Abstract
Background
Mitogen-activated protein (MAP) kinases are well-known molecules that play key roles in mechanical stress signals during skeletal development. To test our hypothesis that the synovium in frozen shoulders is induced by MAP kinases, immunohistochemical analyses for detecting expression and signal transduction of MAP kinases were performed in synovial tissue obtained from the rotator interval (RI) in frozen shoulders.
Methods
Synovial tissues were examined from 10 frozen shoulder patients with a mean age of 55.4 years (46–62 years). Synovial tissues between the long head of the biceps tendon (LHB) and the RI in frozen shoulders were stained with hematoxylin and eosin (H&E) and then examined with immunohistochemical staining. Extracellular signal-regulated (ERK), the Jun N-terminal (JNK), and p38 mitogen-activated protein (MAP) kinases, nuclear factor κB (NF-κB), p50, CD29 (β1-integrin), matrix metalloproteinase (MMP)-3, interleukin-6 (IL-6), CD56, CD68, S-100, and vascular endothelial growth factor (VEGF) were analyzed to detect expression patterns.
Results
H&E showed vascular proliferation with fibrin and fibrous tissue in the synovium of frozen shoulders. ERK was expressed in the epithelial cells of vascular tissue, and JNK was expressed strongly in the interstitial cells around vascular tissue; p38 MAPK was not expressed. NF-κB was expressed in vascular tissue, and IL-6 was expressed around vascular tissue. CD29 (β1-integrin) was expressed in vascular tissue and in superficial cells of synovial tissue. MMP-3 and VEGF were expressed on the surface layer of synovial tissue and vascular tissue, and CD68 was expressed on the surface layer. Nerve-related proteins, CD56 and S-100, were expressed weakly.
Conclusions
Mechanical stress on the LHB and RI in the shoulder may induce ERK and JNK to express NF-κB by CD29 to develop capsule contracture, producing MMP-3, IL-6, and VEGF.
Introduction
Duplay first reported frozen shoulder nearly 150 years ago, but the cause of this disease remains unclear.1 In 1934, Codman described the diagnostic criteria for frozen shoulder, which are the same today.2 Pain in the shoulder comes on slowly and is felt in the deltoid, one cannot sleep on the affected side, and there is a little local tenderness. The range of active and passive motions are restricted in terms of upward movement and rotation.
Studies of cadavers and observations during open and arthroscopic surgery have identified the anatomical area predominantly involved in frozen shoulder to be the rotator interval, which contains the coracohumeral (CH) ligament.3,4 It has been reported that the CH ligament has shown inflammatory, fibrotic, and proliferative myelofibrotic changes.5–7 This process may be mediated by inflammatory cytokine production.8 However, it is not known why the CH ligament undergoes such fibrotic changes and developments around the joint capsule in the shoulder. We hypothesize that mechanical stress induces fibrosis-related molecules to develop contracture in the shoulder joint, not only in the CH ligament but in other capsular ligaments as well.
It has been reported that mechanical stress induces mitogen-activated protein (MAP) kinases through cell adhesion molecules, such as β1-integrin.9 MAP kinases can induce cytokine cascades, such as tumor necrosis factor-α (TNFα) or interleukin-6 (IL-6) expression, which increases the proliferation of fibroblasts.9 The aim of our study was to identify the key cells that signal the molecules of MAP kinases involved in the pathology of frozen shoulder, taking advantage of previously unused immunohistochemical techniques and antibodies to induce mechanical stress molecules. We hope that having this information may lead to the development of a new medical treatment to inhibit the progression of frozen shoulder.
Patients and methods
Patients
Ten consecutive frozen shoulder patients with an average age of 55.4 years (46–62 years), seven men and three women, underwent arthroscopic capsular release. Preoperative treatment for frozen shoulder included range of motion (ROM) exercising and/or hyaluronic acid intraarticular injections. There was no acromial deformity with spur formation in any of the patients. All patients were right-handed and suffered from either frozen right shoulders (four patients) or frozen left shoulders (six patients). Pain was controlled by non-steroidal antiinflammatory drugs (NSAIDs) before surgery.
The criteria for inclusion in this study were severe night pain, no improvement of less than 90° flexion and 0° external rotation, and poor responsiveness to ROM exercise for at least 6 months prior to surgery. Exclusion criteria were no complete rotator cuff tear, no acromioclavicular subluxation, and no biceps tendon rupture on magnetic resonance imaging (MRI) scans.
The average Japanese Orthopaedic Association (JOA) score before surgery, 42.8 (40–47), improved to an average of 87.6 (75–94) 1 month after surgery. Partial-thickness tears were found by arthroscopy at the bursal side of the cuff in six patients, whereas no partial tears were found on the glenohumeral joint side in any of the patients. All patients gave informed consent for this study.
Arthroscopic capsular release
After placing the patient in the beach-chair position under general anesthesia, the shoulder was manipulated to assess the ROM in flexion, external rotation at 0° abduction, external rotation at 90° abduction, and internal rotation at 90° abduction. After introducing a 4-mm arthroscope through the standard posterior portal and performing an initial diagnostic arthroscopy, we created an anterior portal just superior to the subscapularis tendon using the outside-in technique to facilitate maneuvers by instruments such as shavers and a radiofrequency instrument (VAPR; Mitek, Norwood, MA, USA). Next, we investigated how persistently the long head of the biceps tendon (LHB) adhered to the interval intrashoulder joint. After arthroscopically observing the joint, we moved the scope into the subacromial space via a lateral and anterolateral portal, shaved the synovium in the subacromial bursa, and carefully observed the rotator cuff. Then we placed the scope in the glenohumeral joint before capsular release.
Our first step in capsular release was to eliminate the adhesion of the LHB to the interval using the radiofrequency instrument and rasp. Next, we resected the joint capsule just next to the labrum using the radiofrequency instrument and rasp from 5 o’clock to 1 o’clock of the right-side shoulder. Thus, we released the anterior, anteroinferior, superior, and superoposterior capsules in addition to eliminating the LHB adhesion to the interval. A rasp conventionally used for arthroscopic Bankart repair proved useful for moving the capsule into the neck of the glenoid without nervous complications. After arthroscopic capsular release, we arthroscopically assessed the movement of the LHB at the position of the maximum internal rotation at 0° abduction and the maximum external rotation at 0° abduction as well as the recovery of sliding of the LHB to the rotator interval in all groups.
Once the scope and instruments were removed, the shoulders were manipulated for external rotation at 0° abduction, external rotation at 90° abduction, internal rotation at 90° abduction, and flexion in the plane of the scapula. At the end of the capsular release we measured the ROM obtained after the manipulation was performed.
Postoperative protocol
Passive and assisted-active exercises and stooping exercises were commenced for forward flexion and external rotation 1 day after surgery with the assistance of a physical therapist. After 1 week of passive exercise, the patients began active exercise to strengthen the rotator cuff and scapular stabilizers. After rehabilitation for 4 weeks, the patients were back on normal work schedules without any limitations to daily activity. Rehabilitation was continued for 3 months after surgery to obtain complete muscle strength. We measured the JOA scores before and after surgery.
Histological examination
Serial paraffin sections (5 μm) of synovial tissue were stained with hematoxylin and eosin (H&E). For immunohistochemistry tests, deparaffinized, ethanol-dehydrated tissue sections were placed in an autoclave at 121°C for 20 min in a 10 mM sodium citrate buffer (pH 6.4), allowed to cool to room temperature, and then rinsed in a detergent solution [0.05M phosphate-buffered saline (PBS), pH 7.6] three times for 3 min each time. Tissue sections were blocked for 10 min in PBS containing 20% rabbit serum, followed by incubation overnight at 4°C with the following antibodies: anti-human phospho-p38 MAPK (Tyr180/Tyr182) mouse monoclonal antibody (1: 500) (3216S; Cell Signaling Technology, Danvers, MA, USA); anti-human phospho-p44/42 MAPK (Tyr202/Tyr204, ERK1/ERK2) mouse monoclonal antibody (1: 500) (9106S; Cell Signaling Technology); anti-human phosphor-JNK (1: 500) (G7) (sc-6254; Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-human NF-κB p50 rabbit polyclonal antibody (1: 200) (sc-7178; Santa Cruz Biotechnology), anti-TNFα mouse monoclonal antibody (1: 1000) (Biogenesis, Pool, UK), anti-human CD29 (β1-integrin) mouse monoclonal antibody (1: 350) (NCL-CD29; Novocastra, Newcastle upon Tyne, UK), MMP-3(1: 100) (5980-0311; Biogenesis, Mill Creek, WA, USA), IL-6 (1: 6000) (109-401-310; Rockland Immunochemicals, Rockland, ME, USA); CD56 (1: 1000) (NCL-CD56; Novocastra); CD68 (1: 1000) (M0814; Dako, Carpenteria, CA, USA); S-100 (1: 5000) (Z0311; Dako); and VEGF (1: 800) (05-443; Upstate/Millipore, Billerica, MA, USA).
After rinsing (0.05 M PBS, pH 7.6), endogenous peroxidase was blocked for 10 min with 0.3% hydrogen peroxide in Tris-buffered saline (10 mm Tris HCl, 140 mM NaCl, pH 7.4). This was followed by a 30-min incubation with a biotinylated species-specific anti-immunoglobulin G (anti-IgG) secondary antibody (Vector, Burlingame, CA, USA). The sections were then incubated for another 30 min with the appropriate Vectastain ABC reagent (Vector) using 3,3′-diaminobenzidine 4HCl (DAB) (Sigma) for a color reaction, which resulted in brown staining of antigen-expressing cells.
All slides were examined by light microscopy, and the presence of labeled cells was documented and tabulated. The absence of staining was documented as a negative result (−) and the presence of staining as a positive result (+) using a scale based on the number of cells per high-power field (×200), as previously reported:+, 1–4; ++, 5–10; +++, >10; ++++, >100.10 Statistical analysis was performed using the Mann–Whitney U-test for continuous variables and the chi-squared test for categorical variables for histological analysis. P < 0.05 was considered significant.
Results
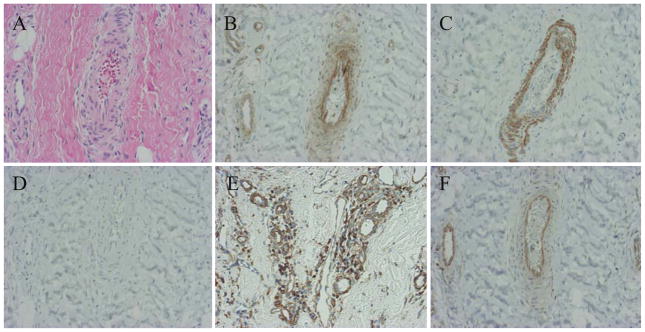
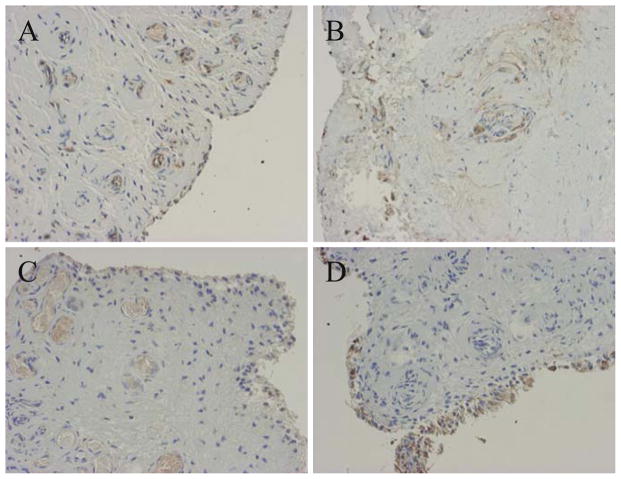
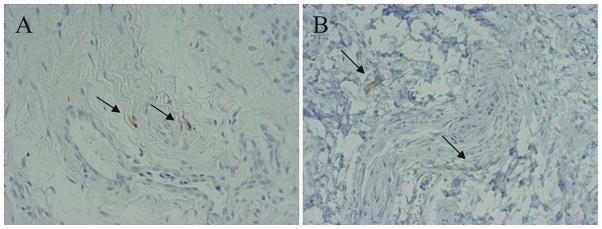
Histologically, H&E staining revealed vascular proliferation with fibrin and fibrous tissue (Fig. 1A). CD29 (β1-integrin), which is related to mechanical stress, was expressed in the vascular tissue and the superficial cells of synovial tissue (Fig. 1B). ERK1/ERK2 were strongly expressed in the epithelial cells of vascular tissue, but p38 MAPK was not expressed (Fig. 1C,D). JNK was strongly expressed in interstitial cells around vascular tissue (Fig. 1E). NF-κB was expressed in the epithelial cells of vascular tissue (Fig. 1F). Therefore, the β1-integrin and ERK expression patterns were similar in frozen shoulders. MMP-3 was expressed on the surface layer of synovial tissue and the epithelial cells of vascular tissue in the synovium (Fig. 2A). IL-6 was expressed in the interstitial cells and epithelial cells of vascular tissue in the synovium (Fig. 2B). VEGF was weakly expressed on the surface layer of synovial tissue and in the epithelial cells of vascular tissue in the synovium (Fig. 2C). CD68 was expressed on the surface layer of synovial tissue (Fig. 2D). Peripheral nerve-related proteins, CD56 and S-100, were not expressed in the synovium (Fig. 3A, B). Therefore, synovial tissues in a mechanical stress environment at the LHB and RI, β1-integrin, ERK1/ERK2, and NF-κB were expressed in the same pattern in epithelial cells, but JNK was strongly expressed in synovium, related to the production of MMP-3, IL-6, and VEGF in frozen shoulders. Detailed analysis of all samples revealed that p38, ERK1/ERK2, JNK, NF-κB, β1-integrin, and IL-6 were significantly increased in the synovium of frozen shoulders (Table 1). We did not detect a significant difference in the expression of ERK1/ERK2 or JNK between the right and left shoulders. We also found that the synovium of the recurrent dislocation of the shoulder as a control sample showed negative vascular proliferation with positive β1-integrin and ERK expression.
Fig. 1.
Immunohistochemical examination of synovial tissues in a frozen shoulder. A H&E stain. B CD29 β1-integrin. C ERK. D p38. E JNK. F NF-κB. (×200)
Fig. 2.
Immunohistochemical examination of synovial tissues in a frozen shoulder. A Matrix metalloproteinase-3. B Interleukin-6. C Vascular endothelial growth factor. D CD68. (×200)
Fig. 3.
Immunohistochemical examination of synovial tissues in a frozen shoulder. A S100. B CD56. Arrows indicate positive cells. (×200)
Table 1.
Results for immunohistochemical staining of synovial tissue in frozen shoulders
| Antibody | Resulta | No. of patients positive (n = 10) |
|---|---|---|
| p38 MAPK | − | 0 |
| p44/42 MAPK (ERK) | ++++ | 10 (100%) |
| JNK MAPK | ++++ | 10 (100%) |
| NF-κBp50 | ++++ | 10 (100%) |
| IL-6 | ++ | 6 (60%) |
| MMP-3 | +++ | 10 (100%) |
| CD29 (β-1 integrin) | ++++ | 10 (100%) |
| CD56 (nerve) | + | 4 (40%) |
| S100 (nerve) | + | 4 (40%) |
| CD68 (macrophage) | +++ | 10 (100%) |
| VEGF (vascular) | ++ | 6 (60%) |
Number of cells counted per high-power field x200
−, none; +, 1 to 4; ++, 5 to 10; +++, >10; ++++, >100
Discussion
Mechanical stress may be considerable in the subacromial space of a shoulder joint in the presence of an impingement syndrome. Nobuhara et al. reported that the major cause of frozen shoulder in 2027 patients was subacromial bursitis related to positive impingement signs.11 Therefore, impingement can be thought of as mechanical stress between the subacromial space and the coracoacromial (CA) ligament, during which the rotator cuff, RI, CH ligament, and LHB develop into frozen shoulder.
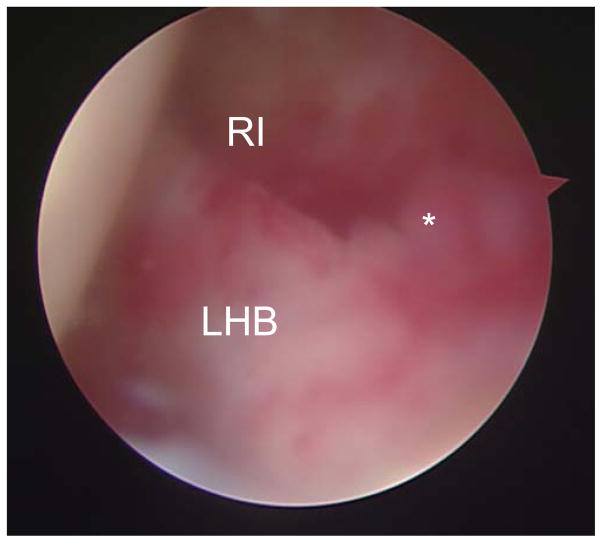
We recognized synovial proliferation at the RI in the impingement syndrome arthroscopically before contracture of the shoulder joint (Fig. 4). We took samples of the synovial tissue from the RI capsule of frozen shoulders for immunohistochemical examination of mechanical stress gene expression and cell signaling of MAP kinases. Previously, we reported that cartilage matrix deformity played an important role in transducing cell signaling in chondrocytes to cell signaling in the skeletal system.12 The dynamic movement of the LHB in a frozen shoulder is restricted to external rotation, with synovial proliferation of the RI, by contracture of the glenohumeral joint. However, at the molecular level, the transduction mechanism by the mechanical stress of impingement remains unclear in regard to the development of frozen shoulder.
Fig. 4.
Synovial proliferation at the rotator interval in a frozen shoulder. *Location of tissue sample obtained for histological examination. LHB, long head of the biceps tendon; RI, rotator interval
One limitation of this study is the lack of evidence of how to transduce the mechanical stress in the subacromial bursa and the development of expression of the mechano-transduction genes such as MAP kinases in the synovium of the RI in shoulder.
β1-Integrin has been reported to be one of the receptors of mechanical stress. We found that synovial tissue in the RI showed expression of β1-integrin in the epithelial cells of vessels. There are several MAP kinase molecules involved in mechanical stress (e.g., p38, ERK, JNK). It is reported that the ERK and JNK pathways of the MAP kinases are related to IL-6 and IL-8 expression after signaling nuclear translocation of NF-κB in fibroblasts in synovial tissues of patients with rheumatoid arthritis.13 JNK, a stress-activated protein kinase, is activated in cardiac myocytes after stretching and in endothelial cells in response to flow.14,15 Furthermore, it has been reported that cyclic mechanical stretching of human patellar tendon fibroblasts activate JNK and modulate apoptosis.16 We found ERK and JNK expression without p38 in the synovium of the RI with positive β1-integrin. Therefore, mechanical stress may induce the specific expression of ERK and JNK through β1-integrin for cell signaling. Furthermore, the transcription factor of IL-6, NF-κB, increased in the epithelial cells. Thus the mechanical stress on the RI due to impingement may induce the expression of β1-integrin to transduce the signaling of ERK for IL-6 production. Interestingly, JNK expression around vascular tissue with epithelial cells extended more widely than ERK and β1-integrin expression. Thus, ERK is specific for expression in epithelial cells, and fibroblastic cells and epithelial cells expressed JNK. Both mechanical stress molecules were expressed simultaneously, which might facilitate gene expression of NF-kB for IL-6 signaling. IL-6 is known to be an inflammation factor, and it induces fibroblast proliferation. Therefore, the contracture and fibroplasia of frozen shoulders may be related to IL-6 signaling. We speculated that the mechanical impingement between the acromion and the RI might induce the expression of mechanical stress-related cytokines.
We found partial rotator tears on the bursal side in 6 of 10 patients. The partial tear of the rotator cuff also might be involved in that mechanical change. The ERK and JNK of MAP kinase signaling of frozen shoulder may be one of the important factors for the development of contracture and fibroplasia in these shoulders. It is reported that the inhibitors of ERK — apigenin (Calbiochem, San Diego, CA, USA) and PD 98059 (Alexis Biochemicals, Lausen, Switzerland) — could block IL-6 production by inhibition of ERK cascades with no effect on cell viability.14 Therefore, those drugs related to inhibiting MAP kinase signaling can be developed for the treatment of frozen shoulder to inhibit ERK and JNK pathways, thereby decreasing IL-6. However, we have not used patients with synovial inflammation without joint contracture as a control, nor have we examined MAP kinases in the subacromial bursa. These are also limitations of our study.
It has been reported that neovascularization is important for the pathology of frozen shoulder, showing positive staining of VEGF and CD34.10 We also found VEGF in the epithelial cells of vascular tissue and the surface of the synovium. Peripheral nerve-related protein S100 and CD56 were weakly positive in our sample, and it has been reported that tests for S100 in RI biopsy specimens have been positive.10 Furthermore, the pain of frozen shoulder may be related to the production of neurally related protein S100, CD56, MMP-3, IL-6, and VEGF via ERK and JNK signaling in the presence of synovial proliferation due to mechanical stress in frozen shoulders. Therefore, mechanical stress may transduce cell signaling of MAP kinase by β1-integrin to change cytokine production and MMP in the fibroblasts of frozen shoulders, with the development of fibrosis and contracture of the shoulder joint.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) (C) (19591769) by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) and the Japan Society for the Promotion of Science (JSPS) and by grants-in-aid from the Aichi D.R.G. Foundation in Japan.
Footnotes
The authors did not receive and will not receive any benefits or funding from any commercial party related directly or indirectly to the subject of this article.
References
- 1.Duplay S. De la peri-arthrite scapulo-humerale et des raideurs de l’epaule qui en sont la consequence. Arch Gen Med. 1872;20:513–4. [Google Scholar]
- 2.Codman EA. The shoulder: rupture of the supraspinatus tendon and other lesions in or about the subacromial bursa. Boston: Thomas Todd; 1934. [Google Scholar]
- 3.Neer CS, II, Satterlee CC, Dalsey RM, Flatow EL. The anatomy and potential effects of contracture of the coracohumeral ligament. Clin Orthop. 1992;280:182–5. [PubMed] [Google Scholar]
- 4.Ozaki J, Nakagawa Y, Sakurai G, Tamai S. Recalcitrant chronic adhesive capsulitis of the shoulder: role of contracture of the coracohumeral ligament and rotator interval in pathogenesis and treatment. J Bone Joint Surg Am. 1989;71:1511–5. [PubMed] [Google Scholar]
- 5.Neviaser JS. Adhesive capsulitis of the shoulder: a study of the pathological findings in periarthritis of the shoulder. J Bone Joint Surg. 1945;27:211–22. [Google Scholar]
- 6.Lundberg BJ. The frozen shoulder: clinical and radiographical observations — the effect of manipulation under general anesthesia; structure and glycosaminoglycan content of the joint capsule; local bone metabolism. Acta Orthop Scand Suppl. 1969;119:1–59. [PubMed] [Google Scholar]
- 7.Bunker TD, Lagas K, Deferme A. Arthroscopy and manipulation in frozen shoulder. J Bone Joint Surg Br. 1994;76:53. [Google Scholar]
- 8.Bunker TD, Reilly J, Baird KS, Hamblen DL. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J Bone Joint Surg Br. 2000;82:768–73. doi: 10.1302/0301-620x.82b5.9888. [DOI] [PubMed] [Google Scholar]
- 9.Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, et al. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta 1-integrin and focal adhesion kinase. J Mol Cell Cardiol. 2007;43:137–47. doi: 10.1016/j.yjmcc.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hand GC, Athanasou NA, Matthews T, Carr AJ. The pathology of frozen shoulder. J Bone Joint Surg Br. 2007;89:928–32. doi: 10.1302/0301-620X.89B7.19097. [DOI] [PubMed] [Google Scholar]
- 11.Nobuhara K, Sugiyama D, Ikeda H, Makimura M. Contracture of the shoulder. Clin Orthop. 1990;254:105–10. [PubMed] [Google Scholar]
- 12.Kanbe K, Yang X, Wei L, Sun C, Chen Q. Pericellular matrilins regulate activation of chondrocytes by cyclic load-induced matrix deformation. J Bone Miner Res. 2007;22:318–28. doi: 10.1359/jbmr.061104. [DOI] [PubMed] [Google Scholar]
- 13.Neff L, Zeisel M, Sibilia J, Scholler-Guinard M, Klein JP, Wachsmann D. NF-κB and the MAP kinases/AP-1 pathways are both involved in interleukin-6 and interleukin-8 expression in fibroblast-like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell Microbiol. 2001;3:703–12. doi: 10.1046/j.1462-5822.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- 14.Komuro I, Kudo S, Yamazaki T, Zou Y, Shiojima I, Yazaki Y. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. FASEB J. 1996;10:631–6. doi: 10.1096/fasebj.10.5.8621062. [DOI] [PubMed] [Google Scholar]
- 15.Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells: role of β 1 integrins and tyrosine kinases. Circ Res. 1996;79:310–6. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 16.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc. 2003;11:122–9. doi: 10.1007/s00167-002-0322-y. [DOI] [PubMed] [Google Scholar]