Abstract
Objective
Applied electrical current flows preferentially along rather than across muscle fibers, a characteristic called anisotropy. In this study, we investigate the alteration in muscle anisotropy after denervation.
Methods
Eight adult male rats underwent sciatic nerve crush and the gastrocnemius was harvested from 1 to 2.5 weeks later. Muscle from 12 additional healthy rats was also obtained. Multifrequency electrical impedance measurements were made on the tissue and its conductivity and relative permittivity (i.e., its polarizability) calculated. Anisotropy of the tissue was determined by calculating conductivity and permittivity differences, subtracting transverse from longitudinal values. Muscle fiber and blood vessel quantification were also performed.
Results
The mean conductivity difference for sciatic crush animals was higher (p <0.05) than for the healthy animals across the frequency spectrum, due to a greater increase in longitudinal conductivity than in transverse conductivity. For example, at 10 kHz, the conductivity difference was 0.15 S/m for healthy animals and 0.29 S/m for post-crush animals. Relative permittivity difference values, however, were similar between groups. There was a strong correlation of conductivity anisotropy to muscle fiber size but not to blood vessel area.
Conclusions
Anisotropy of muscle conductivity increases markedly after subacute denervation injury.
Significance
This alteration in anisotropy has direct relevance to the clinical application of electrical impedance myography. We also speculate that it may impact other forms of diagnostic testing, including needle electromyography and magnetic resonance imaging.
Keywords: impedance, denervation, anisotropy, conductivity, muscle
INTRODUCTION
Skeletal muscle is highly anisotropic with applied electrical currents flowing more easily along muscle fibers than across them. This unique property of skeletal muscle was first appreciated in the early 1960s in studies assessing excised animal tissue (Burger and van Dongen, 1961; Rush, 1962; Rush et al., 1963; Fatt, 1964). In the 1990s, Shiffman and Aaron identified that muscle anisotropy could be assessed non-invasively through the application of surface impedance methods (Aaron et al., 1997). More recent work has suggested that the anisotropic characteristics of the tissue may be effective in distinguishing neurogenic from myopathic injury and could be used as an indicator of disease progression (Garmirian et al., 2009). However, the mechanisms underlying anisotropy and its alteration in diseased states remain poorly understood. In addition, how this property of muscle may impact standard electrophysiologic testing and magnetic resonance imaging (MRI) has not been explored.
As part of a larger research effort focused on the application of electrical impedance techniques to rat models of neuromuscular disease, we have been studying the effects of neurogenic injury on the electrical properties of skeletal muscle (Nie et al., 2006; Ahad and Rutkove, 2009). Although most of this work has focused on surface measurements, we can also study the electrical properties of the tissue directly, after animal sacrifice. We recently assessed the effect of acute denervation injury on the electrical properties of skeletal muscle, demonstrating consistent changes in its conductivity (Ahad et al., 2009) 1 week to 2.5 weeks after sciatic nerve crush. In this supplemental analysis, we specifically assess the resulting alteration in the tissue’s anisotropic characteristics and the possible mechanisms underlying this change. Moreover, since these characteristics impact the propagation of electrical signals through tissue and are indicative of changes in intra- and extra-cellular water content, we also discuss their potential relevance to more standard clinical diagnostic testing, including needle electromyography and MRI.
METHODS
Rats
All animal studies were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center, Boston, MA. A total of 20 rats were studied: 12 control rats and 8 rats after sciatic crush. All were adult male Wistar Rats, weighing between 420 and 480 gm, obtained from Charles River Laboratories, Wilmington, MA and were acclimated for 48 hours after arrival at our facility before any measurements were obtained. In the 8 sciatic crush animals, an incision was made proximally in the thigh and the sciatic nerve exposed, being careful not to disturb surrounding tissues, with the animal anesthetized under isoflurane. The nerve was then crushed using a jeweler’s forceps by applying pressure for approximately 30 seconds. The incision was then sutured closed and the animals allowed to recover until sacrifice, 1 to 2.5 weeks later (this time course, in part, a product of the complexity of the procedures, with 1 animal being studied per day).
Impedance Measurement System
Impedance measurements were made using a lock in amplifier, Signal Recovery Model 7280, Advanced Measurement Technology Inc., Oak Ridge, TN coupled with a very low capacitance active probe (Model 1103 of Tektronix, Beaverton, OR) as previously described (Esper et al., 2006).
Sacrifice and measurements of the electrical constant
After obtaining surface impedance measurements (reported elsewhere), the entire gastrocnemius muscle was immediately excised at its proximal extent just below the knee and distally by cutting the gastrocnemius tendon at its insertion into the calcaneus. The animal was then immediately killed with anesthetic overdose. From this muscle, an approximately 1cm × 1cm square of about 0.4cm height was further excised with a scalpel.
This tissue was immediately placed into a 1cm × 1cm × 2cm plastic measuring cell between two stainless steel current electrodes with the fiber orientation perpendicular to the metal electrodes (providing longitudinal measurements), similar to the approach of Baumann et al (Baumann et al., 1997), as previously described (Ahad et al., 2009). After longitudinal measurements were performed, the muscle was rotated 90 degrees such that the muscle fibers were parallel to the stainless steel plates and measurements repeated (transverse measurements). To ensure consistent temperature, the entire cell was maintained at 37°C through the use of a heating pad surrounding the cell.
Quantitative histology
Measurements were performed on 8 normal animals and all of the crush animals. After impedance measurements were completed as above, the muscle tissue was immediately frozen in isopentane cooled in liquid nitrogen for histological analysis (see below) and stored at −80°C until ready for use. The frozen tissue was cut into 10 μm thick sections and stained with Hematoxylin and Eosin. Standard non-biased, blinded stereological measurements (Mayhew et al., 1997) were made on a Zeiss Axiophot microscope with a LUDL motorized stage interfaced with a Dell Optiflex 380 computer running Stereo Investigator software (MBF Biosciences, Inc, Williston, VT) as previously described (Ahad et al., 2009). For each slide, a histogram of cell size (in cross-sectional area and diameter) is obtained.
These sections were also used to quantify vascular lumen area. Images were captured at 40X from one section of gastrocnemius muscle per animal and all vessels were used for quantification using the public domain NIH image program (http://rsb.info.nih.gov/nih-image/), measuring the entire area inside the endothelial cell layer.
Data Analysis
Calculation of the electrical constants, the conductivity and relative permittivity, was performed as previously described (Ahad et al., 2009). The conductivity is a measure of the inherent ability of electrical current to flow freely through the muscle. The relative permittivity is a measure of the inherent polarizability of the tissue. Anisotropy for these 2 parameters at each frequency was assessed by defining a conductivity difference (longitudinal – transverse conductivity) and a relative permittivity difference (longitudinal-transverse relative permittivity). Standard two-group methods (Mann-Whitney and unpaired t-tests) were used for 2 group comparisons. Spearman rank correlation was used to assess the relationship between anisotropy values and muscle fiber and blood vessel area. All analyses were two-tailed, with alpha = 0.05 and were performing using SPSS Statistical Software, Version 17.0 (SPSS, Inc, Chicago, IL).
RESULTS
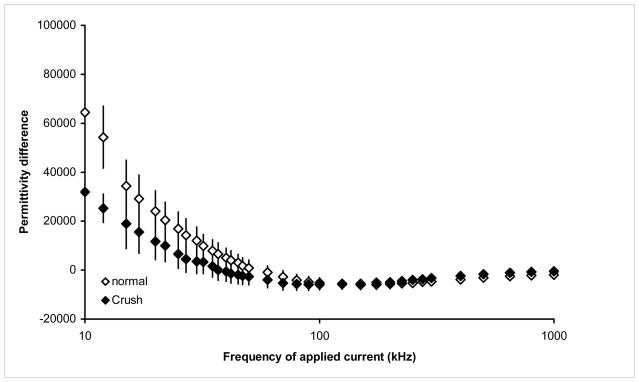
Figures 1 and 2 provide the overall results for the conductivity and relative permittivity differences for the healthy animals and the animals post-crush. Tables 1 and 2 provide the corresponding values for selected frequencies, including the raw longitudinal and transverse data from which the anisotropy values were calculated. Technical problems interfered with the measurement of one of the 12 healthy animal’s tissue and thus this data is excluded from analysis. Similarly, in the crush animals, conductivity measurements were completed in all 8, but the relative permittivity measurements were corrupted in one animal and thus this data is excluded. As can be seen, there is a significant elevation in the conductivity difference at most frequencies, due to elevations in both the longitudinal and transverse conductivity, but with the longitudinal increasing more than the transverse. For example at 100 kHz, the conductivity difference is 0.22 S/m on average for the healthy animals and 0.34 S/m for the sciatic crush, representing a 58% increase in the anisotropy of the tissue. At 10 kHz, the difference is even more substantial, nearly doubling from 0.148 S/m in the healthy animals to 0.29 S/m in the crush. Although there is some subtle alteration in the relative permittivity difference across the frequency spectrum these are non-significant at all the same frequencies (see Table 1). We also note that there were no significant differences between those animals measured earlier as compared to those measured later, and thus we performed only a group analysis of all the data obtained.
Figure 1.
Conductivity differences in normal and sciatic crush animals from 2 kHz to 1 MHz, mean ± standard error
Figure 2.
Relative permittivity differences in normal and sciatic crush animals from 2 kHz to 1 MHz, mean ± standard error
Table 1.
Summary of changes in conductivity* at selected frequencies
| Frequency | 10 kHz | 50kHz | 100kHz | 300kHz | 500kHz | 1MHz |
|---|---|---|---|---|---|---|
| Healthy Raw Values (Longitudinal, Transverse) | 0.28, 0.09 | 0.38, 0.15 | 0.45, 0.22 | 0.58, 0.39 | 0.64, 0.46 | 0.70, 0.52 |
| Conductivity Difference | 0.17 ± 0.022 | 0.22±0.025 | 0.22±0.028 | 0.16±0.035 | 0.14±0.032 | 0.086±0.044 |
| Sciatic Crush Raw values (Longitudinal, Transverse) | 0.45, 0.15 | 0.55, 0.21 | 0.62, 0.28 | 0.75, 0.47 | 0.82, 0.56 | 0.91, 0.67 |
| Conductivity difference | 0.31 ± 0.033 | 0.34 ±0.033 | 0.34±0.033 | 0.29±0.031 | 0.26±0.0.30 | 0.24±0.030 |
| p value | 0.006 | 0.010 | 0.021 | 0.048 | 0.069 | 0.048 |
Raw conductivity values and conductivity differences are all given in Siemens/meter
Table 2.
Summary of changes in relative permittivity* at selected frequencies
| Frequency | 10 kHz | 50kHz | 100kHz | 300kHz | 500kHz | 1MHz |
|---|---|---|---|---|---|---|
| Healthy Raw Values (Longitudinal, Transverse) | 136900, 77,700 | 47400, 47600 | 29,200, 35,100 | 12400, 17200 | 8400, 11400 | 5300, 7000 |
| Permittivity Difference | 64400 ± 6470 | 890 ±3380 | −5170±2180 | −4700±1140 | −3140±1090 | −1880±1140 |
| Sciatic Crush Raw Values (Longitudinal, Transverse) | 111,000,78700 | 45,800, 48500 | 29,900, 35,800 | 14804,18,000 | 10500,12,200 | 7100, 7600 |
| Permittivity Difference | 31950 ± 13800 | −2680±3270 | −5870±2090 | −3220±1860 | −1662±1770 | −468±1570 |
| p value | 0.55 | 0.66 | 0.93 | 0.25 | 0.48 | 0.29 |
Raw relative permittivity values and permittivity differences are unitless.
Mean muscle fiber area, as expected, was significantly lower in the crush group (2180 ± 68 μm2) as compared to the control group (3262 ± 144μm2, p = 0.01). Blood vessel lumen area, rather than showing the expected increase in the crush animals, showed instead a reduction, with a mean lumen area of 996 ± 259 μm2 in the control animals and 710 ± 205 μm2 in the crush animals (p = 0.022). Conductivity difference had a moderate strength correlation to muscle fiber area (r = 0.56, p = 0.030). However, no relationship was identified between vessel lumen area and the conductivity difference (r = 0.030, NS).
DISCUSSION
These findings demonstrate that the electrical anisotropy of skeletal muscle increases significantly within 2.5 weeks of neurogenic injury, due to a greater elevation in longitudinal than in transverse conductivity. Changes in relative permittivity, by contrast, are non-significant. A reason for the absence of changes in relative permittivity may be related to the fact that calculation of relative permittivity depends upon the measured reactance of the tissue, which is about one-tenth the magnitude of the resistance, from which the conductivity is calculated and thus more subject to random noise. It is possible that with considerably larger numbers of animals or improved measurement technique a consistent difference in relative permittivity would also have been identified.
In regard to electrical impedance myography testing, this alteration in anisotropy could help explain changes in surface electrical anisotropy measurements we have observed in ALS patients. These patients have demonstrated an elevated surface anisotropy (Garmirian et al., 2009) possibly correlated to the changes observed here. Such changes have the potential to distinguish between neurogenic disease and myopathic disease and could also be potentially utilized to help localize neurogenic injury to specific muscles.
To effectively explain these changes in the conductivity values in the longitudinal and transverse directions and their alteration after denervation injury, it is useful to consider what confers muscle with electrical anisotropy in the first place. When applied longitudinally, travels in a relatively straight line, through the extracellular space immediately along the muscle fibers. When applied transversely, the current must take a circuitous path around the fibers, making for a longer total distance over which it must travel, and thus a cumulatively higher resistance and lower conductivity. This simple model is valid at low frequencies of applied current but is less so at higher frequencies, since as frequency increases, the current can begin to penetrate the sarcolemma and pass through the fibers directly (Grimnes and Martinsen, 2000). At those higher frequencies, the proportion of intracellular free water may start to play a more important role.
Thus, a combination of pathological effects likely explains our findings after denervation. Increasing extracellular water leads to elevations in conductivity in both longitudinal and transverse directions, with the longitudinal being more affected since the relative circuitous path for extracellular current flow in the transverse direction will persist. At higher frequencies, the effect of extracellular water continues to predominate, but changes in intracellular free water may also begin to play a role.
Although speculative, we believe that MRI studies evaluating changes in muscle after denervating injury support this basic idea. After denervation, muscle typically shows an elevation in T2 signal (Kikuchi et al., 2003; Bendszus et al., 2004; Wessig et al., 2004; Zhang et al., 2008; Polak et al., 1988). Polak et al (Polak et al., 1988) first described this increased T2 signal and ascribed it to the presence of increased extracellular water, possibly related to increased muscle membrane permeability. These changes can be observed within 10 days of nerve injury (Zhang et al., 2008). Others have suggested that changes in muscle vascular perfusion may account for this change (Wessig et al., 2004), though others have not found evidence for this (Zhang et al., 2008). Earlier pathological work has also identified a shift in free water from the intra- to extra-cellular compartments (Kotsias et al., 1985). Perhaps, the most comprehensive explanation for both the MRI findings is that suggested by Kamath and colleagues’ that alterations in the internal structure of muscle fibers along with elevations in extracellular free water contribute to these findings (Kamath et al., 2008). An intracellular aspect to this finding is appealing since it is well established that shortly after denervation injury, there is a rapid breakdown of intracellular protein components (Furuno et al., 1990; Jagoe et al., 2004).
Although we did not perform MRI measurements as part of this study, we speculate that our data are consistent with this overall view. At lower frequencies of applied current, conductivity will increase mainly due to increased extracellular water. At higher frequencies, although the extracellular component may still predominate, increased intracellular water may also start to play an important role. Like Zhang et al (Zhang et al., 2008), we did not find pathological evidence to support a vascular origin for these changes. Moreover, it should be noted that although the degree of anisotropy changes correlates with muscle fiber size, this does not imply direct causation. Reduction in muscle fiber diameter quickly follows after denervation and any one of a number of processes can contribute to the observed changes in conductivity. Indeed, reductions in muscle fiber diameter may simply represent a marker for changes in the intra- and extra-cellular compartments.
Another interesting speculation based on the finding of increased conductivity in general and an increased conductivity difference bears upon both standard electrophysiologic studies. Since conductivity actually increases in both longitudinal and transverse directions, denervated muscles may, as a whole, be more capable of transmitting weaker (ie, lower amplitude) electrical potentials, whether they be in the form of applied current or natively generated. For example, denervated muscle may be conveniently “primed” for the volume conduction of distantly generated potentials, including fibrillation potentials. Although we could not easily evaluate lower frequencies where these findings would be most meaningful (e.g., a fibrillation potential of 1 ms duration has a frequency of 1kHz) due to limitations in our measurement techniques, the conductivity changes are present across the frequency spectrum, and there is no reason that similar alterations are not to be found at these lower frequencies as well. The alteration in anisotropy itself could impact these measurements in even more complex ways, by conferring a direction-dependence to this effect. These results thus help support the general recommendation to sample multiple areas of muscle with a needle electrode since abnormal spontaneous activity (e.g. fibrillation potentials) may not be evenly distributed (Daube and Rubin, 2009). Although the patchiness of fibrillation potentials is generally considered simply a result of a lesion being incomplete, the lower conductivity of non-denervated fibers could actually serve as a barrier to the volume conduction of distant potentials. Thus, a more specific, practical result from this analysis is the recognition of the importance of traversing as many muscle fibers as possible with a needle electrode since volume conduction of these signals in the longitudinal direction will be better than in the transverse.
In summary, muscle shows a significant increase in electrical conductivity as a whole and an elevation in its anisotropy subacutely after sciatic nerve crush. These observations suggest that electrical impedance data can be used as a novel means of exploring mechanisms underlying the pathologic signatures of other diagnostic modalities. Similarly, these results also assist in formulating enhanced analysis methods for the interpretation of surface electrical impedance data.
Acknowledgments
This work was funded by grant R01055099 from the National Institutes of Health/National Institute of Neurological Diseases and Stroke.
The authors appreciate the assistance of Mary Hochman, MD, and Jim Wu, MD in exploring the mechanisms underlying signal change in MRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron R, Huang M, Shiffman CA. Anisotropy of human muscle via non-invasive impedance measurements. Phys Med Biol. 1997;42:1245–62. doi: 10.1088/0031-9155/42/7/002. [DOI] [PubMed] [Google Scholar]
- Ahad M, Rutkove SB. Electrical impedance myography at 50 kHz in the rat: technique, reproducibility, and the effects of sciatic injury and recovery. Clinical Neurophys. 2009;120:1534–8. doi: 10.1016/j.clinph.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahad MA, Fogerson PM, Rosen GD, Narayanswami P, Rutkove SB. Electrical characteristics of rat skeletal muscle in immaturity, adulthood, and after sciatic nerve injury and their relation to muscle fiber size. Physiol Meas. 2009;30:1415–1427. doi: 10.1088/0967-3334/30/12/009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SB, Wozny DR, Kelly SK, Meno FM. The electrical conductivity of human cerebrospinal fluid at body temperature. IEEE Trans Biomed Eng. 1997;44:220–3. doi: 10.1109/10.554770. [DOI] [PubMed] [Google Scholar]
- Bendszus M, Wessig C, Solymosi L, Reiners K, Koltzenburg M. MRI of peripheral nerve degeneration and regeneration: correlation with electrophysiology and histology. Exp Neurol. 2004;188:171–7. doi: 10.1016/j.expneurol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Burger HC, van Dongen R. Specific electrical resistance of body tissues. Phys Med Biol. 1961;5:431–447. doi: 10.1088/0031-9155/5/4/304. [DOI] [PubMed] [Google Scholar]
- Daube JR, Rubin DI. Needle electromyography. Muscle Nerve. 2009;39:244–70. doi: 10.1002/mus.21180. [DOI] [PubMed] [Google Scholar]
- Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34:595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- Fatt P. An analysis of the transverse electrical impedance of striated muscle. Proc R Soc Lond B Biol Sci. 1964;159:606–51. doi: 10.1098/rspb.1964.0023. [DOI] [PubMed] [Google Scholar]
- Furuno K, Goodman MN, Goldberg AL. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem. 1990;265:8550–7. [PubMed] [Google Scholar]
- Garmirian LP, Chin AB, Rutkove SB. Discriminating neurogenic from myopathic disease via measurement of muscle anisotropy. Muscle Nerve. 2009;39:16–24. doi: 10.1002/mus.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimnes S, Martinsen OG. Bioimpedance and Bioelectricity Basics. London: Academic press; 2000. [Google Scholar]
- Jagoe RT, Tawa NE, Goldberg AL. Protein and amino acid metabolism in muscle. In: Engel AG, editor. Myology. New York: McGraw-Hill; 2004. p. 555. [Google Scholar]
- Kamath S, Venkatanarasimha N, Walsh MA, Hughes PM. MRI appearance of muscle denervation. Skeletal Radiol. 2008;37:397–404. doi: 10.1007/s00256-007-0409-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Nakamura T, Takayama S, Horiuchi Y, Toyama Y. MR imaging in the diagnosis of denervated and reinnervated skeletal muscles: experimental study in rats. Radiology. 2003;229:861–7. doi: 10.1148/radiol.2293020904. [DOI] [PubMed] [Google Scholar]
- Kotsias BA, Muchnik S, Arrizurieta EE, Losavio AS, Sosa M. Influence of trophic substances in the regulation of resting membrane potential and ionic concentration in skeletal muscle. Exp Neurol. 1985;88:56–67. doi: 10.1016/0014-4886(85)90113-x. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Pharaoh A, Austin A, Fagan DG. Stereological estimates of nuclear number in human ventricular cardiomyocytes before and after birth obtained using physical disectors. J Anat. 1997;191 (Pt 1):107–15. doi: 10.1046/j.1469-7580.1997.19110107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie R, Chin AB, Lee KS, Sunmonu NA, Rutkove SB. Electrical impedance myography: transitioning from human to animal studies. Clin Neurophys. 2006;117:1844–9. doi: 10.1016/j.clinph.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Polak JF, Jolesz FA, Adams DF. Magnetic resonance imaging of skeletal muscle. Prolongation of T1 and T2 subsequent to denervation. Invest Radiol. 1988;23:365–9. doi: 10.1097/00004424-198805000-00007. [DOI] [PubMed] [Google Scholar]
- Rush S. Methods of measuring the resistivities of anisotropic conducting media. J Res J Res NBS. 1962;66C:217–22. [Google Scholar]
- Rush S, Abildskov JA, McFee R. Resistivity of body tissues at low frequencies. Circ Res. 1963;12:40–50. doi: 10.1161/01.res.12.1.40. [DOI] [PubMed] [Google Scholar]
- Wessig C, Koltzenburg M, Reiners K, Solymosi L, Bendszus M. Muscle magnetic resonance imaging of denervation and reinnervation: correlation with electrophysiology and histology. Exp Neurol. 2004;185:254–61. doi: 10.1016/j.expneurol.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang G, Morrison B, Mori S, Sheikh KA. Magnetic resonance imaging of mouse skeletal muscle to measure denervation atrophy. Exp Neurol. 2008;212:448–57. doi: 10.1016/j.expneurol.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]