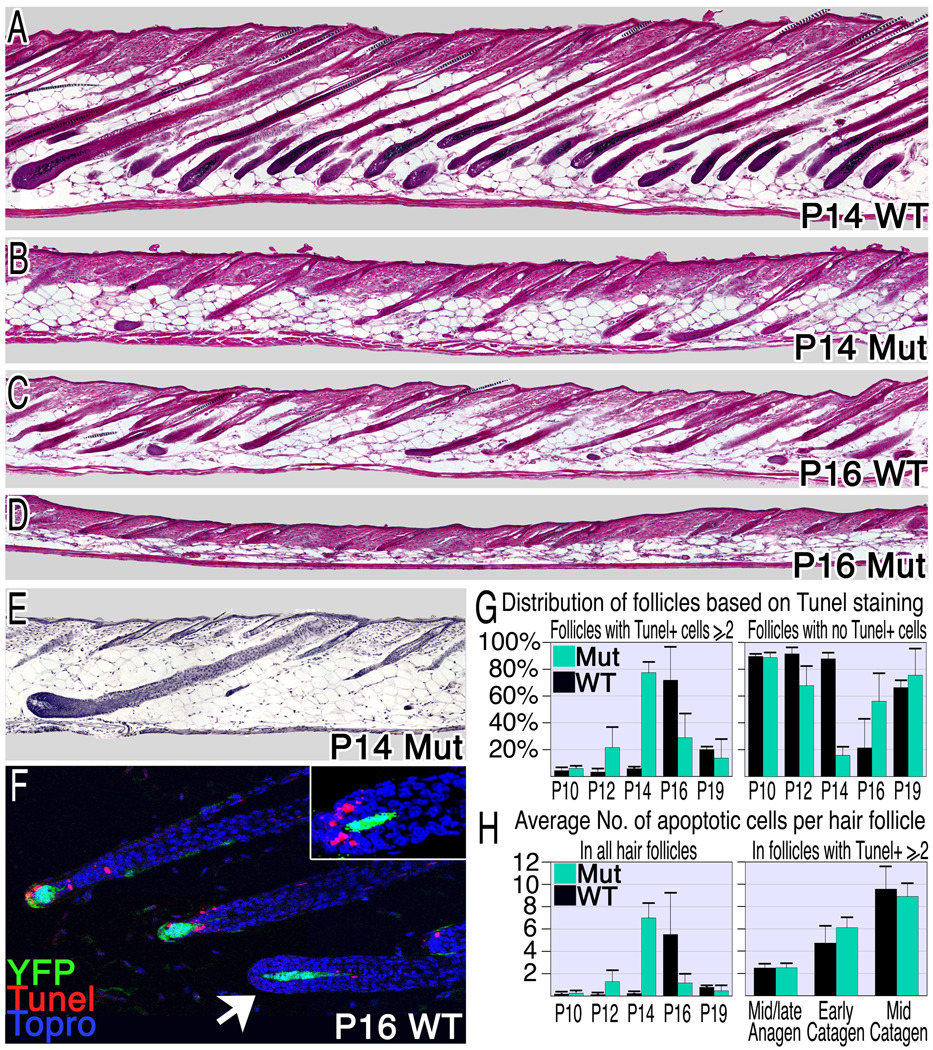
The length of the anagen phase also contributes to hair length. The duration of anagen was also decreased in the mutant mice (Fig. 4). When scored by morphology, catagen onset occurs synchronously in wild type follicles at P16 (Fig. 4C) and follicular regression is largely complete by P19 (data not shown). Catagen onset in the mutant occurs at P12 and is less synchronized. Consequently, a range of follicular morphologies from very early catagen to telogen can be found interspersed in P14 mutant skin and most follicles are in the telogen phase by P16 (Fig. 4B,D,E). The number of apoptotic cells in the hair matrix of the follicle is a reliable measure for catagen onset and progression. The distribution of apoptotic cells per hair follicle was analyzed from P10-19 (Fig. 4F–H). This analysis confirms the premature induction of catagen in the mutant.
Figure 4. Compromising β-catenin in the DP induces premature catagen.
(A–D) Hematoxylin and eosin stained sections from wild type and mutant mice reveal premature and asynchronous induction of the catagen phase in mutant mice. (E) An anagen follicle surrounded by late catagen and telogen follicles in P14 mutant skin. (F–H) TUNEL analysis of apoptosis. Follicles in catagen exhibit 2 or more apoptotic cells, while the absence of apoptotic cells in the hair matrix or bulge was used as a marker for anagen or telogen follicles respectively. (F) TUNEL staining (red) of P16 wild type skin at catagen onset reveals the rapid transition from TUNEL negative follicles (arrowhead) to those with multiple apoptotic cells/follicle. YFP expression marks the DP (green) and nuclei are blue. Inset shows a follicle morphology typical of the transition from late anagen to early catagen where abundant TUNEL staining reveals entry to the catagen phase. Many catagen follicles were observed in mutant skin at P12 (G: left), 4 days earlier than wild type. However, anagen-follicles are still predominant at this stage (G: right). (H) The average number of apoptotic cells per hair follicle per section was calculated. On the left all follicles were included and on the right only those follicles that have entered catagen were scored to eliminate the diluting effects of follicles that remain in anagen. See also Figure S3.
The appearance of late catagen and telogen follicles by P14 (Figure 4B,E) is expected if follicles that entered catagen at P12 progress through this phase at the normal rate. Although the number of apoptotic cells increases more slowly in the mutant averaged over all follicles (Fig. 4H, left), the increase in the number of apoptotic cells during the transition from anagen to mid-catagen is very similar in mutant and wild type when only those follicles that have entered catagen are scored to eliminate the diluting effects of follicles that remained in anagen (Fig 4H, right). This analysis and the distribution of follicular morphologies observed at successive time points suggest that although the initiation of the regression process is asynchronous and premature, the subsequent progression of individual follicles through the catagen phase to telogen is normal when β-catenin activation is compromised in the DP.
β-catenin activity in the DP is required for normal regeneration of the hair follicle
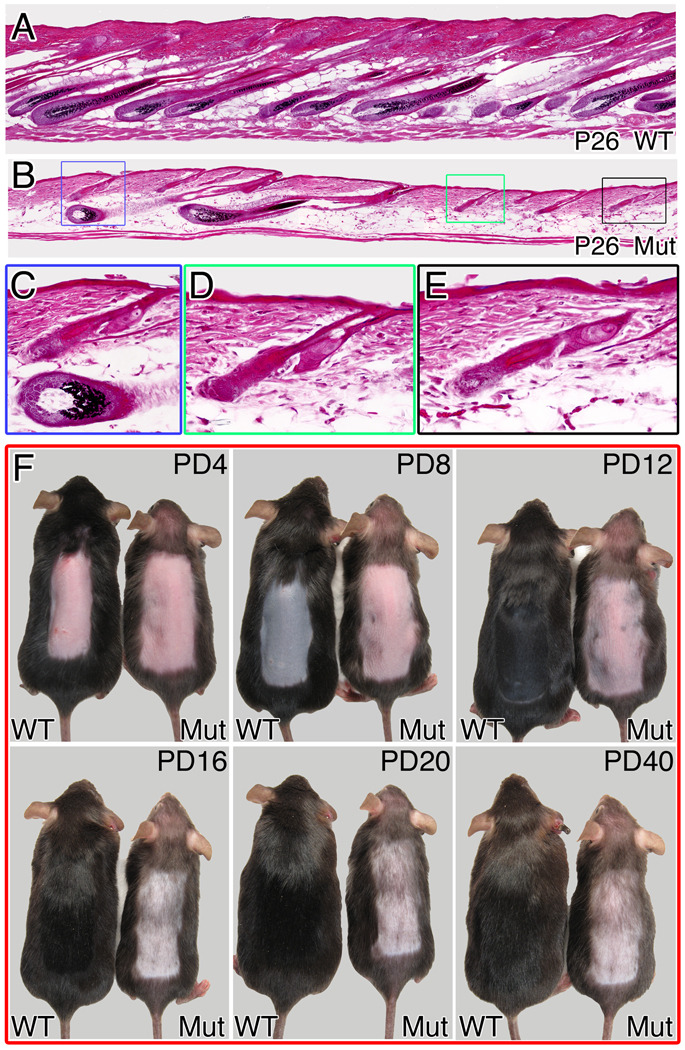
Hair follicles in mutant mice assume normal telogen morphology at the end of the premature catagen phase (Fig 4D, Fig S3). Regeneration of the lower follicle is thought to occur in response to an initiating signal from the dermal papilla (Sun et al., 1991). Fur was clipped during the first telogen phase to follow the growth of the second hair coat formed by activation of stem cells in the permanent follicle. The clipped region remains largely devoid of hairs in the mutant, with only sparse escaper hairs observed even weeks after the hair coat has completed growth in the controls (data not shown). This defect arises early in the hair cycle. Anagen re-entry is normally synchronous in unperturbed mice during the first post-natal hair cycle and fully-regenerated follicles, all at a similar stage of the hair cycle, are observed at P26 in control mice (Fig. 5A). Hair follicles in a similar advanced anagen stage are occasionally identified in mutant animals at P26, but these are widely interspersed among follicles exhibiting morphologies characteristic of telogen or an abnormal early anagen phase (Fig. 5B–E).
Figure 5. β-catenin activity in the DP is involved in follicular regeneration.
Hematoxylin and eosin staining of P26 skin sections from wild type (A) and mutant (B) unperturbed mice. A higher magnification of the colored squares in (B) identifies follicles in advanced anagen (C: lower) and early anagen (C: upper, D, E) in the same region. (F) Anagen-inducing signal was provided by depilation at P70. Pictures of the same wild type and mutant mice were taken every 4 days until hair coat was fully regenerated in the wild type. Note that in the mutant, the depilated region is largely devoid of hairs with only sparse escapers and this remains unaltered even 40 days post depilation. PD-post depilation. See also Figure S5.
The failure of regeneration implies either that the signal initiating anagen or the response to it is defective when β-catenin is deleted in the DP, or that maturation after anagen onset is blocked. Telogen follicles in the mutant at P50 are morphologically normal and immunostaining with markers for follicular bulge stem cells (CD34) and the secondary germ population (P-cadherin) confirms that both populations are present and appear normal (Fig S4). Depilation at P70 can be employed to provide an anagen-inducing signal to the follicles after an extended telogen phase. In wild type mice, a new hair coat was fully regenerated 20 days after depilation (Fig 5F, Fig S5A). However, when depilation was performed on mutant mice, no hair coat was generated even 40 days after depilation (Fig 5F, Fig S5B). In skin sections from mutant mice 12 days post-depilation, all but a few escaping follicles remained in abnormal early anagen when wild type follicles had been fully regenerated (Fig S5C). Thus defective progression through the early anagen phase downstream of an anagen-initiating signal is largely sufficient to account for the phenotypes observed in the second hair cycle. β-catenin activity in the DP is required for normal regeneration of the hair follicle in both natural and induced hair cycles.
Discussion
While tools to manipulate gene expression in keratinocytes have allowed rapid progress towards characterizing epithelial-mesenchymal interactions at the molecular level from the perspective of the epithelium in this important model system, the lack of tools to alter gene expression specifically in the DP has hampered progress towards defining the role of these cells. The Cor-cre line that expresses cre recombinase specifically in the DP after hair follicle formation provides an important tool to study the role of the mesenchyme in the hair follicle. By employing this line, we have detected a role for β-catenin in the DP of the hair follicle subsequent to its postulated role in early follicle development. When β-catenin activity is blocked, there is a rapid decrease in the proliferation of adjacent MPADs and their progeny and concomitant reduction in the growth of hair. The maintenance of the transient MPAD pool is also prematurely curtailed. There is no apparent role for β-catenin signaling in the DP during the catagen or telogen phases of the hair cycle. Although the follicular bulge stem cell compartment and secondary germ are apparently unperturbed, the regeneration of the follicle from the stem cell compartment in the permanent follicle is also abnormal in the absence of β-catenin in the DP.
β-catenin activity and the hair cycle
Despite growth effects as early as P5 and efficient deletion of β-catenin in the DP by P9, follicles nonetheless remain in anagen until P12. Thus, if β-catenin activity in the DP directly regulates the duration of anagen, either very few DP cells with an intact β-catenin gene are sufficient to sustain anagen with reduced proliferation, or there is a significant lag between deletion of the β-catenin gene and reduction in the activity of β- catenin dependent signals required to maintain the anagen phase. Alternatively, the early entry to catagen is a consequence of other alterations in the hair bulb and is not directly regulated by β-catenin activity in the DP. In this context, it is noteworthy that Nerve growth factor receptor (Ngfr) expression is increased in DP lacking β-catenin (Fig 3J). Ngfr mutant mice enter catagen later than wild type, while over-expression of Ngf in keratinocytes results in premature catagen entry (Botchkarev et al., 2000). Augmented Ngfr expression may sensitize mutant DP to a signal promoting catagen.
The synchrony of catagen entry in wild type skin may be in part due to tissue-wide signals that coordinate activity between follicles. The variable and uncoordinated entry into catagen in the mutant skin suggests that if such signals exist, either the magnitude of their effect is insufficient to overrule follicle autonomous influences on the hair cycle, or that the timing of their expression is independent of follicular progression to catagen. In the latter case, they would be expressed too late to influence follicles that had entered catagen autonomously.
Regeneration of the follicle is blocked in mutant mice. The “bulge activation” hypothesis proposes that signals from the DP activate bulge stem cells to initiate anagen (Sun et al., 1991). Depilation either provides or circumvents this initiating signal. Despite this, similarly abnormal regeneration is observed in both depilation and natural hair cycle studies. This confirms a role for β-catenin in the DP very early in the anagen phase, but precludes evaluation of its role, if any, in producing an anagen-inducing signal using the indirect score of hair cycle progression. A molecular definition of anagen initiation will be required for direct evaluation of this question.
β-catenin regulates signals from the DP that orchestrate keratinocyte behavior
The data presented here demonstrates that β-catenin is required in the DP of all hair follicle types. Furthermore, all hair types in the mutant are thinner near their distal tips. This suggests that signals from the DP are quantitatively limiting for hair growth, such that blocking production of β-catenin dependent signals in even a subset of cells within a DP impacts matrix proliferation rates. The variable length of the first segment in mutant zigzag hairs is also consistent with this hypothesis. The first zigzag segment is formed while deletion of β-catenin is likely to be variably mosaic in the DPs of different hair follicles.
Among the genes differentially expressed between mutant and wild type DP, Fgf7 and Fgf10 and Igfbp5 stand out as likely mediators of the proliferation-promoting activity of the DP during anagen. Fgf7 expression increases in the DP prior to anagen onset and exogenous Fgf7 or 10 stimulates proliferation of hair follicle keratinocytes (Greco et al., 2009). Manipulations that impair the function of the Fgf7/10 receptor in keratinocytes result in sparse thin hair (Grose et al., 2007; Petiot et al., 2003). However, no hair growth phenotype was reported for mice lacking a functional Fgf7 gene (Guo et al., 1996), and skin grafts from Fgf10 mutant mice show normal hair growth (Suzuki et al., 2000). This contrasts with the apparent quantitatively limiting nature of signals from the DP and suggests that not only functional redundancy between these Fgfs, but additional feedback mechanisms to regulate growth factor signaling from the DP would be required to explain these observations. If such feedback exists, disruption of β-catenin in the DP interrupts this regulatory loop.
Igfbp5 was increased in the mutant DP. IGF signaling regulates the size of the hair shaft in vivo and is mitogenic for keratinocytes in vitro. Overexpression of Igf1 in hair follicles increases the size of some hair shafts and results in transformation from zigzag to awl-like hairs (Schlake, 2005; Su et al., 1999; Weger and Schlake, 2005). In contrast, forced expression of Igfbp3 or Igfbp5, secreted proteins that decrease Igf1 signaling, results in thinner and slightly shorter hairs (Schlake, 2005; Weger and Schlake, 2005). The increase in Igfbp5 expression in mutant DP (Fig. 3J) may contribute to the reduced hair growth observed in the mutant.
Although the functional importance of Wnt/β-catenin signaling in anagen DP was predicted by our previous in vitro studies, they also suggested the likelihood of context dependent activity (Kishimoto et al., 2000; Shimizu and Morgan, 2004). In contrast to the result reported here in vivo, in vitro studies using alternative enrichment and culture conditions for DP cells reported decreased expression of Fgf7 and Fgf10 when the β-catenin pathway was activated (Rendl et al., 2008). These observations emphasize the importance of performing these studies in the context of an otherwise intact signaling milieu in vivo.
The gene expression analysis presents clear evidence that β-catenin mediated transcriptional activity plays a critical role in DP cell function. While β-catenin also functions in cell adhesion and adhesion defects could in principle contribute to the phenotype, the morphology of the DP is not discernibly different between wild type and mutant at any time in the hair cycle, including catagen and telogen when the DP is no longer constrained within the confines of the hair bulb and yet forms a compact ball of cells in intimate contact with the secondary germ (Fig S3). Severe adhesion defects are often accompanied by apoptosis (Frisch and Francis, 1994). However, most follicles in both wild type and mutant mice are devoid of apoptotic cells in the DP throughout the hair cycle including the regression phase (Fig S3A–B), and the rare apoptotic cells in some DPs during the catagen phase are observed in similar frequency in wild type and mutant (data not shown).
Signals acting on the DP
These results suggest that Wnt signaling to the DP regulates its activity. Several Wnts expressed in the hair matrix or differentiating keratinocytes of the proximal follicle have been shown to maintain the inductive properties of enriched dermal papilla cell preparations isolated from anagen skin and cultured in vitro. In contrast, the only Wnt known to be expressed in the DP, Wnt5a, was ineffective in this assay (Kishimoto et al., 2000; Shimizu and Morgan, 2004). This suggests that β-catenin activity in the DP is part of a reciprocal signaling loop whereby Wnts expressed in keratinocytes induce activation of β-catenin in the DP to generate the signals that maintain MPAD activity in the anagen phase.
The DP as niche
The regeneration of the follicle at the end of the telogen phase depends on a stem cell pool in the permanent portion of the follicle. In the prevalent model, signals from the DP activate stem cells resident in the follicular bulge to initiate the production of TA cells that then regenerate the follicle (Sun et al., 1991). In this model, the DP may act as niche for follicular bulge stem cells in the more abstract use of this term by providing a signaling environment conducive to their maintenance and regulating their activation, but it is not a physical niche for these cells. Even during the telogen phase when the DP is closest to the follicular bulge, it remains physically separated from it (Fig S4). In contrast, the DP provides a physical niche for the secondary germ and the MPAD progenitor population that arises from it. At the onset of anagen, a subset of the secondary germ closest to the DP undergoes rapid proliferation and adopts the characteristics of the MPADs that reconstitute the hair bulb. Throughout the anagen phase, physical apposition to DP is one defining character for this progenitor population that sustains production of the HS and IRS. The work presented here demonstrates that trophic signals regulating MPAD proliferation and maintenance are provided by the DP niche and provides insight into the more general mechanisms that sustain and regulate stem and progenitor cell populations in the adult.
Experimental Procedures
Mice
ROSA26 YFP reporter (r26YFP), β-actin cre, and conditional knock-out allele of β-catenin (Ctnnb1Flox/Flox) strains were obtained from F Costantini (Columbia), G Martin (UCSF), and Jackson Labs respectively. The DP-specific Cre (Cor-cre) mouse was generated by inserting sequences of Cre recombinase into the Corin locus and will be described in detail elsewhere. A knock-out allele of β-catenin (Ctnnb1Del/+) was generated by crossing the floxed allele (Ctnnb1Flox/Flox) to the β-actin cre line.
TUNEL and BrdU analysis
For TUNEL analysis, the In situ cell death detection kit was used (Roche). For BrdU analysis, mice at P10 were injected i.p. with BrdU (100ug/g body weight) (Molecular Probes). 30 min later, mid-dorsal skins were harvested, fresh-frozen, embedded in OCT and immunostained with anti-BrdU antibody conjugated with Alexa Fluor 546 (Molecular Probes).
Cell sorting, genotyping and real-time PCR
YFP-positive cells were FACS sorted from whole back skins twice. Purities of about 75% and 95% were obtained after the first and second sort respectively. To prepare RNA or genomic DNA from sorted cells, the “RNeasy Plus Micro kit” (Qiagen) and “QAIamp DNA investigator kit” (Qiagen) were used respectively. For genotyping, YFP-positive and YFP-negative cells were sorted from the same individual mouse (See SI). For gene expression analysis, 8 P9 mice per genotype from 4 different litters were used individually to sort for DP-YFP positive cells. To prepare cDNA, total RNA was reverse transcribed using random hexamer primers and SuperScript III First-Strand synthesis system (Invitrogen). For real-time PCR, primer pairs from SuperArray were employed and differences were quantified based on the ΔΔCt method.
Supplementary Material
Acknowledgments
We thank Eleanor Wu and Ying Zheng for technical assistance and F. Costantini and G. Martin for providing mice. This work was supported by grants from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01AR055256, B.A.M.) and Shiseido, Ltd. (B.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Barreca A, De Luca M, Del Monte P, Bondanza S, Damonte G, Cariola G, Di Marco E, Giordano G, Cancedda R, Minuto F. In vitro paracrine regulation of human keratinocyte growth by fibroblast-derived insulin-like growth factors. J Cell Physiol. 1992;151:262–268. doi: 10.1002/jcp.1041510207. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Albers KM, Chen LH, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB J. 2000;14:1931–1942. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R, Fantl V, Werner S, Chioni AM, Jarosz M, Rudling R, Cross B, Hart IR, Dickson C. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. Embo J. 2007;26:1268–1278. doi: 10.1038/sj.emboj.7601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Rosenquist T, Gotz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Wright EA. Inductive capacity of irradiated dermal papillae. Nature. 1977;265:733–734. doi: 10.1038/265733a0. [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol. 1993;101:584–590. doi: 10.1111/1523-1747.ep12366039. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Noramly S, Freeman A, Morgan BA. beta-catenin signaling can initiate feather bud development. Development. 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- Petiot A, Conti FJ, Grose R, Revest JM, Hodivala-Dilke KM, Dickson C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development. 2003;130:5493–5501. doi: 10.1242/dev.00788. [DOI] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlake T. Segmental Igfbp5 expression is specifically associated with the bent structure of zigzag hairs. Mech Dev. 2005;122:988–997. doi: 10.1016/j.mod.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Morgan BA. Wnt signaling through the beta-catenin pathway is sufficient to maintain, but not restore, anagen-phase characteristics of dermal papilla cells. J Invest Dermatol. 2004;122:239–245. doi: 10.1046/j.0022-202X.2004.22224.x. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HY, Hickford JG, The PH, Hill AM, Frampton CM, Bickerstaffe R. Increased vibrissa growth in transgenic mice expressing insulin-like growth factor 1. J Invest Dermatol. 1999;112:245–248. doi: 10.1046/j.1523-1747.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- Sun TT, Cotsarelis G, Lavker RM. Hair follicular stem cells: the bulge-activation hypothesis. J Invest Dermatol. 1991;96:77S–78S. doi: 10.1111/1523-1747.ep12471959. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yamanishi K, Mori O, Kamikawa M, Andersen B, Kato S, Toyoda T, Yamada G. Defective terminal differentiation and hypoplasia of the epidermis in mice lacking the Fgf10 gene. FEBS Lett. 2000;481:53–56. doi: 10.1016/s0014-5793(00)01968-2. [DOI] [PubMed] [Google Scholar]
- Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger N, Schlake T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005;125:873–882. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161–2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, Birchmeier W, Paus R, Piccolo S, Mikkola ML, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.